Research Article - Journal of Experimental Stroke & Translational Medicine (2022) Volume 14, Issue 4
Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine
Tayeb Salih*
Department of Biotechnology, Faculty of Applied and Computer Sciences, Vaal University of Technology, Vanderbijlpark 1900, South Africa
Received: 04-Jul-2022, Manuscript No. JESTM-22-40113; Editor assigned: 07-Jul-2022, PreQC No. JESTM-22-40113 (PQ); Reviewed: 21-Jul-2022, QC No. JESTM-22-40113; Revised: 27-Jul-2022, Manuscript No. JESTM-22-40113 (R); Published: 31-Jul-2022, DOI: 10.37532/ jestm.2022.14(4).72-78
Abstract
Abstract
Humans and animals lose tissues and organs due to congenital defects, trauma, and diseases. The human body has a low regenerative potential as opposed to the urodele amphibians commonly referred to as salamanders. Globally, millions of people would benefit immensely if tissues and organs can be replaced on demand. Traditionally, transplantation of intact tissues and organs has been the bedrock to replace damaged and diseased parts of the body. The sole reliance on transplantation has created a waiting list of people requiring donated tissues and organs, and generally, supply cannot meet the demand. The total cost to society in terms of caring for patients with failing organs and debilitating diseases is enormous. Scientists and clinicians, motivated by the need to develop safe and reliable sources of tissues and organs, have been improving therapies and technologies that can regenerate tissues and in some cases create new tissues altogether. Tissue engineering and/or regenerative medicine are fields of life science employing both engineering and biological principles to create new tissues and organs and to promote the regeneration of damaged or diseased tissues and organs. Major advances and innovations are being made in the fields of tissue engineering and regenerative medicine and have a huge impact on three-dimensional bioprinting (3D bioprinting) of tissues and organs. 3D bioprinting holds great promise for artificial tissue and organ bioprinting, thereby revolutionizing the field of regenerative medicine. This review discusses how recent advances in the field of regenerative medicine and tissue engineering can improve 3D bioprinting and vice versa. Several challenges must be overcome in the application of 3D bioprinting before this disruptive technology is widely used to create organotypic constructs for regenerative medicine.
Introduction
Tissue and organ shortages have been identified as a major public health challenge with only a small percentage of deserving patients receiving transplantations. Most waiting lists for tissues and organs do not capture the magnitude of the crisis well as only those who are sick seek such assistance [1]. The terms regenerative medicine and tissue engineering are used with appreciable overlap by scientists and clinicians and in this review are used as synonyms. The promise of regenerative medicine is founded on the potential and ability to regenerate and replace damaged tissues and organs. Regenerative medicine has shown promising results for the regeneration and replacement of a variety of tissues and organs including skin, heart, kidney, and liver and the potential to even correct some congenital flaws. The traditional reliance on donated tissues and organs for transplantations faces the problem of donor shortages and possible immunological rejection of the donated body parts. Some of the organ transplants performed in developing nations includes cases of transplant tourism where foreigners, with enough money and influence, are given priority over the local populace [2]. Such practices have been condemned as it can result in the exploitation of defenseless people. Despite differences in national economic powers and therefore differences in healthcare infrastructure, overcoming burdens such as the low supply of organs and the practical hurdles of collecting and storing them can help in increasing the number of people who can undergo organ transplantations. Therefore, strategies and technologies that can increase the supply of tissues and organs for transplantation must be developed further. In most cases, tissues and organs are required immediately for transplantation as is the case when people are wounded in accidents, wars, and natural disasters. The shortage of tissues and organs not only hampers the treatment of patients but also hinders scientific research. The development of an endless supply of tissues and organs therefore represents the most challenging task of our generation. Many initiatives have been undertaken to increase organ donations and better usage of the donated organs. One solution is the advent of laboratory-grown tissues, humanized animal organs, and bioartificial organs. Regenerative medicine may help in solving some of these challenges [3].
