Review Article - International Journal of Clinical Rheumatology (2020) Volume 15, Issue 3
An autoimmune storm: A case based literature review of successful management of lupus-polymyositis overlap syndrome complicated by multiple organ failure
- Corresponding Author:
- Ali Hameed Ali
The University of California San Francisco
Fresno Medical Education Program, Department of Internal Medicine
Division of Rheumatology, Fresno, California, USA
E-mail: aali@fresno.ucsf.edu
Abstract
Overlap syndromes of Connective tissues diseases is a rare and under-studied disorder. This syndrome can be complicated by multiple life-threatening complications like hepatitis, pancreatitis, macrophage activation syndrome and Acute Respiratory Distress syndrome. We report a 22-year-old Hispanic male with no remarkable past medical history who presented to the hospital with abdominal pain, myalgia, arthralgia, and persistent fever for two weeks and was found to have tachycardia, pancytopenia, transaminitis and an elevated lipase. The patient was initially evaluated for acute pancreatitis and hepatitis due to binge drinking and presumptive viral infection. Infectious work up was unrevealing, and the patient continued to deteriorate. The autoimmune panel came back significant for highly elevated Antinuclear Antibody (ANA) and Anti Double Stranded DNA antibody (dsDNA), low complement, high inflammatory markers, ferritin, elevated Creatine Kinase (CK), positive Anti Mi 2 antibody, low Natural Killer (NK) cell activity and elevated CD25. Patient was subsequently diagnosed with Lupus- Polymyositis overlap syndrome. Acute lupus flare was considered as the trigger for pancreatitis and hepatitis. The hospital course was complicated by Acute Respiratory Distress Syndrome (ARDS) due to Lupus pneumonitis, encephalopathy, and Macrophage Activation Syndrome (MAS). Patient was treated successfully with steroid pulse therapy, Cyclophosphamide, plasmapheresis, artificial ventilation, and extracorporeal membrane oxygenation (ECMO). We believe that this case is unique as it represented a diagnostic challenge as the Lupus-Polymyositis overlap syndrome is a rare entity, and it initially manifested in an extensively complicated pattern which is unusual. Moreover, this case was not only a diagnostic challenge but also a treatment conundrum due to multiple life-threatening complications the patient had during this hospitalization. In this review, we intend to discuss the management of this challenging and rarely reported connective tissue disease overlap syndrome.
Keywords
lupus-polymyositis overlap syndrome • lupus hepatitis • lupus related pancreatitis • macrophage activation syndrome • acute respiratory distress syndrome
Abbreviations
ARDS: Acute Respiratory Distress Syndrome; ANA: Antinuclear Antibody; dsDNA: Anti Double Stranded DNA antibody, Antiphospholipid Syndrome (APS), Anti-Smooth Muscle Antibody (ASMA); AIHA: Autoimmune hemolytic anemia ; AIH: Autoimmune Hepatitis; ATN: Acute Tubular Necrosis; CT: Computed Tomography; CTDs: Connective Tissue Diseases; CK: Creatine Kinase; CMV: Cytomegalovirus; CRP: C-Reactive Protein, DM: Dermatomyositis; DAH: Diffuse Alveolar Hemorrhage; DCTD: Definitive Connective Tissue Disease; EBV: Epstein Barr Virus; ECMO: Extracorporeal Membranous Oxygenation; ELSO: Extracorporeal Life Support Organization; FHLH: Familial Hemophagocytic Lymphohistiocytosis; HLH: Hemophagocytic Lymphohistiocytosis; LDH: Lactate Dehydrogenase; LFTs: Liver Function Tests; MAS: Macrophage Activation Syndrome; MCTD: Mixed Connective Tissue Disorder; PM: Polymyositis; PSC: Primary Sclerosing Cholangitis; OS: Overlap Syndrome; RNP: Ribonucleoprotein; SSc: Systemic Sclerosis; SLE: Systemic Lupus Erythematosus; UCTD: Undifferentiated Connective Tissue Disease; VZV: Varicella Zoster virus; VVECMO: Veno-Venous Extracorporeal Membranous Oxygenation
Case report
A 22-year-old Hispanic male presented to the hospital with abdominal pain, watery diarrhea, nausea, vomiting, and subjective fever. Initial vital signs showed a heart rate 106 bpm and blood pressure 90/60 mmHg but no fever. Physical examination was remarkable for conjunctival pallor and mild generalized abdominal tenderness.
Complete blood count revealed pancytopenia. Complete metabolic panel was pertinent for elevated creatinine, hyperbilirubinemia, transaminitis and significant elevation of lipase in addition to deranged coagulopathy (Table 1, Figures 1 and 2). No bacterial growth was appreciated on blood cultures. Viral hepatitis panel was not remarkable.
Table 1. Laboratory Trend.
| Test | Day 1 | Day6 | Day 21 | Outpatient follow up visit Day 42 |
|---|---|---|---|---|
| HB | 13.2 | 6.2 | 8.4 | 10.6 |
| WBC | 1.5 | 6.4 | 10.4 | 9.7 |
| ANC | 1.1 | 2.5 | 17.4 | = |
| Platelets | 66 | 40 | 213 | 219 |
| ALT | 261 | 397 | 293 | 114 |
| AST | 566 | 1973 | 167 | 40 |
| Total Bilirubin | 1.2 | 19.9 | 2.2 | 0.6 |
| Lipase | 510 | 5417 | 1660 | 211 |
| Creatinine | 6959 | 1300 | 65 | 55 |
| kinase | ||||
| Alkaline | 105 | 105 | 76 | 66 |
| Phosphatase | ||||
| INR | 1.4 | 3.9 | 1.3 | 1.1 |
| Fibrinogen | 176 | 80 | 235 | 270 |
| Triglyceride | 119 | 380 | 382 | = |
| Ferritin | Not checked | 5764 | 2252 | = |
HBG: Hemoglobin; WBC: White blood cell; ANC: Absolute Neutrophil count; ALT: Alkaline Phosphatase; AST: Alanine Aminotransferase; CK: Creatinine Kinase; ALP: Alkaline Phosphatase; INR: International Normalised Ratio
Figure 1. Day 1- Chest radiograph: normal lung appearance (Chest X-ray one).
Initial chest X-ray was unrevealing. Ultrasound of abdomen and pelvis showed hepatomegaly and heterogenicity of liver parenchyma with suspicion of acute hepatitis. CT abdomen without contrast was remarkable for acute pancreatitis. Magnetic resonance cholangiopancreatography MRCP was unremarkable. The patient was diagnosed initially with acute pancreatitis and hepatitis secondary to binge drinking and treated conservatively. He received a short course of piperacillin/tazobactam for 4 days and discontinued 4 days later due to negative blood cultures.