Several 3D-bioprinted constructs and stem cell therapies have been approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in the last 10 years. These therapies and products range from biologics and medical devices to biopharmaceuticals. Biomolecules and growth factors can be tethered to the materials and can provide sustained stimuli to promote cellular differentiation and regeneration of damaged tissue. Growth factors that have been used this way include the bone morphogenetic proteins (BMP) for bone formation and platelet-derived growth factor for wound healing [4]. The lack of growth factor release control once the material has been transplanted can result in complications during the use of such materials. Products approved by the FDA generally perform better than preexisting products, but the efficiency of these products varies. Most products are, however, unable to fully resolve complex injuries and disease. New biomaterials and stem cell products tend to take time to be introduced into the market mainly due to the number of policies required to get FDA approval and also the lack of monetary funding for these products. Normally, it takes more than 10 years for a product to reach the market whilst more than a billion dollars would have been spent to develop the product. Generally, it is much easier and cheaper to introduce a new medical device than it is for drugs and biologics. This has favoured the development of non-cell-based regenerative products than cell-based ones.
Methodology
A literature search from the databases PubMed, Google Scholar, and Science Direct was done from 2000 to October 2017, although we mainly focused on the later years to provide the latest technological advances, for the following key words: biologics, biomaterials, innovation, medicine, native tissue, organs, regenerative medicine, stem cells, tissue engineering, transplantation, and 3D bio printing. These databases specialize in novel technologies, innovation, human diseases and conditions requiring organ and tissue transplantation, and innovative technologies and mostly use English as the main language. Duplicate articles were removed, and only full articles with the above searched words were included. Articles cited outside of these criteria are to cater for origins of technologies and theories.
Replacement of Human Body Tissues and Organs
Body tissues and organs have both structure and function, and therefore, any engineered material must be able to recapitulate the morphology and characteristics of the target tissue and organ. Several methods have been used to combine both structure and function in engineered tissue or organs [5]. Decellularization of tissues and organs and recellularization before transplantation have shown great promise as they remove immunogenic cells whilst maintaining the structure and material composition of the native extracellular matrix. Decellularization of organs is normally done when the organ is too old to be used for transplantation. Decellularised ECM has also been used as a bioink in 3D bioprinting. Decellularised ECM has the advantage of recapitulating tissuespecific properties and therefore provides the right cues for cellular proliferation and differentiation. Issues such as retention of some decellularizing detergent must be addressed. Limitations to this procedure include the use of detergents to wash off all cells so that only the extracellular matrix remains. Cells can then be seeded onto the matrix to restart the process of recellularization. Either stem cells or patientspecific cells can be used in the process [6]. Stem cells are the cells of choice as they can differentiate into several types of cells whereas differentiated cells will only attach and start growing when they find a suitable environment. Together with the use of bioreactors, the approach of decellularization has been used to successfully treat several diseases in animal models. Decellularised tissues and organs can be used as medical devices if the recellularization step is omitted. That would shorten the time needed for the product to reach the market as it is considered a cellular. There exist several methods of decellularization of tissue and/or organs.
3D Bioprinting
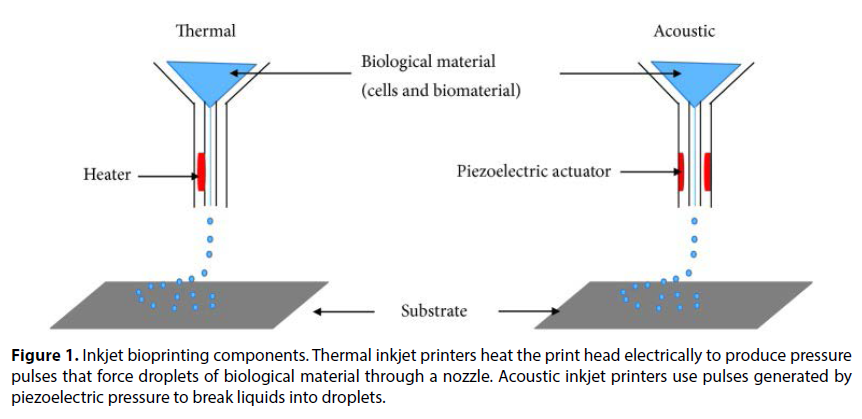
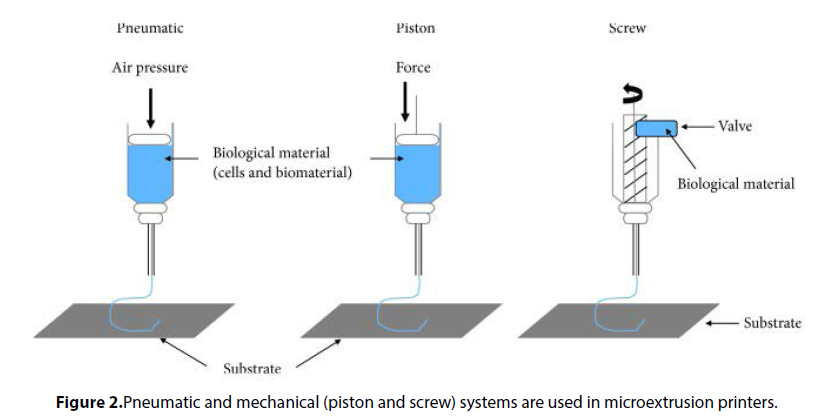
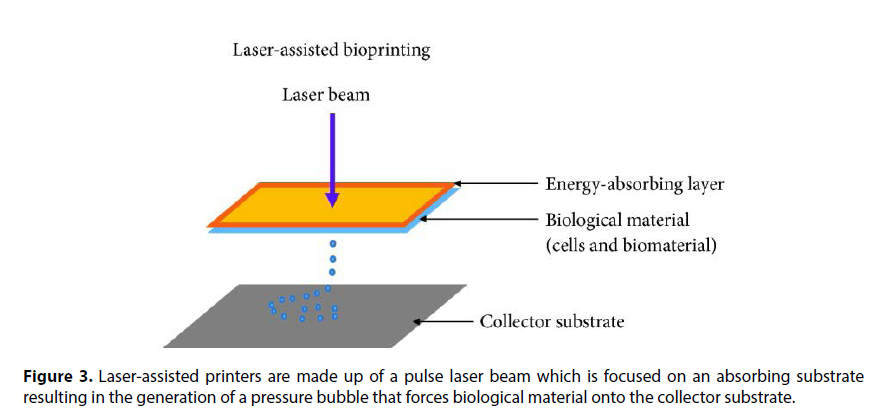
One major challenge associated with populating scaffolds with cells is the uncontrolled placement of cells. 3D bioprinting has revolutionized the mixing of scaffolds and cells as it can result in structures with some control over material and cell placement in grafts and constructs. 3D bioprinting strategies currently in use include the inkjet, micro extrusion, and laser-assisted printing methods. Droplets of scaffolds or hydrogel containing cells are sprayed in the inkjet bioprinting method whilst a continuous stream of ink or scaffold containing cells is dispensed onto a stage in the micro extrusion method. These 3D bioprinting methods have resulted in the fabrication of several 3D tissues including cartilage, aortic valves, and blood vessels, with placed cells able to produce ECM proteins such as collagens and fibronectin. Several bioprinting machines have been manufactured, and these have different capabilities [7]. Challenges still remain, however. One major drawback of 3D bioprinting is the low viability of placed cells. In a recent study, Huang and colleagues showed that a graphene-polyurethane Nano composite hydrogel is a possible bioink for 3D bioprinting of tissue constructs laden with cells. The hydrogel maintained its shear thinning behavior and retained positive effects of graphene or graphene oxide on neural tissue regeneration. Another major drawback of 3D bioprinting tissues is the lack of vascular tissues, resulting in the death of cells due to lack of nutrients and oxygen. Miller and colleagues printed a rigid 3D filament network of carbohydrate glass which they used as a template to generate cylindrical networks that were lined with endothelial cells and extracellular matrices. The perfused vascular channels even sustained the metabolism of rat hepatocytes in tissue constructs. Three main technologies are used in bioprinting of materials of biological origin. These are inkjet printing sometimes called dropon- demand printing and microextrusion printing where a microextrusion head is used for the printing onto the scaffold and is done by a robot and laser-assisted printing, where laser pulses are used to generate bubbles under pressure, and this sprays the bubble onto the scaffold. A detailed description of these printing technologies is beyond the scope of this review [8]. Sometimes referred to as drop-ondemand printers, inkjet printing can be used for both biological and nonbiological applications. Commercially available inkjet paper printers were basically converted into printers of biological material. Volumes of biological material in liquid form are sprayed onto defined surfaces with increased resolution and precision and at high speeds. Liquids are ejected from the printer using thermal or acoustic forces onto a scaffold or substrate which is usually part of the graft that will be transplanted onto the tissue (Figure 1). In the case of thermal inkjet printers, a heated print head releases drops of biological material onto the scaffold. The heating does not affect the quality or integrity of the biological material. Thermal inkjet printers are the cheapest of the three bioprinting techniques and are used widely. Inkjet printers are also compatible with many biological materials. Acoustic printers have a piezoelectric crystal that generates acoustic waves. The size of the droplet of the biological material can be controlled by adjusting the duration and amplitude of the wave generated in the printer head. It is very easy to control the size of the biological material droplet as well as the direction of ejection using acoustic inkjet printers. One of the drawbacks of using inkjet printers is the need to maintain a certain viscosity of the biological material being printed. Above certain viscosities, the printer head can be clogged. To maintain biological materials as liquids, usually the number of cells included and therefore printed is lowered. High cell concentrations can jeopardize droplet formation and increase the chances of printer head clogging. Several biological materials are compatible with microextrusion printing, and these materials can have a range of viscosities. Unlike inkjet printing, microextrusion bioprinting can be used with high cell densities and therefore achieve cell densities similar to those found under physiological conditions. Microextrusion bioprinting can also print cellular spheroids, and these can then self-assemble into several 3D structures. Scientists believe that cellular spheroids have the same properties as tissue ECM (Figure 2). Vascular tissue spheroids have been generated using the self-assembly of spheroids in 3D bioprinted organs.