On Day 5, the patient developed a fever of 103 F, became more lethargic and had an episode of epistaxis. Transaminitis was noted to be worsening. Further work up showed Elevated sedimentation rate (90 mm) C-Reactive protein (CRP) (75.1 mg/l), and hypocomplementemia. The immunology tests were significant for an ANA of 1:1280 with homogenous pattern and dsDNA (6224 IU/L, normal range < 5 IU/L), Creatine kinase is 6959 ( normal range 38-174 U/L), myositis panel was positive anti-MI-2 alpha ab (13 SI) but negative for sRNP, Mitochondrial antibody and ribosomal antibody. Total IgG level was normal. CT guided liver biopsy was done and showed nonspecific hepatitis and unremarkable for autoimmune hepatitis. Bone marrow biopsy and cytogenetics was done due to pancytopenia which showed normal cellular exam.
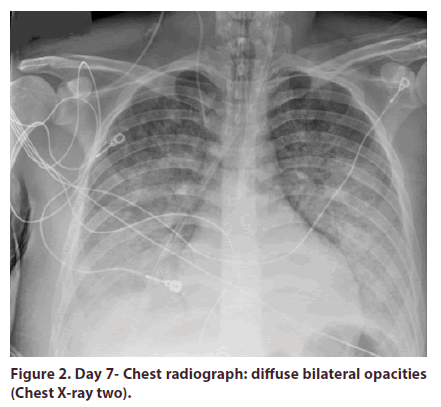
The diagnosis of lupus-overlap syndrome was made. Initial therapy included plasmapheresis for 5 days and pulse steroid therapy with methylprednisolone 1000 mg. However, on day 6, the patient started developing dyspnea and became more hypoxic. Repeated chest radiograph showed bilateral pulmonary opacities. CT chest with contrast demonstrated bilateral pulmonary opacities with suspected acute respiratory distress syndrome. Transthoracic Echocardiography was remarkable for grade 1 diastolic dysfunction with preserved ejection fraction. He was intubated due to persistent hypoxia and then placed on Extracorporeal Membrane Oxygenation (ECMO) therapy due to persistent and resistant hypoxemia.
Bronchoscopy was done with no alveolar hemorrhage appreciated. Bronchoalveolar lavage was negative for bacterial and fungal growth. At the same time, his markers of kidney function continued to worsen, and the patient became anuric. Urine analysis showed proteinuria >500 mg/dl, hematuria 3+ and muddy brown cast. Continuous renal replacement therapy was initiated due to anuria. Kidney biopsy was done after the patient started to get better and found to have WHO class II lupus nephritis and extensive Acute Tubular Necrosis (ATN) attributed to rhabdomyolysis.
Due to high suspicion of MAS in the setting of high ferritin levels, pancytopenia, we checked the Natural Killer cell activity which was decreased 3 LU30 (Ref range 7-125), and CD25 which showed an elevation with 3507 pg/ml (ref range 532-1891 pg/ml). Both helped to confirm the diagnosis of MAS.
Extensive virology tests were done to rule out any viral etiology of his hepatitis. The viral testing showed an elevated Epstein Barr Virus (EBV) DNA PCR in the serum of 2592 copies/ml and EBV Nuclear Antigen IgG of 465 U/L, and elevation of both Cytomegalovirus CMV IgG and IgM of 240 Au/ml and 6.40 U/L but negative DNA PCR. Varicella Zoster virus (VZV) IgG 1.62 (positive), IgM >5.00 (positive) but patient had history of VZV vaccination. Based on the above virology work up, active EBV infection and defined as possible trigger of both lupus and MAS. EBV infection was treated conservatively.
Lupus-Polymyositis overlap syndrome complicated by multiple organ failure was treated with pulse methylprednisolone 1,000 mg for 3 days, then methylprednisolone 1 mg/kg daily along with Trimethoprim-sulfamethoxazole for Pneumocystis Jiroveci prophylaxis. Cyclophosphamide 500 mg with Mesna once with a plan to repeat every two weeks for a total of 6 doses. Patient was decannulated from ECMO three days later and extubated on day 12 and eventually downgraded to a step-down unit. Patient was discharged from the hospital two weeks later on prednisone 60 mg, hydroxychloroquine 200 mg twice daily, and did not receive further cyclophosphamide infusion given that his nephritis was only Class II. He was seen in our Rheumatology, Pulmonary, and Nephrology outpatient clinic 2 weeks after discharge and was doing remarkably well with no signs of flare or disability. Eventually, tapered-off steroids and discontinuation of hemodialysis after gaining kidney function.
Introduction
Connective Tissue Diseases (CTDs) are systemic inflammatory autoimmune disorders characterized by a large spectrum of clinical and laboratory features. The CTD spectrum includes, however is not limited to dermatomyositis (DM), Polymyositis (PM), Systemic Sclerosis (SSc), Systemic Lupus Erythematosus (SLE), and Sjögren’s syndrome [1]. Although each disease can manifest in a specific entity, they can overlap with each other, creating the category of Overlap Syndrome (OS). The concept of Overlap Syndromes (OSs) implies the occurrence of two or more well-defined CTD in the same patient [1,2]. OSs are not frequent, and their descriptions in the literature are limited to a few case reports and case series [3]. CTD can be present with a broad range of complications. In this case, pancreatitis and acute liver failure was the initial presentation.
Acute pancreatitis is an uncommon but life-threatening complication in many CTDs, of which, lupus is the most common etiology [4]. Hepatic dysfunction is a reported finding in lupus and polymyositis, although a true liver disease triggered by SLE itself is a controversial issue, as with 25% to 50% of lupus patients may present with transaminitis [5]. In polymyositis, transaminitis can often be mistaken for hepatic involvement, but truly it is due to the underlying inflammatory myopathy [6]. To the best of our knowledge, literature of noninfectious hepatitis in polymyositis or lupus-PM overlap syndrome is insufficiently reported. Another lifethreatening challenge we experienced with our patient was acute hypoxic respiratory failure secondary to Acute Respiratory Distress Syndrome (ARDS). ARDS, which is characterized by bilateral inflammation of the lung, is a recognized complication of CTDs, including both lupus and polymyositis. It can happen either secondary to autoimmune pneumonitis, Diffuse Alveolar Hemorrhage (DAH), or due to infection [7]. If all these complications were not enough, in addition to the above multiorgan dysfunction, our patient also developed Macrophage Activation Syndrome (MAS). MAS is the secondary form of Hemophagocytic Lymphohistiocytosis (HLH) that happens due to an underlying autoimmune disease. The latter is characterized by severe, uncontrolled, hyperinflammatory immune activation that can occur in all age groups [8].
In this case review, we will discuss the overlap syndrome and the cytokine storm complications we dealt with. We hope that by sharing our unique complicated patient experience, our reported management approach might help to treat others in such a clinical conundrum.