Novel Considerations in Regenerative Medicine and Tissue Engineering
Several factors such as the biomaterial to be used and the cellular source must be considered during tissue or graft manufacture. Such considerations will allow for proper cell-cell and cell-biomaterial (cellmatrix) interactions, thus enhancing the function of the scaffold. Regenerated tissue for transplantation must recapitulate normal tissue in having a specific cell type, with a specific function [9]. Just as in normal tissues and organs, different cells play different roles such as providing structural and supportive roles as provided by endothelial cells. Thus, the cells used during 3D bioprinting will determine the function of the resulting graft or scaffold. The integration of the transplanted graft or scaffold requires that it must selfrenew and maintain homeostasis. The most desired source of cells is autologous cells to avoid a host immune response. Autologous cells can be passaged in vitro and induced to differentiate into the desired cells before the 3D bioprinting process or transplantation. Several drawbacks are associated with the use of autologous cells. These include the limited regeneration capacity of primary cells and the technical restrictions to the in vitro culture of cells (Figure 3). 3D bioprinting is considered more manageable than acellular printing which would require seeding of cells after printing. For grafts to successfully become integrated within the body there is need for proper integration with the patient’s vasculature. Cells in the body are situated near blood vessels to allow for the exchange of nutrients and oxygen. Traditional methods such as biomimetic scaffold fabrication or designing of tissues and organs are unsuccessful when it comes to fulfilling the need for blood vessels and nerves in tissues and organs. Several angiogenic growth factors including VEGF, bFGF, and PDGF have been used in engineered tissues to stimulate blood vessel formation. These growth factors are presented to the scaffolds, and this stimulates the body to initiate angiogenesis. The challenge with the use of growth factors is their short half-lives and their potential for toxic effects. Continuous release of growth factors has been shown to reverse necrosis in some tissues. Prevascularisation of grafts before transplantation is one way to promote graft vascularisation. There are several issues that need improvements regarding cells used for 3D bio printing [10]. There is a need for cells to survive the actual 3D bioprinting process, remain robust, and continue proliferating and be able to differentiate as in the case of stem cells. Once the scaffold or graft has been transplanted, there is need for cells to have the same cellular function as normal cells. Lastly, all cells used during the 3D bioprinting process must be able to interact directly or through release of biomolecules such as growth factors and cytokines. Cells that can self-renew and have the capacity to generate multiple other cells such as embryonic and adult stem cells are therefore appealing. Adult stem cells are considered safer to use for transplantation than any other cells and remain robust after 3D bioprinting. To overcome this problem, cells can be encapsulated with material such as hydrogel, and this can lead to a prolonged presence of the cells within the grafted tissue and possibly prevent rejection. By coating transplanted cells with specific antibodies and peptides, such cells can home in to specific tissues and organs. Although the immune system is involved in rejecting grafts or new tissues, it can actively promote the regeneration of damaged tissues as well as enhance engraftment of transplanted grafts. Technological advancement means that the alteration of scaffold characteristics can minimize graft rejection and encourage graft tolerance.