Discussion
Autoimmune inflammatory rheumatic diseases, as the name suggests, are a group of illnesses arising from a combination of loss of tolerance to self-antigens and shifting of the immune system into self-destructive overdrive. The vast data on clinical spectrum and antibodies associated with various phenotypes have led to the classification of these systemic autoimmune tdiseases under five mutually exclusive diseases which include:
• Systemic Lupus Erythematosus (SLE)
• Systemic Sclerosis (SSc)
• Dermatomyositis (DM)
• Polymyositis (PM) and
• Primary Sjögren syndrome.
These diseases are labelled as Definitive Connective Tissue Diseases (DCTD) [9]. However, some of the cases of systemic connective tissue disease do not fulfil the criteria of a DCTD or has the criteria of more than one disease. This led to the existence of Undifferentiated Connective Tissue Disease (UCTD), Mixed Connective Tissue Disorder (MCTD), and Overlap Syndrome (OS).
UCTD is often referred to patients who do not have specific clinical features but possess positive autoantibodies. Work up for this subsect does not satisfy the proposed classification criteria of any DCTD. However, the diagnosis of UCTD should not be made unless the patient is unclassifiable for at least 3 years. Interestingly, a significant percentage of early UCTD gradually evolves to DCTD or an overlap syndrome (35%).
On the other hand, OS is diagnosed when the patient meets the classification criteria of two different DCTD. It differs from MCTD which is an evolved overlap syndrome but strongly associated with ant-U1 Ribonucleoprotein (RNP) antibody. Several criteria to classify MCTD have been compared and one study reviewing four sets of diagnostic criteria (Sharp, Alarcon- Segovia, Kasukawa, and Kahn) concluded that those of Alarcon-Segovia and Kahn were better compared to others [10]. The criteria utilized by Alarcon-Segovia had a sensitivity and specificity of 63% and 86%, respectively. These criteria will not be discussed in this review. Our patient meets the diagnosis of overlap syndrome as he had Lupus and polymyositis with negative U1-RNP antibody. This is important to distinguish as patients with U1 RNP antibodies have higher chance to develop pulmonary hypertension, interstitial lung disease, myocarditis, renovascular hypertension and cerebral edema but rarely develop glomerulonephritis.
Inflammatory myositis is relatively rare (4%-16%) in lupus. Patients with SLE-myositis overlap are reported generally at a younger age, and the duration of the disease was inversely correlated for the occurrence of myositis in SLE, suggesting it is an early manifestation of the disease (as what we noticed in this case). Although both Dermatomyositis and PM have been reported with SLE, the former is reported more often. In the largest published series of this phenotype, the occurrence of SLE was followed by an average of 14.3 months for the development of myositis in six out of ten patients. In the remaining four patients, two developed myositis after the diagnosis of SLE at an average of 8 months and two had simultaneous occurrence of the SLE and myositis [11]. In terms of PM autoantibody panel, Anti- Mi-2 was positive. Although Anti-Mi-2 is highly associated with DM and its classic skin finding such as Gottren’s papules and heliotropic rash. Our patient did not develop any dermatological manifestations. Fortunately, this antibody is associated with low lung involvement, good prognosis, and has a favorable response to steroids [12]. In OS, this particular antibody is insufficiently reported.
PM is characterized by symmetrical, proximal, muscle weakness, that involves both the upper and lower limbs. Trunk muscles are often involved in the later stages of the disease course. If treated promptly, patients with myositis in SLE have been reported less likely to have pharyngeal weakness, neck flexor weakness, and involvement of respiratory muscles. Clinical manifestation of skin rash, alopecia, vasculitis, oral ulcerations, and pericarditis are reported to be significantly higher in overlap of SLE with myositis compared to SLE alone. Comparing overlap syndrome with SLE alone, Raynaud’s phenomenon and pulmonary involvement in the form of clinically relevant ILD are reported more with overlap syndromes. The occurrence of erosive arthritis is more commonly described in SLE-myositis overlap and the occurrence of lupus nephritis and neuropsychiatric lupus is similar in both the groups [13]. Laboratory investigations across SLE-myositis cohorts have shown higher incidence of leukopenia and thrombocytopenia in the overlap subgroups. Among the myositis specific antibodies, antijo- 1 antibody is reported in up to 30% cases. In regard to Lupus-specific antibodies such as anti-Sm antibody, they are reported more commonly in the overlap syndromes compared to SLE alone. ANA positivity, Anti-dsDNA, and antiphospholipid antibody positivity and occurrence of hypocomplementemia do not differ between SLE and SLE-myositis overlap. Muscle biopsy findings of SLE include inflammation, vasculitis, type II fiber atrophy, vessel wall thickening, vacuolar myopathy, and rarely, inclusion body myositis [14]. Patients with overlap syndrome have muscle biopsy features with predominance of muscle inflammation and necrosis [9]. The other two big categories of overlap syndrome include Lupus- RA overlap “Rhupus” and Lupus- SSC “lupderma” which will not be discussed in this review.
Patients with overlap syndrome are usually excluded from the trials involving DCTD and hence the data on management is mainly from an expert opinion or from the recommended treatment of SLE, SSc, and inflammatory myositis. Management of SLE with myositis overlap in general does not differ from the management of inflammatory myositis or SLE with glucocorticoids being the mainstay of treatment. The majority of patients of SLE show an adequate response to glucocorticoids but will need an alternative DMARD in order to effectively taper off steroids. In patients with inadequate response, adverse drug reaction, or persons of any other major organ involvement with SLE, an alternative immunosuppressant agent is required upfront. Methotrexate, azathioprine, cyclosporine, cyclophosphamide, and mycophenolate are the usually preferred second line immunosuppressants in refractory myositis with SLE [15]. We treated our patient with steroid pulse therapy with methylprednisolone 1 gm for 5 days. Cyclophosphamide was started due to the severe complications. Moreover, the initial impression of kidney impairment was attributed to lupus before the result of kidney biopsy came back which showed benign class II lupus nephritis. The patient underwent five sessions of plasmapheresis as adjunctive therapy.
The prognosis of myositis varies greatly and factors that affect prognosis in PM should be considered the same in overlap syndrome SLE/myositis. These include the patient's age, the severity of myositis, the presence of dysphagia or cardiopulmonary disease and the initial response to corticosteroid therapy [15].
Lupus hepatitis
Although a true liver disease induced by SLE itself is a controversial etiology, 25% to 50% of SLE flare patients may present with alterations in the Liver Function Tests (LFTs). In polymyositis, deranged liver enzymes can be wrongly diagnosed as liver involvement which may be elevated in inflammatory muscle diseases. To the best of our knowledge, academic literature about noninfectious hepatitis in polymyositis or lupus-PM overlap syndrome is insufficiently reported [5]. Therefore, most of the literature we have reviewed discuss hepatitis in Lupus only.