Medicinal Remedies in Regenerative Medicine and Tissue Engineering
Most 3D bioprinting processes and stem cell therapies require the use of synthetic and natural biological molecules such as growth factors to enhance the proliferation and differentiation of stem cells. Reports of severe side effects and toxicity from the use of these substances have surfaced, and scientists are searching for alternatives. Most of the current stimulants are of nonhuman origin and therefore may be rejected when used. In addition, the use of these purified biological molecules is an expensive option. The need to replenish growth factors during stem cell differentiation, due to their short half-lives, makes their use an expensive option, especially in developing countries. Medicinal or herbal plants are used mostly in the developing world for primary health care. The last 5 years has seen an increase in the use of medicinal plants for health promotion and treatment of diseases in developed countries. Indeed, many medicinal plant extracts are now used as prescription drugs in many developed countries such as the United Kingdom, Germany, and France. Our data show that resveratrol treatment up regulates collagen type II in chondrocytes and can increase chondrocyte viability. Resveratrol, therefore, can be used during 3D bioprinting of cartilage constructs to enhance chondrocyte viability after the printing process. Ethanol and dichloromethane extracts of Pleurostylia capensis Turcz (Loes) were shown to have antimicrobial, antioxidant, and anti-inflammatory activities. Many medicinal plant extracts have shown anticancer activities through inhibition of cancer cell proliferation and growth. Of late, several medicinal plant extracts have been used to promote stem cell proliferation and differentiation and to encourage tissue regeneration leading to rehabilitation of damaged or diseased tissues. Several studies have been undertaken to study the effect of medicinal plant extracts on stem cell differentiation with the hope of providing nontoxic and affordable stem cell therapy and tissue and organ transplantation. Promising results have been shown in the treatment of several pathological conditions such as osteoporosis, neurodegenerative disorders, and degenerative ailments using medicinal plant extracts. Medicinal plant extracts are an affordable and readily available option since they have been in use since time immemorial. The only drawback on the use of medicinal extracts is the lack of knowledge on the mechanism of action of these extracts. Issues such as variability, toxicity [11], and complexity of the medicinal extracts have limited their clinical use in stem cell therapy and tissue engineering procedures. It is hoped that once purified and standardized, medicinal extracts can be used in many applications requiring tissue regeneration and enhanced stem cell growth. In addition, understanding the mechanisms and signaling pathways involved in medicinal extracts’ healing potential or power is a necessity before they can successfully be used in 3D bioprinting and regenerative and reparative therapies. The advantages of using these medicinal extracts stem from their availability, low cost, and nontoxicity if taken in certain doses. Most of these extracts are already in use to treat several ailments. The health benefits of using plant-based remedies are known to include the prevention of certain ailments such as headaches and the common cold. Most medicinal plant extracts are used as cocktails, and the combination of different phytochemicals is thought to have an additive or synergistic effect on different pathological conditions. Another important component of Radix angelica sinesis extract, ferulic acid, was shown to decrease neurotoxic β-amyloid peptide aggregation in several animal models [12]. An extract from another medicinal plant, Salvia miltiorrhiza, induced neurogenic differentiation of Wharton’s jellyderived MSCs with significant up regulation of markers such as nestin, a glial fibrillary acidic protein. Curcumin is a major component of Curcumin longa L. extract and has antiinflammatory properties. An ethanol extract of Curcumin longa L. induced endothelial differentiation of adipose-derived MSCs. The commonly used olive leaf extract induced endothelial differentiation and formation of tubular structures in mesenchymal stem cells, suggesting it is important for blood vessel formation.