The frequent association between SLE and LFT alterations may be accounted for by three possibilities, namely
• The existence of intraparenchymal injury associated with SLE alone, often referred to as “lupus hepatitis”
• The occurrence of an overlap syndrome by which SLE shows additional features of another autoimmune liver disease like Autoimmune Hepatitis (AIH) and
• The concurrency of comorbidity of SLE with a non-autoimmune hepatopathy, e.g., drug-induced liver damage, viral hepatitis or thrombotic liver disease, among others [16].
Lupus hepatitis has been reported in 3%-9% of patients affected by SLE [17-19]. Although it is still a controversial issue, there is compelling evidence in the literature that lupus itself is not associated with a specific, severe, and progressive liver injury. However, several authors have highlighted a role for SLE in triggering an often subclinical hepatopathy, referred to as “lupus hepatitis”. They described this disease entity as an asymptomatic transaminitis frequently associated with exacerbations of the lupus disease, which returns to normal values after corticosteroid therapy [19-21].
Another entity that needs to be considered and ruled out among SLE patients with transaminitis is an overlap with AIH, which represents a separate disease process from lupus, primarily because of its distinct pathogenic mechanism and its distinctive biochemical, serological, and histological characteristics that allow for a clear differentiation. Hypergammaglobulinemia, [autoantibodies Antinuclear Antibody (ANA), Anti-Smooth Muscle Antibody (ASMA), anti-liverkidney microsome antibodies], a histological profile characterized by piecemeal necrosis (interface hepatitis), and a rich plasma cells infiltrate are highly distinctive aspects of AIH. It is important to mention that the serological markers might overlap between lupus hepatitis and AIH. In this situation, liver histology is the decisive tool to define diagnosis. The presence of cirrhosis or periportal hepatitis associated with lymphocytes and plasma cell infiltration, as well as rosette formation of liver cells, tips the scales in favor of AIH being the etiology. On the other hand, the presence of mainly lobular and occasionally portal inflammation with a paucity of lymphoid infiltrates are more compatible with SLE [22].
On the other hand, if a lupus patient presents with evidence of progressive non-autoimmune chronic hepatitis characterized by persistent severe inflammatory damage, we need to consider other probable diagnosis of chronic liver injury, such as hepatitis B or C, or other autoimmune diseases overlapping with lupus. The discrimination is further complicated by the fact that liver histopathological features in patients with lupus hepatitis are miscellaneous and nonspecific, similar to those in other liver diseases. It is therefore important, before diagnosing lupus hepatitis, to rigorously rule out other liver diseases, including drug-induced liver injury, alcohol liver disease, viral hepatitis (common offenders being hepatitis A, B, C, D, E, Epstein-Barr virus or cytomegalovirus), and other autoimmune- associated liver diseases such AIH, PBC, Primary Sclerosing Cholangitis (PSC) [16]. A study by Zheng et al. [19] reported a 9.3%incidence of lupus hepatitis among 504 SLE patients that were evaluated. They also reported that the prevalence of lupus hepatitis in patients with active SLE was higher than those with inactive SLE (11.8% vs 3.2%). Patients with lupus hepatitis mostly showed mild to moderate elevations of serum transaminase levels, though six patients had jaundice as the predominant feature. ALP and Gamma-glutamyl transferase elevations were far less frequent.
In patients with suspected lupus hepatitis, it has often been reported a correlation between hepatic enzymes abnormalities and autoantibodies to ribosomal P proteins (anti-ribosomal P), a highly specific marker for SLE [23-25]. Indeed, several reports suggest that SLE-related hepatitis may be associated with, or even caused by antiribosomal P which occurs in 12%-16% of patients with lupus [24-26] .The proportion of serum anti-ribosomal P occurrence raised to 44% among SLE patients with liver dysfunction and of them 70% had SLE-associated hepatitis, a far higher value as compared with SLE patients suffering from other hepatic alterations, such as fatty liver (29%), drug-induced hepatitis (17%), or SLE-AIH overlap syndrome (20%) [27]. Furthermore, Koren et al. [28] reported the development of chronic active hepatitis in a patient with SLE followed several months later by the appearance of high serum levels of anti-ribosomal P antibodies which suggests a possible causal relationship. As for the mechanism of explaining this causal relationship, anti- ribosomal P positive serum from SLE patients was found to react strongly “in vitro” with a polypeptide antigenically related to a 38 kD ribosomal P0 protein present on the plasma membrane of hepatoma cells [29].
Although these findings help link anti-ribosomal P antibodies to liver damage in SLE patients, the association is still highly controversial. For example, lack of a clear association between lupus hepatitis and antiribosomal P levels was reported in another published retrospective study of 73 patients with SLE, where 12 of them (16%) were reported to have lupus hepatitis. In this group, 6 patients had a concurrent liver involvement with the diagnosis of SLE, and it occurred later during an exacerbation of the disease in the remaining 5 patients [23]. Despite elevated liver enzymes being noted in 11 cases and cholestasis in 8, the presence of anti-ribosomal P antibodies was observed only in one case. Although the authors showed clear evidence of immunosuppressive therapy response in most patients, liver biopsy was performed in less than half of them, and their description was not detailed enough to clearly differentiate lupus hepatitis from AIH.
Moreover, Calich et al. [30] reported the presence of anti-ribosomal P antibodies in patients having AIH not associated with lupus (9.7%; 9/93), further suggesting that this antibody predicts a worse prognosis of the disease. This finding suggests that anti-ribosomal P antibodies maybe involved in the pathogenesis of other hepatic autoimmune diseases, apart from lupus hepatitis and maybe used to help prognosticate patients in such a clinical setting. The debate is still open, and it is apparent that we need more data to support the role and impact of anti-ribosomal P antibodies in both SLE and AIH pathogenesis. In general, the levels of liver enzymes seen in lupus hepatitis return to normal following glucocorticoid therapy [16].
Our patient did have severe hepatitis that led to acute liver failure in the setting of encephalopathy and coagulopathy. Autoimmune hepatitis was ruled out based on the serology work up and most importantly, the nonspecific liver biopsy results. Although antiribosomal antibody has a high association with lupus hepatitis, our patient's test result came back negative. All virology work up came back negative except from EBV infection. However, the latter was not considered as the cause of this hepatitis because liver function starts to improve after starting the steroids, which make lupus hepatitis as the most likely underlying etiology of the acute liver failure.
Lupus-related pancreatitis
Acute pancreatitis is a rare complication of SLE. It is seen in approximately 0.2%-8.2% of all lupus patients. In the majority of instances, it presents as a lupus flare [31,32]. Occasionally, it can be a major presenting symptom of SLE [33]. To the best of our knowledge, acute pancreatitis was never reported in polymyositis or in lupus-PM overlap syndrome. Most of the discussion in this part of the review will focus on pancreatitis in lupus patients. The underlying cause of pancreatitis in lupus may or may not be related to lupus itself. Gallstones are the most common cause of acute pancreatitis with alcohol abuse coming in second. However other less common etiologies must be considered in a subset of patients, specifically patients with lupus. Two separate etiologies have been classified in patients who present with pancreatitis and have a history of lupus: Lupus related pancreatitis and autoimmune pancreatitis.
Autoimmune pancreatitis is a form of pancreatitis that can be seen concomitantly in patients with lupus. It has its distinct pathogenic mechanism and its distinctive biochemical, serological, and histological characteristics that allow for a clear differentiation. In Autoimmune pancreatitis, the diagnostic criteria proposed by the Mayo Clinic (the "HISORt" criteria) are most commonly used in the United States and include the presence of one or more of the following: Diagnostic Histology (eg. more than 10 IgG4-positive cells with at least two of the periductal lymphoplasmacytic infiltrate. Characteristic Imaging on Computed Tomography (CT) (diffusely enlarged pancreas with featureless borders and delayed enhancement with or without a capsule-like rim) and/or pancreatography, Elevated serum IgG4 levels on Serologic testing , Other organ involvement including the salivary glands (Sjögren's syndrome), lung nodules, autoimmune thyroiditis, obstructive jaundice and kidney (interstitial nephritis), and lastly Response of pancreatic and extra pancreatic manifestations to glucocorticoid Therapy [34-36].
In our case, no pancreatic biopsy was done, CT imaging did show subtle pancreatitis with diffuse involvement, IgG4 level was low, other organ involvement was not specific for autoimmune pancreatitis. However, our patient did show significant response to steroid and other immunosuppressive therapy and without other suggestive findings of autoimmune pancreatitis, given this we came to the conclusion that our patient probably had lupus related pancreatitis.
There have been increasing reports of association between Autoimmune Hemolytic Anemia (AIHA) and pancreatitis in SLE [37,38]. The exact cause is not known. In fact, any condition that causes massive hemolysis can give rise to acute pancreatitis secondary from bilirubin stones. In a retrospective study by Druml et al. it was found that the prevalence of pancreatitis was 20% in forty patients who had massive hemolysis (which was defined as 12% drop of hematocrit in 12 h) [39].
Antiphospholipid Syndrome (APS) syndrome is another entity that thought to be linked with the development of acute pancreatitis in lupus [40,41]. However, according to Hopkins lupus cohort, Antiphospholipid antibodies were not found to be associated, meanwhile a history of thrombosis, cutaneous vasculitis, secondary Sjogren’s syndrome, and triglyceridemia were all suggested as potential mechanisms. Only triglyceridemia remained significant in the multiple regression model for a strong association with pancreatitis attributable to SLE [32]. The method of diagnosing pancreatitis in SLE does not differ as compared to non-SLE patients. Two of the following three help in cliniching the diagnosis: Burning epigastric pain with or without radiation to the back, Increased lipase (> 3x ULN), and or Ultrasound/CT imaging findings concerning pancreatitis.
The management of acute pancreatitis in SLE can be quite challenging. With the probable association between SLE and APS and/or AIHA, patients should be investigated for these conditions as early as possible. Initiating specific treatment early in the course of disease may help in preventing mortality and morbidity. Corticosteroids are indicated in autoimmune hemolytic anemia and lupus related pancreatitis. Apart from steroids, increasing evidence supports the use of rituximab in AIHA, particularly warm agglutinin AIHA [42]. In a study by Birgens et al. a satisfactory response was observed in 75% of the patients treated with rituximab and prednisolone, whereas it was observed only in 36% of those given prednisolone alone (P=0.003). After 36 months, about 70% of the patients who had received rituximab and prednisolone were still in remission, compared with only 45% of those in the prednisolone group [43]. In patients with APS also, apart from anticoagulation, rituximab has been suggested to have a significant role [44,45]. The European registry suggested an essential role of rituximab in catastrophic APS [46]. The rate of complications if lupus pancreatitis remains untreated is as large as 57%, with mortality of up to 45%, higher than those observed in non-SLE populations [47].
Steroid treatment for acute pancreatitis in SLE patients is controversial because of steroid-induced toxic effect; however, this concern is regarded as minimal. Because the immunosuppressive effect of steroids can significantly improve prognosis in patients with acute pancreatitis, recent studies recommend the administration of steroids during the acute episode lupus related pancreatitis. Studies have shown mortality among patients who received glucocorticoids following the diagnosis of pancreatitis was 20%, compared to 61% of those who did not receive this therapy [31,48].
Macrophage activation syndrome
It is a life-threatening hyperinflammatory syndrome characterized by a set of non-specific clinical and laboratory features with inconstant histiocyte proliferation and hemophagocytosis [49]. It is a subset of secondary Hemophagocytic Lymphohistiocytosis (HLH) that happens secondary to rheumatic diseases. The main manifestations are high grade fever, hepatosplenomegaly, and lymphadenopathy. The most typical laboratory abnormalities are cytopenias, elevated levels of ferritin, triglycerides, transaminitis, Lactate Dehydrogenase (LDH), and low fibrinogen [50,51]. HLH is categorized as primary or familial HLH (FHLH), when there is a familial history of HLH or a known underlying genetic defect [52]. Secondary HLH can occur during systemic infections (in particular with EBV or CMV [53], malignancy and rheumatic disorders such as Still disease or SLE [54]. MAS is a well-recognized complication in Lupus [49] and it’s also reported in PM but less frequently [55]. Although the occurrence of MAS is possible in the setting of overlap syndrome, to the best of our knowledge, this is the first case of overlap syndrome with MAS. There is paucity of data on MAS in overlap syndrome.
In adult onset SLE, MAS has been reported with frequency of up to 5% but the accuracy is unclear due to a significant number of underdiagnosed MAS [52,56]. Mortality associated with MAS in SLE is as high as 50 % [57]. A recent large retrospective cohort showed that half of the cases where identified as African American [58]. An important characteristic of lupus associated MAS is that it tends to happen during severe flare or in with the very onset of the disease [59].
In SLE, MAS can mimic an acute exacerbation of the lupus because both share some common features, such as fever and cytopenia. This overlap in clinical presentations can delay the diagnosis and, consequently, the selection of the most appropriate therapeutic approach of MAS. Delayed diagnosis has been associated with more ICU admission and mortality irrespective of SLE flare [49]. And to complicate MAS presentation in Lupus, it is found to be usually triggered by viral infection [49,56,58,60].
Although MAS and lupus flare presentations share clinical and laboratory similarities, thrombocytopenia was found to be a discriminative feature between lupus flare and MAS as it happens more frequently in MAS [49,57]. Interestingly, number of cohorts did show significant association between lupus with renal involvement and the occurrence of MAS [49,56-58]. Importantly, a negative bone marrow aspirate for Hemophagocytosis should not rule out the diagnosis of MAS as this is not present in all patients evaluated [50,52,61].
Regarding treatments options, high dose intravenous steroids remain the cornerstone of treatment [50,56,58]. Several approaches have been used after the initial steroids, which includes the use of cyclophosphamide, mycophenolate or azathioprine [49,56,58,60]. However, in the setting of infection, and to avoid further immune suppression, IVIG was found as a successful option in achieving remission in multiple series [52,62].
MAS has been reported in patients with idiopathic inflammatory myopathy, mainly in DM. MAS may be the presenting feature [55], and may be associated with pulmonary [63], as well as CNS [64] complication. Favorable response to treatment with calcineurin inhibitor and steroids, with and without plasma exchange, have been reported in this clinical setting [65,66].
Applying the above to our case, in the setting of this storm of complications, there was a high clinical suspicion of MAS. Moreover, our patient had met both Ravelli and HLH-2004 criteria as he had sustained fever, pancytopenia, significant elevation of ferritin, high triglyceride and low fibrinogen, decreased NK cell activity and increased CD25 cell activity. No hemophagocytes were noted on liver or bone marrow biopsy. There is a frequent notice that MAS happens early in the disease and renal involvement in lupus is a possible risk factor and this is what we noticed in our case also. We did treat the underlying overlap syndrome with steroid pulse therapy, plasmapheresis, and cyclophosphamide infusion with significant improvement.
Acute respiratory distress syndrome
Acute Respiratory Distress Syndrome (ARDS) is an acute, diffuse, inflammatory form of lung injury that is associated with a variety of etiologies. Recognizing and promptly treating ARDS is critical to reduce the associated high mortality. ARDS should be suspected in patients with progressive symptoms of dyspnea, an increasing requirement for oxygen, and alveolar infiltrates on chest imaging within 6 to 72 hours of an inciting event. Arterial Blood Gas (ABG) analysis shows hypoxemia, which is often initially accompanied by acute respiratory alkalosis, and an elevated alveolararterial oxygen gradient. Autoimmune disease is of the recognized etiologies of ARDS [67].
In polymyositis, ARDS occurs usually secondary to well recognized association with interstitial lung disease or due to pneumonia and sepsis. On the other hand, in lupus patients, ARDS is well reported in a number of case reports which is mostly attributed to Diffuse Alveolar Hemorrhage (DAH), interstitial pneumonitis, or sepsis and/or Pneumonia [68]. ARDS secondary to lupus-polymyositis syndrome is insufficiently reported.
Mild to moderate ARDS is usually managed by addressing the underlying problem in addition to supportive measures. However, Veno-venous ECMO (VV-ECMO) can be a life-saving bridge to clinical recovery in severe ARDS. Furthermore, it may provide a mechanical cardiopulmonary platform to allow further diagnostic testing, lung rest, and ultimate full clinical resolution. Extracorporeal membrane oxygenation is one of the several terms for an extracorporeal circuit that oxygenates and removes CO2 from the blood. When used in patients with the ARDS, a central vein is cannulated, and the blood is withdrawn from the vein into the extracorporeal circuit by a mechanical pump before entering an oxygenator. This specific technique is called venovenous ECMO because blood is both withdrawn and replaced into a central vein [7]. The primary outcome of mortality or severe disability occurred in 47% of those who were referred to ECMO when compared to 63% who underwent a conventional ventilation strategy as shown by hemorrhage randomized clinical trial [69].
Data from the Extracorporeal Life Support Organization (ELSO) document bleeding to be a major non-ECMO circuit related complication. Specifically, surgical incisions and cannulation sites had the most bleeding, 19.1% and 17.1%, respectively [3]. Pulmonary, gastrointestinal, and intracranial hemorrhage occur in 8.1%, 5.1%, and 3.8%, respectively. Systemic anticoagulation with unfractionated heparin is required to prevent clotting in the circuit. A generally accepted absolute contraindication for ECMO is any condition that precludes the use of systemic anticoagulation [70]. Despite this, ECMO has been used successfully in both pediatric and adult patients with refractory hypoxemia from pulmonary haemorrhage [71-73].
ECMO was used in ARDS related to lupus related DAH [74] and pneumonitis [68] with very good outcome. In our case, using ECMO helped to provide supportive treatment and buy some time for the immune suppressive treatment to start showing its effect.
Conclusion
Overlap syndrome is an understudied category compared with other definitive connective tissue disease. This reveals the need for dedicated clinical trials in patients of overlap syndromes for better understanding of the natural history, outcome, and treatment strategy required for these syndromes. Lupus- polymyositis might manifest initially as well as hepatitis and pancreatitis. We recommend early consideration of this syndrome in the proper clinical setting. Early detection of this syndrome might prevent life threatening complications like macrophage activation syndrome and acute respiratory distress syndrome related to lupus pneumonitis. This case sheds light on the role of extracorporeal membrane oxygenation in the treatment of severe acute respiratory distress syndrome in the setting of lupus polymyositis overlap syndrome which is rarely reported to the best of our knowledge.
Funding Source
None to report.
Financial disclosure
None to report.
Conflict of Interest
No Conflict of interest was reported by the authors.
References
- Iaccarino L, Gatto M, Bettio S et al. Overlap connective tissue disease syndromes. Autoimmun. Rev. 12(3), 363–373 (2013).
- Jury EC, D’Cruz D, Morrow WJW. Autoantibodies and overlap syndromes in autoimmune rheumatic disease. J. Clin. Pathol. 54(5), 340–347 (2001).
- Aguila LA, Lopes MRU, Pretti FZ et al. Clinical and laboratory features of overlap syndromes of idiopathic inflammatory myopathies associated with systemic lupus erythematosus, systemic sclerosis, or rheumatoid arthritis. Clin. Rheumatol. 33(8), 1093–1098 (2014).
- Jadhav P, Desai S, Jadhav J et al. Acute pancreatitis in rheumatology practice, with emphasis on systemic lupus erythematosus: A case series and newer concepts. Indian. J. Rheumatol. 14(3), 229 (2019).
- Chowdhary VR, Crowson CS, Poterucha JJ et al. Liver involvement in systemic lupus erythematosus: Case review of 40 patients. J. Rheumatol. 35(11), 2159–2164 (2008).
- Abraham S, Begum S, Isenberg D. Hepatic manifestations of autoimmune rheumatic diseases. Ann. Rheum. Dis. 63(2), 123–129 (2004).
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N. Engl. J. Med. 365(20), 1905–1914 (2011).
- Davì S, Minoia F, Pistorio A et al. Performance of current guidelines for diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Arthritis. Rheumatol. 66(10), 2871–2880 (2014).
- Mosca M, Tani C, Vagnani S et al. The diagnosis and classification of undifferentiated connective tissue diseases. J. Autoimmun. 48-49, 50–52 (2014).
- Alarcon-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patients. J. Rheumatol. 1(2), 81–83 (1989).
- Dayal NA, Isenberg DA. SLE/myositis overlap: Are the manifestations of SLE different in overlap disease? Lupus. 11(5), 293–298 (2002).
- Satoh M, Tanaka S, Ceribelli A et al. A Comprehensive Overview on Myositis-Specific Antibodies: New and Old Biomarkers in Idiopathic Inflammatory Myopathy. Clin. Rev. Allergy. Immunol. 52(1), 1–19 (2017).
- Liang Y, Leng RX, Pan HF et al. Associated variables of myositis in systemic lupus erythematosus: A cross-sectional study. Med. Sci. Monit. 23, 2543–2549 (2017).
- Jakati S, Rajasekhar L, Uppin M et al. SLE myopathy: A clinicopathological study. Int. J. Rheum. Dis. 18(8), 886–891 (2015).
- Maazoun F, Frikha F, Snoussi M et al. Systemic lupus erythematosusmyositis overlap syndrome: report of 6 cases. Clin. Pract. 1(4), 89 (2011).
- Bessone F, Poles N, Roma MG. Challenge of liver disease in systemic lupus erythematosus: Clues for diagnosis and hints for pathogenesis. World. J. Hepatol. 6(6), 394 (2014).
- Piga M, Vacca A, Porru G et al. Liver involvement in systemic lupus erythematosus: incidence, clinical course and outcome of lupus hepatitis. Clin. Exp. Rheumatol. 2010.
- Piga M, Vacca A, Porru G et al. Two different clinical subsets of lupus hepatitis exist. Mimicking primary autoimmune liver diseases or part of their spectrum? Lupus. 20(13), 1450–1451 (2011).
- Zheng R hua, Wang J hui, Wang S bing et al. Clinical and immunopathological features of patients with lupus hepatitis. Chin. Med. J (Engl). 2013.
- Gibson T, Myers AR. Subclinical liver disease in systemic lupus erythematosus. J. Rheumatol. 1981.
- Miller MH, Urowitz MB, Gladman DD et al. The liver in systemic lupus erythematosus. Q. J. Med. 62(5), 327 (1984).
- Usta Y, Gurakan F, Akcoren Z et al. An overlap syndrome involving autoimmune hepatitis and systemic lupus erythematosus in childhood. World. J. Gastroenterol. 13(19), 2764 (2007).
- Arnett FC, Reichlin M. Lupus hepatitis: An under-recognized disease feature associated with autoantibodies to ribosomal P. Am. J. Med. 99(5), 465–472 (1995).
- Carmona-Fernandes D, Santos MJ, Canhão H et al. Anti-ribosomal P protein IgG autoantibodies in patients with systemic lupus erythematosus: Diagnostic performance and clinical profile. BMC. Med. 11(1) 2013.
- Bonfa E, Golombek SJ, Kaufman LD et al. Association between Lupus Psychosis and Antiribosomal P Protein Antibodies. N. Engl. J. Med. 317(5), 265–271 (1987).
- Schneebaum AB, Singleton JD, West SG et al. Association of psychiatric manifestations with antibodies to ribosomal p proteins in systemic lupus erythematosus. Am. J. Med. 90(1), 54–62 (1991).
- Ohira H, Takiguchi J, Rai T et al. High frequency of anti-ribosomal P antibody in patients with systemic lupus erythematosus-associated hepatitis. Hepatol. Res. 28(3), 137–139 (2004).
- Koren E, Schnitz W, Reichlin M. Concomitant development of chronic active hepatitis and antibodies to ribosomal p proteins in a patient with systemic lupus erythematosus. Arthritis. Rheum. 36(9), 1325–1328 (1993).
- Koren E, Reichlin MW, Koscec M et al. Autoantibodies to the ribosomal P proteins react with a plasma membrane- related target on human cells. J. Clin. Invest. 89(4), 1236–1242 (1992).
- Calich AL, Bonfa E. The anti-ribosomal P antibodies and prognosis in autoimmune hepatitis. Liver. Int. 34(2), 324 (2014).
- Derk CT, DeHoratius RJ. Systemic lupus erythematosus and acute pancreatitis: A case series. Clin. Rheumatol. 23(2), 147–151 (2004).
- Makol A, Petri M. Pancreatitis in systemic lupus erythematosus: Frequency and associated factors - A review of the Hopkins Lupus Cohort. J. Rheumatol. 37(2), 341–345 (2010).
- Wang F, Wang NS, Zhao BH et al. Acute pancreatitis as an initial symptom of systemic lupus erythematosus: A case report and review of the literature. World. J. Gastroenterol. 11(30), 4766 (2005).
- Chari ST, Takahashi N, Levy MJ et al. A Diagnostic Strategy to Distinguish Autoimmune Pancreatitis From Pancreatic Cancer. Clin. Gastroenterol. Hepatol. 7(10), 1097–1103 (2009).
- Chari ST, Smyrk TC, Levy MJ et al. Diagnosis of Autoimmune Pancreatitis: The Mayo Clinic Experience. Clin Gastroenterol. Hepatol. 4(8), 1010–1016 (2006).
- Kawa S, Kamisawa T, Notohara K et al. Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2018: Revision of Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2011. Pancreas. 49(1), e13–e14 (2020).
- Masoodi I. The simultaneous incidence of acute pancreatitis and autoimmune hemolytic anemia: A rare duo in a patient with SLE. GMS. Ger. Med. Sci. (2014).
- Cairoli E, Pérez G, Briva A et al. Fatal acute pancreatitis complicated by pancreatic pseudocysts in a patient with systemic lupus erythematosus. Rheumatol. Int. 30(5), 675–678 (2010).
- Druml W, Laggner AN, Lenz K et al. Pancreatitis in acute hemolysis. Ann. Hematol. 63(1), 39–41 (1991).
- Wang CR, Hsieh HC, Lee GL et al. Pancreatitis related to antiphospholipid antibody syndrome in a patient with systemic lupus erythematosus. J. Rheumatol. (1992).
- Yeh TS, Wang CR, Lee YT et al. Acute pancreatitis related to anticardiolipin antibodies in lupus patients visiting an emergency department. Am. J. Emerg. Med. 11(3), 230–232 (1993).
- Dierickx D, Kentos A, Delannoy A. The role of rituximab in adults with warm antibody autoimmune hemolytic anemia. Blood. 125(21), 3223–3229 (2015).
- Birgens H, Frederiksen H, Hasselbalch HC et al. A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br. J. Haematol. 163(3), 393–399 (2013).
- Erkan D, Vega J, Ramón G et al. A pilot open-label phase II trial of rituximab for non-criteria manifestations of antiphospholipid syndrome. Arthritis. Rheum. 65(2), 464–471 (2013).
- Bern MM. Rituximab Immunotherapy for the Antiphospholipid Syndrome. Blood. 106(11), 4163 (2005).
- Berman H, Rodríguez-Pintó I, Cervera R et al. Rituximab use in the catastrophic antiphospholipid syndrome: Descriptive analysis of the CAPS registry patients receiving rituximab. Autoimmun. Rev. 12(11), 1085–1090 (2013).
- Goel R. Pancreatitis in Systemic Lupus Erythematosus-Case Series from a Tertiary Care Center in South India. Open. Rheumatol. J. 6(1), 21–23 (2012).
- Nesher G, Breuer GS, Temprano K et al. Lupus-associated pancreatitis. Semin. Arthritis. Rheum. 35(4), 260–267 (2006).
- Ahn SS, Yoo BW, Jung SM et al. In-hospital mortality in febrile lupus patients based on 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome. Semin. Arthritis. Rheum. 47(2), 216–221 (2017).
- Parodi A, Davì S, Pringe AB et al. Macrophage activation syndrome in juvenile systemic lupus erythematosus: A multinational multicenter study of thirty-eight patients. Arthritis. Rheum. 60(11), 3388–3399 (2009).
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A et al. Adult haemophagocytic syndrome. In: The Lancet. 383(9927), 1503–1516 (2014).
- Pringe A, Trail L, Ruperto N et al. Macrophage activation syndrome in juvenile systemic lupus erythematosus: An under-recognized complication? Lupus. 16(8), 587–592 (2007).
- Muskardin TLW, Niewold TB. Type i interferon in rheumatic diseases. Nat. Rev. Rheumatol. 14(4), 214–228 (2018).
- Kyttaris VC. Novel Treatments in Lupus. Curr. Rheumatol. Rep. 19(3) 2017.
- Poddighe D, Cavagna L, Brazzelli V et al. A hyper-ferritinemia syndrome evolving in recurrent macrophage activation syndrome, as an onset of amyopathic juvenile dermatomyositis: A challenging clinical case in light of the current diagnostic criteria. Autoimmun. Rev. 13(11), 1142–1148 (2014).
- Gavand PE, Serio I, Arnaud L et al. Clinical spectrum and therapeutic management of systemic lupus erythematosus-associated macrophage activation syndrome: A study of 103 episodes in 89 adult patients. Autoimmun. Rev. 75(Suppl 2), 126.2–127 (2017).
- Liu AC, Yang Y, Li MT et al. Macrophage activation syndrome in systemic lupus erythematosus: a multicenter, case-control study in China. Clin. Rheumatol. 37(1), 93–100 (2017).
- Cohen EM, D’Silva K, Kreps D et al. Arthritis and use of hydroxychloroquine associated with a decreased risk of macrophage activation syndrome among adult patients hospitalized with systemic lupus erythematosus. Lupus. 27(7), 1065–1071 (2018).
- Lambotte O, Khellaf M, Harmouche H et al. Characteristics and long-term outcome of 15 episodes of systemic lupus erythematosus-associated hemophagocytic syndrome. Medicine (Baltimore). 85(3), 169–182 (2006).
- Ruscitti P, Cipriani P, Ciccia F et al. Prognostic factors of macrophage activation syndrome, at the time of diagnosis, in adult patients affected by autoimmune disease: Analysis of 41 cases collected in 2 rheumatologic centers. Autoimmun. Rev. 16(1), 16–21 (2017).
- Borgia RE, Gerstein M, Levy DM et al. Features, Treatment, and Outcomes of Macrophage Activation Syndrome in Childhood-Onset Systemic Lupus Erythematosus. Arthritis. Rheumatol. 70(4), 616–624 (2018).
- Gormezano NWS, Otsuzi CI, Barros D et al. Macrophage activation syndrome: A severe and frequent manifestation of acute pancreatitis in 362 childhood-onset compared to 1830 adult-onset systemic lupus erythematosus patients. Semin. Arthritis. Rheum. 45(6), 706–710 (2016).
- Wakiguchi H, Hasegawa S, Hirano R et al. Successful control of juvenile dermatomyositis-associated macrophage activation syndrome and interstitial pneumonia: Distinct kinetics of interleukin-6 and -18 levels. Pediatr. Rheumatol. 13(1) (2015).
- Lilleby V, Haydon J, Sanner H et al. Severe macrophage activation syndrome and central nervous system involvement in juvenile dermatomyositis. Scand. J. Rheumatol. 43(2), 171–173 (2014).
- Kaieda S, Yoshida N, Yamashita F et al. Successful treatment of macrophage activation syndrome in a patient with dermatomyositis by combination with immunosuppressive therapy and plasmapheresis. Mod. Rheumatol. 25(6), 962–966 (2015).
- Bustos BR, Carrasco AC, Toledo RC. Plasmapheresis for macrophage activation syndrome and multiorgan failure as first presentation of juvenile dermatomyositis. An. Pediatr. 77(1), 47–50 (2012).
- Hervier B, Uzunhan Y. Inflammatory Myopathy-Related Interstitial Lung Disease: From Pathophysiology to Treatment. Front. Med. (2020).
- Noah Kornfield Z, Horak J, Gibbs RM et al. CASE 2-2015: Extracorporeal membrane oxygenation as a bridge to clinical recovery in life-threatening autoimmune acute respiratory distress syndrome. J. Cardiothorac. Vasc. Anesth. 29(1), 221–228 (2015).
- Peek GJ, Mugford M, Tiruvoipati R et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 374(9698), 1351–1363 (2009).
- Zamora MR, Warner ML, Tuder R et al. Diffuse alveolar hemorrhage and systemic lupus erythematosus: Clinical presentation, histology, survival, and outcome. Medicine (Baltimore). 76(3), 192–202 (1997).
- Virdi RPS, Bashir A, Shahzad G et al. Diffuse Alveolar Hemorrhage: A Rare Life-Threatening Condition in Systemic Lupus Erythematosus. Case. Rep. Pulmonol. 2012, 1–4 (2012).
- Hohenforst-Schmidt W, Petermann A, Visouli A et al. Successful application of extracorporeal membrane oxygenation due to pulmonary hemorrhage secondary to granulomatosis with polyangiitis. Drug. Des. Devel. Ther. 2013.
- Patel JJ, Lipchik RJ. Systemic lupus-induced diffuse alveolar hemorrhage treated with extracorporeal membrane oxygenation: A case report and review of the literature. J. Intensive. Care. Med. 29(2), 104–109 (2014).
- Pais F, Fayed M, Evans T. The Successful Use of Extracorporeal Membrane Oxygenation in Systemic Lupus Erythematosus-Induced Diffuse Alveolar Haemorrhage. Eur. J. Case. Reports. Intern. Med. 4(1) (2017).