Conclusion
A number of diseases and conditions are now being treated via the use of regenerative medicine. The continual manipulation of both scaffolds and cells will allow for the control of the host’s response to the presence of the scaffold and cells in 3D-bioprinted constructs or organs. Technological advances will allow for the fabrication of patient-specific and tailor-made grafts that will position cells within specific regions of the scaffold and possibly mimic native tissues. Most importantly, graft integration with host tissue will improve with new knowledge on graft vascularization and innervation. Improved techniques with regard to the release of growth factors within 3D-bioprinted constructs and organs once transplanted will allow the controlled healing and regeneration process. Modulation of the immune system can lower the rejection of 3D-bioprinted tissues and organs or at least allow scientists to achieve a desirable immune response. Increased knowledge on stem cell behavior and controlled differentiation of the cells can be achieved, allaying fears of their safety. Alteration of the host environment to prevent rejection of 3D-bioprinted constructs and organs and to provide the right niche for the transplanted cells will allow cells to grow under their “normal conditions,” thus improving outcomes of regenerative medicine strategies. The latest research also points to the microbiome affecting almost all cellular processes of the body; thus, knowledge of the role the microbiome plays in construct or graft integration is important. 3D-bioprinted models of human diseases and conditions must continue to improve to allow for the translation of promising regenerative medicine strategies. The future of regenerative medicine and tissue engineering relies on the ability of scientists and clinicians to “mimic nature” or “work with nature” in coming up with innovative biomaterials and technologies such as nanotechnology to advance this field.
Conflicts of Interest
The authors declare no conflict of interest regarding the publication of this manuscript.
References
- Giwa S, Lewis JK, Alvarez L et al. The promise of organ and tissue preservation to transform medicine. Nat Biotechnol. 35, 530–542 (2017).
- Jones B, Bes M Keeping kidneys. Bull World Health Organ. 90, 718-719 (2012).
- Colvin M, Smith JM, Skeans MA et al. OPTN/SRTR 2015 annual data report: heart. Am J Transplant. 17, 286–356 (2017).
- Hart A, Smith JM, Skeans MA et al. OPTN/SRTR 2015 annual data report: kidney. Am J Transplant. 17, 21–116 (2017).
- Israni AK, Zaun D, Bolch C et al. OPTN/SRTR 2015 annual data report: deceased organ donation. Am J Transplant. 17, 503– 542 (2017).
- Kasiske BL, Asrani SK, Dew MA et al. The living donor collective: a scientific registry for living donors. Am J Transplant.17, 3040–3048 (2017).
- Nagral S, Hussain M, Nayeem SA et al. Unmet need for surgery in south Asia. BMJ. 357, 1423-1425 (2017).
- Ott HC, Matthiesen TS, Goh SK et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 14, 213–221 (2008).
- Atala A Advances in tissue and organ replacement. Stem Cell Res Ther. 3, 21–31 (2008).
- Mendelson A, Frenette PS Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 20, 833–846 (2014).
- Bailey AM, Mendicino M, Au P et al. An FDA perspective on preclinical development of cell-based regenerative medicine products. Nat Biotechnol. 32, 721–723 (2014).
- Knoepfler PS From bench to FDA to bedside: us regulatory trends for new stem cell therapies. Adv Drug Deliv Rev. 82, 192–196 (2015).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref