Review Article - Interventional Cardiology (2013) Volume 5, Issue 6
Advances in echocardiography: insights into the mitral valve and implications for surgical and percutaneous repair
- Corresponding Author:
- Mauro Pepi
Centro Cardiologico Monzino IRCCS
Via Parea, 4 20138 Milan
Italy
Tel: +39 025 800 2581
E-mail: mpepi@ccfm.it
Abstract
Imaging the mitral valve apparatus ▪ In presence of severe mitral regurgitation, early surgery preserves long-term left ventricular function and imaging-based assessment of the mitral valve (MV) is essential to adopt the best surgical strategy. ▪ Percutaneous MV repair requires the clear delineation of intracardiac anatomy and the mechanism of mitral regurgitation, and this can only be achieved using echocardiography. New echocardiographic modalities ▪ 3D echocardiography allows visualization of the MV in an easy way and from multiple planes of the complex structures. ▪ Both experienced and less trained operators benefit from the use of 3D compared with traditional 2D echocardiography. ▪ 3D transesophageal echocardiography is highly accurate in the identification of both simple and complex cases of MV prolapse when compared with surgical inspection. Implications for MV surgery and MV percutaneous repair ▪ 3D echocardiography allows the assessment of the complexity of MV lesions, facilitating the surgical planning. ▪ 3D echocardiography is the ideal technique to evaluate the effect of MV repair on the morphology and function of MV annulus, leaflets and papillary muscles and their interaction with the aortic valve. ▪ Echocardiography plays a role in each phase of the percutaneous MV repair procedure, including patient selection, the delivering and positioning of the device, and follow-up evaluation. ▪ 3D imaging is fundamental for patient selection and prediction of procedural effectiveness.
Keywords
3D echocardiography, mitral valve, mitral valve regurgitation, percutaneous mitral valve repair, surgical mitral valve repair
Why do we need a more accurate and quantitative imaging of the mitral valve apparatus?
Mitral valve (MV) prolapse, with a prevalence of approximately 2-3% [1], is the most frequent etiology of mitral regurgitation (MR) in industrialized countries [2,3]. Since the 1970s, MV repair has become preferential to replacement and, nowadays, this approach is considered the optimal surgical treatment in the overwhelming majority of patients with MV prolapse [4].
Recent studies underlined the importance of early surgical intervention to preserve long-term left ventricular (LV) function in severe MR [4,5], and the early correction of MR is recommended even in asymptomatic patients, when deemed feasible, with a high likelihood of successful repair. A noninvasive preoperative assessment of MV anatomy is fundamental to define the feasibility of surgical repair in differentiating simple from complex lesions and to define the best surgical strategy.
Functional etiology is another common cause of MV insufficiency. Functional MR is known to occur in patients with systolic LV dysfunction, either ischemic or idiopathic, with structurally normal MV leaflets. Mitral annulus (MA) dilatation, tethering of MV leaflets secondary to LV dilatation with outward displacement of papillary muscles, together with a reduced systolic transmitral pressure gradient hampering the correct coaptation of MV leaflets, have been identified as mechanisms of functional MR [6]. Real-time 3D (RT3D) echocardiography largely contributed to the understanding of these complex mechanisms.
In recent years, there has been an increasing interest towards nonsurgical solutions to severe MR, with the MitraClip® system (Abbott Vascular, Inc., CA, USA) being the most well established. The clear delineation of intracardiac anatomy and the accurate evaluation of the mechanisms underlying MR are crucial aspects in percutaneous MV repair (PMVR). Currently, these tasks cannot be accomplished by the use of fluoroscopy and cineangiography without the addition of transesophageal echocardiography (TEE) [7]. The central role of echocardiography in PMVR, and in structural interventional procedures in general, has gained even more strength with the availability of advanced scanners capable of simultaneous biplane or RT3D imaging. This is particularly important during interventional procedures in the cardiac catheterization laboratory, where immediate qualitative and quantitative feedback is fundamental.
New echocardiographic methods for MV evaluation
Recent guidelines stated that RT3D echocardiography is the ideal imaging modality for interrogating the anatomy and function of each of the individual components of the MV apparatus including MA, leaflets, chordae and papillary muscles [8,9]. Both qualitative and quantitative evaluation of valvular heart disease can be improved by 3D echocardiography [10-12]. 3D evaluation may be easily integrated into standard 2D examination in the qualitative assessment of morphology of the MV [13]. Indeed, the possibility of visualizing MV leaflets, commissures, MA calcifications and subvalvular structures from different and unique planes, from both the atrium or ventricle, with access to en face> views, facilitates the understanding of this complex apparatus. Recently, Hien et al. showed that both experienced and less well-trained echocardiographers achieved better results when using 3D TEE rather than traditional 2D TEE in the localization of MV prolapse and in the identification of ruptured chordae [14]. Furthermore, they demonstrated that the benefits of the additional dimension is greater in the less experienced echocardiographer, as the true-life en face> display of the MV is self-explanatory and reduces the need for mental reconstruction and interpretation of multiple 2D planes. This advantage is even more evident when examining complex prolapse. Indeed, RT3D transthoracic echocardiography (TTE) and TEE allow the accurate morphological and functional assessment of the MV in its complexity, and recent data have demonstrated that 3D techniques are superior to the corresponding 2D approaches in the description of MV pathology [14-16]. In addition, as the accuracy of RT3D TTE is comparable to that of 2D TEE, this technique may be an invaluable additional tool to the standard 2D TTE examination.
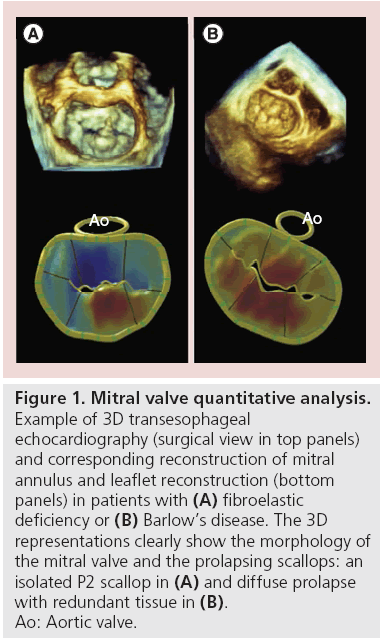
3D echocardiography has further improved our knowledge of MV prolapse, differentiating two forms: fibroelastic deficiency (FED) and Barlow’s disease (BD). Carpentier described the FED condition asassociated with a fibrillin deficiency, often leading to the rupture of one or more chordae, usually involving the middle scallop of the posterior leaflet [17]. By contrast, BD is characterized by diffuse excess tissue. Moreover, valve size is generally quite large with myxomatous alterations affecting multiple scallops, resulting in floppy, thickened and distended leaflets. Diffuse chordal elongation, in addition to chordal rupture, is very common in BD patients as opposed to a simple isolated chordal rupture in FED. Severe MA dilatation with varying degrees of calcification may be observed, and subvalvular fibrosis and calcification of the papillary muscles, usually the anterior, may occur. In terms of epidemiology, patients with BD are generally younger at the time of surgical referral. All of these morphologic characteristics of MV may be well visualized by RT3D echocardiography. A TEE study with a method for the static assessment of the MV leaflet and annulus not only showed differences in FED and BD before surgery, but also provided the description of immediate postoperative changes after MV repair (Figure 1) [18].
Figure 1: Mitral valve quantitative analysis.
Example of 3D transesophageal
echocardiography (surgical view in top panels)
and corresponding reconstruction of mitral
annulus and leaflet reconstruction (bottom
panels) in patients with (A) fibroelastic
deficiency or (B) Barlow’s disease. The 3D
representations clearly show the morphology of
the mitral valve and the prolapsing scallops: an
isolated P2 scallop in (A) and diffuse prolapse
with redundant tissue in (B).
Ao: Aortic valve.
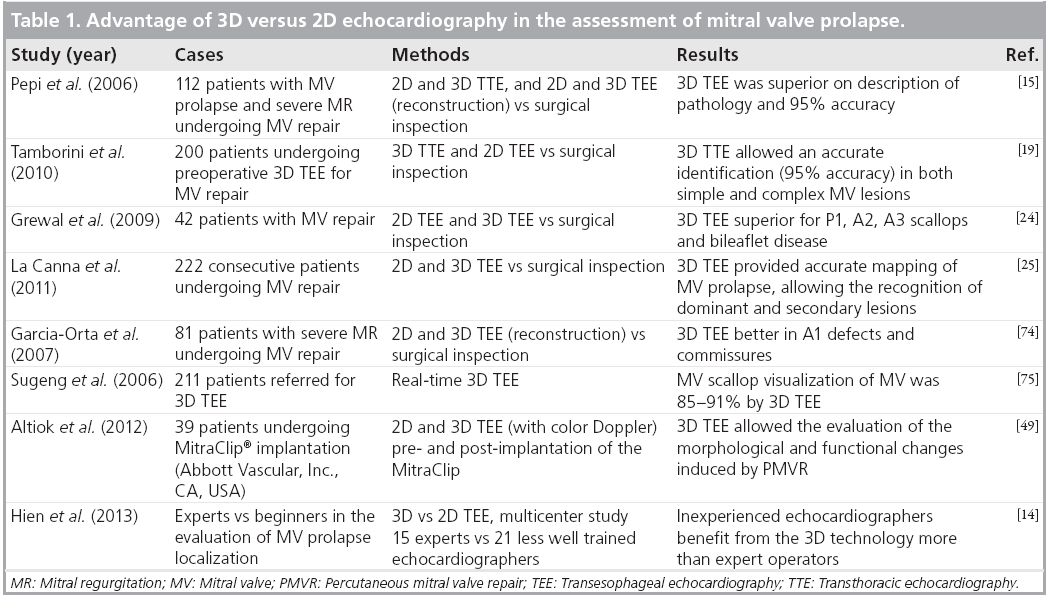
In terms of accuracy, RT3D TTE has recently been investigated versus surgical inspection in a consecutive series of 200 cases [19]. Overall, performance of the method was high (sensitivity: 92.5%; specificity: 96%; and accuracy: 95%). Sensitivity of RT3D TTE was slightly lower for the anterolateral commissure, lateral posterior (P1) and anterior (A1) scallops, while specificity was very high in all segments. The accuracy was very high in both simple and complex lesions (98 and 93%, respectively), even if slightly lower in complex lesions. All of these data were in agreement with previous findings [13,15,20]. Regarding RT3D TEE, there is already a substantial body of literature demonstrating the additional value of 3D over 2D echocardiography in the evaluation of patients with MV prolapse [15,21-23], with an accuracy of 90-95% in both simple and complex cases [13,15,19,20]. Grewal et al. underlined the importance of the method, particularly in cases with complex lesions [24]. Very recently, La Canna et al. demonstrated that RT3D TEE provided more accurate mapping of MV prolapse than 2D and 3D TTE imaging, adding quantitative recognition of dominant and secondary lesions and MV details in a large series of cases [25]. Table 1 summarizes the main studies demonstrating the advantages of 3D versus 2D echocardiography in the evaluation of MV prolapse.
Table 1: Advantage of 3D versus 2D echocardiography in the assessment of mitral valve prolapse.
Regarding functional MV regurgitation, Watanabe et al. clearly demonstrated the alterations induced in MV leaflets and annulus by ischemic MR [26,27], using a dedicated software for 3D image processing [28]. The tenting length was longer and tenting volume was larger in patients with ischemic MR than in controls and the maximum tenting site was located in anterior leaflet. Otsuji et al. first described in an animal model that LV dysfunction, even when severe, fails to produce important MR without LV dilatation [29]. Using 3D echocardiographic-based models, geometric changes, including an increased distance over which the MV leaflets are tethered from the papillary muscles to the anterior MA ring and increased MA areas, were demonstrated. Other studies showed that functional MR is associated with MA dilatation and reduced cyclic variation in MA shape and area [30,31].
From a clinical point of view, Ben Zekry et al. compared 2D and 3D TTE and TEE in patients with MR and showed that RT3D TEE had the advantage of better localizing the underlying mechanisms [32]. Moreover, in a RT3D TEE study, Saito et al. demonstrated that coaptation length at each region is related to papillary muscle displacement and that the obtained measures of coaptation are associated with MR severity [28].
Regarding MA evaluation, 2D echocardiography only allows a suboptimal assessment of this structure as the MA is saddle shaped, with higher points in the anterior and posterior regions, it is oval and its morphology may be markedly affected in several pathologic conditions [33]. The complex oval shape of the MA can be better appreciated by RT3D imaging, especially from the 3D surgical view of the MV.
Implications for MV surgery and MV percutaneous repair
The work of the heart team in the operating room and during interventional procedures has been greatly influenced by the new imaging modalities, especially by RT3D echocardiography. Cardiac surgeons, anesthesiologists and cardiologists are greatly facilitated in evaluating and discussing morphologic data by means of a direct 3D visualization of cardiac structures, avoiding cumbersome and potentially misleading mental reconstruction from several 2D views. Therefore, RT3D echocardiography facilitates training and communication not only between experts and nonexperts, but also between different specialists [21].
Guidelines on the management of valvular heart diseases underlined how MV repair should be considered, even in asymptomatic patients, when the likelihood of a successful repair is high and in the presence of a low operative risk [4,5]. It is clear that the likelihood of a durable and safe MV repair strongly depends on the anatomy of the MV and, similarly, the complexity of surgical procedures directly related to the complexity of MV lesions. In a recent paper, the relationship between the extent of MV lesions and the complexity of the surgical procedures has been investigated [19]. The results clearly demonstrated that simple lesions, as assessed by RT3D echocardiography, are very likely to be treated with simple techniques, while in almost half of the patients with complex lesions, complex surgical repair was performed by the surgeon. Thus, an accurate preoperative RT3D TTE evaluation, eventually using ad hoc postprocessing software, may facilitate surgical planning allowing a tailored approach to each case. While the contribution of 3D echocardiography in the diagnosis of MV insufficiency and in the assessment of the surgical repair outcome is clear, its role in monitoring surgical procedures is still an open issue and there is little evidence supporting its usefulness. Gripari et al. investigated the role of RT3D TEE in the routine monitoring of cardiac surgery and demonstrated that this imaging modality facilitates the intraoperative monitoring, with an additional clinical value in more than a third of the patients undergoing MV repair, allowing an optimal definition of the scallops with an impact on the repair strategy [21].
MV annuloplasty can be categorized as complete or partial annuloplasty, as suture versus ring annuloplasty, and as rigid versus flexible ring annuloplasty. Partial and suture annuloplasty may be useful in children owing to the potential for the unsutured annulus to grow and avoid mitral stenosis. In the case of myxomatous MV prolapse, similarly good results have been reported using rigid or flexible rings. Flexible rings have the potential advantage of allowing the base of the left ventricle to contract, whereas rigid rings are more resistant to central leakage in patients with LV dilation. Caiani et al. investigated, by RT3D TTE, the changes in MA dynamics and the long-term effects induced by annuloplasty in MV organic prolapse undergoing repair using either an incomplete flexible band or a complete semi-rigid ring [34]. Both rings showed similar dynamic adaptation and, at 6 months, the MA area changes during the cardiac cycle were reduced compared with controls, independently of the implanted prosthetic ring. Thus, the main factor affecting MA function is the undersizing due to the ring, which restricts MA geometry and limits the natural MA motion [34].
Even though most of the investigations based on RT3D echocardiography were focused on MV morphology, Veronesi et al. investigated, using custom software, the relationship between MV and the aortic annulus [35]. They demonstrated that, in patients with MR, the dynamic mitral-aortic coupling was preserved despite the altered morphology and function of the MV. In these patients, MV repair with annuloplasty led to smaller and less pulsatile MA, with altered spatial displacement of the MV-aortic annulus complex.
Implications for percutaneous MV repair
Despite surgical correction of MR demonstrating optimal outcomes in terms of efficiency, safety and residual MR, this option is not often feasible in high-risk patients. According to the results of the EuroHeart survey, approximately half of the symptomatic patients with severe MR are denied surgery, and the likelihood of surgery denial increases with LV dysfunction, age and the presence of comorbidities, and is higher in functional than in degenerative MR [3,36]. Therefore, several interventional options have been investigated during the last decade. Chiam and Ruiz classified the proposed technologies into those targeting the chordae, leaflets or MA, both indirectly via the coronary sinus or directly through an edge-to-edge repair [37]. Among the latter, the MitraClip system is the more diffused and studied, and it is currently under CE mark in Europe and under investigation in the USA and parts of Asia. The MitraClip system mimics the surgical MV repair procedure introduced by Alfieri et al., who treated a patient with an anterior prolapse by placing a pledgeted stitch to approximate the middle portions of the MV leaflets, creating a double orifice MV and successfully reducing the MR [38]. The EVEREST trials (I and II) and other pivotal studies established the feasibility, safety and hemodynamic improvement of the MitraClip system in the large majority of patients, despite this being less effective at reducing MR than conventional surgery and thus having a higher rate of reintervention and/or surgical operation [39-44].
As for all other structural interventions, such as transcatheter aortic valve implantation or percutaneous MV commissurotomy, edgeto- edge PMVR requires a clear delineation of intracardiac anatomy to allow a safe and longlasting procedural success. Currently, these aspects cannot be investigated by fluoroscopy and cineangiography and, thus, echocardiography is fundamental in PMVR. Echocardiography largely contributes in each phase of the procedure, including patient selection, the successful delivering and positioning of the device, as well as the early and follow-up evaluation of the procedural outcome.
Proper patient selection is of critical importance for the success of PMVR and, beyond clinical aspects, detailed TTE and TEE examination should be performed in all the candidates to determine not only the MR mechanism and severity, but also the structural and anatomic suitability for the procedure. Inclusion criteria require that the MR jet originates by the central scallops of the MV (A2 to P2 scallops) and not at the commissures. For patients with functional MR, the coaptation length must be at least 2 mm and the coaptation depth less than 11 mm. For patients with flail leaflet, the flail gap must be 10 mm and the flail width 15 mm. In addition, calcification of the grasping area of the leaflets constitutes a contraindication to the procedure because of the potential risk of embolization. Preliminary data suggest the feasibility of a systematic screening of candidates for MitraClip based on TTE and propose a new diagnostic work-up in patient selection using TTE as the first-line imaging technique [45]. This would increase the number of evaluated patients and to widen the referral network towards hospitals experienced in PMVR. The results of this preliminary protocol should be confirmed by larger studies.
Regarding the procedural guidance, echocardiography is the primary imaging modality used at all stages of the MitraClip procedure, complementing the role of cinefluoroscopy. The main steps of echocardiographic monitoring and guidance include: transeptal catheterization; advancing the clip delivery system towards the mitral leaflets; positioning the clip above the regurgitant orifice and orienting the clip arms; entry into the left ventricle and pullback to grasp the leaflets; assessment of MR and MV area before deployment of the device; evaluation of the need for a second clip; and final assessment and detection of complications.
RT3D TEE greatly facilitates all the procedural steps, in particular the positioning of the clip above the MR jet and the orientation of the clip arms as it provides an en face view of the MV leaflets and of the approaching clip. Using RT3D TEE, it is also possible to observe the repaired valve en face from both atrial and ventricular perspectives, documenting the eccentricity, if any, of the dual orifices created by the device. Biner et al. demonstrated that combined 2D and 3D TEE imaging was associated with a shorter time to first clip deployment and with a general reduction in the total procedure time when compared with traditional 2D TEE guidance [46]. They also stressed that RT3D TEE is valuable in determining the precise orientation of the clip arms, guiding the interventional cardiologist until the clip is positioned perpendicularly between the two central scallops, without the need for multple verifications and cross-checks of multiple 2D planes. Moreover, the use of 3D color Doppler could allow for better identification of the site of origin and the degree of any residual MR. These observations are crucial when a second clip has to be implanted. In this regard, Armstrong et al. identified thickened anterior mitral leaflet and more severe MR to be the echocardiographic predictors associated with a higher likelihood of a dual-clips procedure [47].
RT3D TEE data of 55 patients acquired during the MitraClip procedure immediately before and after clip placement have been recently published with the aim of assessing changes in annulus diameter and area [48]. The results demonstrated that MitraClip can produce immediate reductions in MA size and tenting in functional MR. By contrast, similar changes were not observed in organic MR, and this different pattern of remodeling between the two etiologies of MR may be important to improve procedural strategies and could influence the outcome.
Altiok et al. analyzed the procedural effects of PMVR by 2D and 3D TEE in 39 cases [49], demonstrating a significant reduction of MR volume after the procedure. The unique visualization of the MV by 3D TEE indirectly confirmed the arbitrarily applied exclusion criteria for MV area as all the patients with a preprocedural MV area <4.1 cm2 had MV diastolic mean pressure gradient ≥5 mmHg after edge-to-edge repair. Moreover, the MitraClip was less effective in patients with enlarged preprocedural MA, which is in agreement with the previously published surgical reports showing that the edge-to-edge repair is less effective in presence of significant MA dilation. Similar findings were also reported by Scandura et al., who demonstrated that the MitraClip system led to left cardiac chamber reverse remodeling, with significant improvement in LV size and function, similarly to what was observed in surgical restoration [50]. All of these results support the importance of 3D imaging for preprocedural patient selection and prediction of procedural effectiveness.
Other imaging modalities
Cardiovascular magnetic resonance (CMR) could be used to accurately assess MR severity and its influence on LV volumes and function. In the case of isolated MR, evaluation of jet severity via CMR could be performed as the difference between the LV stroke volume and the anterograde aortic flow, or the difference between left and right ventricular stroke volumes [67]. As these approaches do not rely on the direct assessment of the regurgitant jet, CMR is suitable for quantifying multiple or eccentric regurgitant jets [68]. Recently, several studies have explored the potential of CMR in the evaluation of MA and leaflets in the setting of MV prolapse [69-71]. Despite results clearly showing the feasibility of a CMR-based assessment using the same echocardiographic criteria and the incremental value of this technique in the evaluation of myocardial viability, larger clinical studies are needed to better define the role of CMR in the clinical management of patients with MV prolapse.
Advances in computed tomography have substantially improved the temporal resolution of this technique to the point that it can now be applied to quantify the severity of MR [67,72] and in the diagnosis of MV prolapse [73]. However, radiation exposure in computed tomography is still a concern and the use of this imaging modality is not recommended for the direct assessment of MV prolapse [73].
Conclusion
3D echocardiography is a relatively simple imaging modality that offers several advantages over traditional 2D echocardiography in the examination of the MV. Its capability to image and represent the MV apparatus in its intrinsic 3D nature has improved our understanding of normal and abnormal MV. A substantial body of literature evidences the advantages of RT3D echocardiography over conventional 2D echocardiography, underlining the benefits during the diagnostic phase, as well as during interventional and surgical procedures.
Future perspective
This section discusses a series of fields where echocardiography, especially when performed in 3D, could potentially have an impact on interventional MV repair. It is worth noting that most, but not all, of these foreseen innovations derive from previous investigations on surgical MV repair and are expected to evolve into solutions that address the limitations of the percutaneous approach.
First of all, while revising the literature on PMVR, it is clear that additional efforts are needed to identify the best candidates for the procedure. Indeed, while the criteria adopted in the EVEREST trials should ensure the safe and long-lasting positioning of the device, Volker et al. performed the MitraClip procedure in patients not fulfilling the abovementioned criteria and demonstrated that, when performed by experienced and skilled operators, the procedure could be as safe as in those cases compliant with the EVEREST criteria [51]. Regarding the benefit that 3D echocardiography methods could introduce in the patient-selection phase, one should consider the amount of data that, during recent years, have proven the accuracy and usefulness of the 3D modality in the setting of MV evaluation [11,16,18,52-54]. The adoption of the 3D modality in the standard 2D protocol could allow not only a better characterization of the 3D morphology of the prolapsing scallops, presence of ruptured chordae and thickened leaflet, but also to accurately image the left-side cardiac chambers. This approach will allow a more detailed and comprehensive view of the MV and cardiac chambers, and also refinement of the EVEREST criteria. This second task should take into account an accurate postprocedural evaluation of the outcome, ideally in a long-term follow-up. Furthermore, recent innovations in 3D echocardiographic equipment led to the development of a novel approach for the quantitative evaluation of MR on the basis of 3D color Doppler data sets [55-58]. This aspect is of particular relevance not only during patient selection for the evaluation of native MR, but it would constitute the first quantitative and reliable methods for the direct assessment of residual MR. Indeed, the double orifice morphology of the postprocedural MV only allows a qualitative evaluation of the MR and this new 3D-based technique would definitely facilitate the assessment of the procedural results.
Regarding outcome evaluation, RT3D echocardiography could potentially introduce benefits beyond the well-known more accurate evaluation of LV volumes and function, as previously demonstrated in the setting of surgical MV repair. Maffessanti et al. developed an algorithm for the evaluation of 3D LV shape analysis [59], and applied the methodology to asymptomatic patients referred early to surgical MV repair [60]. They demonstrated that, despite the evidence of preoperative LV systolic dysfunction, the left ventricle has already remodeled towards a more globular and less conical morphology compared with controls. A normalization of the same shape indices, describing a reverse remodeling process, was observed in the mid-term follow-up. The application of this morphological analysis, completely based on RT3D echocardiography and otherwise unfeasible, to the settings of PMVR would add important information during patient selection but also in outcome evaluation phases. Encouraging results on positive reverse LV remodeling following PMVR have been proven by Scandura et al., who demonstrated a substantial reduction of LV end-diastolic and -systolic volumes 6 months after MitraClip implantation, which was associated with a significant improvement in LV ejection fraction and decrease in LV sphericity, as evaluated by 2D TTE [50].
Several ongoing protocols aim at describing the alterations that the MA experiences after PMVR, and at correlating morphological and clinical outcomes. Furthermore, the high spatial and temporal resolutions of RT3D TEE made this technique the ideal basis to accurately describe the dynamic behavior of the MA throughout the cardiac cycle [34,52,54]. Moreover, the high quality of the latest generation of RT3D TEE equipment allowed to derive not only a model of the MA but also of the leaflet surface [18,52,53]. Evaluation of leaflet morphology is of particular importance in the setting of the Mitra- Clip, as the device could alter the physiological stress of the leaflet, as previously demonstrated in the relationship between MA shape and leaflet stress [61,62]. All of these new methodologies require a time-consuming manual interaction and, thus, their applicability is now limited to the research field. Preliminary data for the creation of a 3D MV model directly from a 3D data set, requiring minimal user interaction, have been reported [63-66]. Of note, the methods proposed by Sotaquira et al. are the first that are able to quantify local MV thickness, which is considered one of the major determinants of MitraClip detachment [63].
Although the clinical usefulness of these parameters needs to be further supported by evidences in the setting of PMVR, Altiok et al. have already demonstrated the potential of preprocedural quantitative assessment of the MV by TEE as a predictor of the effectiveness of the MitraClip, as they found that less than 50% of MR reduction is associated with larger annuli [49]. Finally, the fascinating results concerning the reciprocal behavior of the MA and aortic annulus reported in healthy subjects could be investigated before and after PMVR, to study whether the presence of the device stiffens the mitral-aortic valve complex [34].
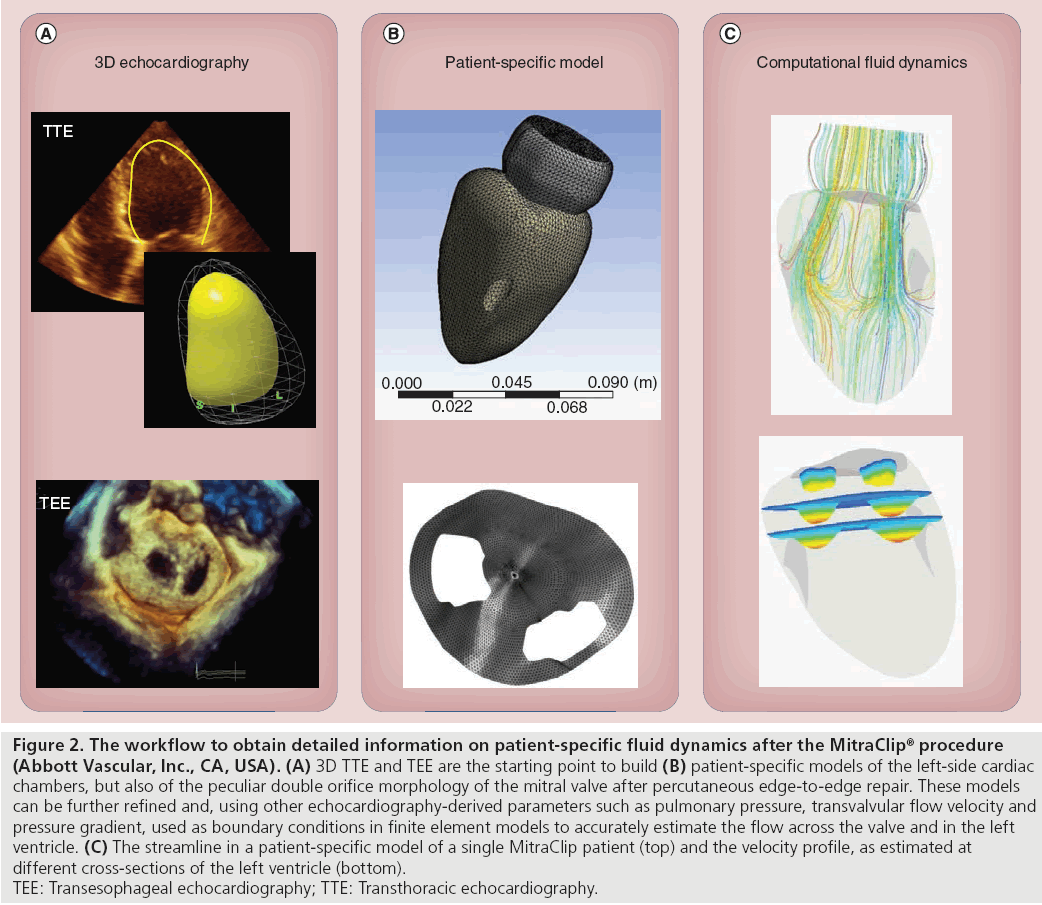
Beyond physiological and clinical potentials, in the near future, all of these 3D parameters and models could be integrated into a patientspecific model of the MV, via finite element modeling, allowing a preprocedural evaluation of the effects of PMVR [65]. This virtual approach, summarized in Figure 2, will make it possible to evaluate how the clip positioning will influence the left-side fluid dynamics, and to simulate and compare different procedural strategies.
Figure 2: The workflow to obtain detailed information on patient-specific fluid dynamics after the MitraClip® procedure
(Abbott Vascular, Inc., CA, USA). (A) 3D TTE and TEE are the starting point to build (B) patient-specific models of the left-side cardiac
chambers, but also of the peculiar double orifice morphology of the mitral valve after percutaneous edge-to-edge repair. These models
can be further refined and, using other echocardiography-derived parameters such as pulmonary pressure, transvalvular flow velocity and
pressure gradient, used as boundary conditions in finite element models to accurately estimate the flow across the valve and in the left
ventricle. (C) The streamline in a patient-specific model of a single MitraClip patient (top) and the velocity profile, as estimated at
different cross-sections of the left ventricle (bottom).
TEE: Transesophageal echocardiography; TTE: Transthoracic echocardiography.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Imaging the mitral valve apparatus
▪ In presence of severe mitral regurgitation, early surgery preserves long-term left ventricular function and imaging-based assessment of the mitral valve (MV) is essential to adopt the best surgical strategy.
▪ Percutaneous MV repair requires the clear delineation of intracardiac anatomy and the mechanism of mitral regurgitation, and this can only be achieved using echocardiography.
New echocardiographic modalities
▪ 3D echocardiography allows visualization of the MV in an easy way and from multiple planes of the complex structures.
▪ Both experienced and less trained operators benefit from the use of 3D compared with traditional 2D echocardiography.
▪ 3D transesophageal echocardiography is highly accurate in the identification of both simple and complex cases of MV prolapse when compared with surgical inspection.
Implications for MV surgery and MV percutaneous repair
▪ 3D echocardiography allows the assessment of the complexity of MV lesions, facilitating the surgical planning.
▪ 3D echocardiography is the ideal technique to evaluate the effect of MV repair on the morphology and function of MV annulus, leaflets and papillary muscles and their interaction with the aortic valve.
▪ Echocardiography plays a role in each phase of the percutaneous MV repair procedure, including patient selection, the delivering and positioning of the device, and follow-up evaluation.
▪ 3D imaging is fundamental for patient selection and prediction of procedural effectiveness.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Freed LA, Levy D, Levine RA et al. Prevalence and clinical outcome of mitral valve prolapse. N. Engl. J. Med. 341(1), 1–7 (1999).
- Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet 373(9672), 1382–1394 (2009).
- Iung B, Baron G, Butchart EG et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on valvular heart disease. Eur. Heart J. 24(13), 1231–1243 (2003).
- Vahanian A, Alfieri O, Andreotti F et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. J. Cardiothorac. Surg. 42(4), S1–S44 (2012).
- Bonow RO, Carabello BA, Chatterjee K et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American college of Cardiology/American Heart Association Task Force on management of patients with valvular. J. Am. Coll. Cardiol. 52(13), e1–e142 (2008).
- Agricola E, Oppizzi M, Pisani M, Meris A, Maisano F, Margonato A. Ischemic mitral regurgitation: mechanisms and echocardiographic classification. Eur. J. Echocardiogr. 9(2), 207–221 (2008).
- Cavalcante J, Rodriguez L, Kapadia S, Tuzcu E, Stewart W. Role of echocardiography in percutaneous mitral valve interventions. JACC Cardiovasc. Imaging 5(7), 733–746 (2012).
- Lang RM, Badano LP, Tsang W et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur. Heart J. Cardiovasc. Imaging 13(1), 1–46 (2012).
- Lang RM, Badano LP, Tsang W et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J. Am. Soc. Echocardiogr. 25(1), 3–46 (2012).
- Lang RM, Mor-Avi V, Sugeng L, Nieman PS, Sahn DJ. Three-dimensional echocardiography: the benefits of the additional dimension. J. Am. Coll. Cardiol. 48(10), 2053–2069 (2006).
- Lang RM, Tsang W, Weinert L, Mor-Avi V, Chandra S. Valvular heart disease: the value of 3-dimensional echocardiography. J. Am. Coll. Cardiol. 58(19), 1933–1944 (2011).
- Salcedo EE, Quaife RA, Seres T, Carroll JD. A framework for systematic characterization of the mitral valve by real-time three-dimensional transesophageal echocardiography. J. Am. Soc. Echocardiogr. 22(10), 1087–1099 (2009).
- Sugeng L, Shernan SK, Salgo IS et al. Live 3-dimensional transesophageal echocardiography initial experience using the fully-sampled matrix array probe. J. Am. Coll. Cardiol. 52(6), 3–6 (2008).
- Hien MD, Großgasteiger M, Med C et al. Experts and beginners benefit from three-dimensional echocardiography: a multicenter study on the assessment of mitral valve prolapse. J. Am. Soc. Echocardiogr. 26(8), 828–834 (2013).
- Pepi M, Tamborini G, Maltagliati A et al. Head-to-head comparison of two- and three-dimensional transthoracic and transesophageal echocardiography in the localization of mitral valve prolapse. J. Am. Coll. Cardiol. 48(12), 2524–2530 (2006).
- Biaggi P, Jedrzkiewicz S, Gruner C et al. Quantification of mitral valve anatomy by three-dimensional transesophageal echocardiography in mitral valve prolapse predicts surgical anatomy and the complexity of mitral valve repair. J. Am. Soc. Echocardiogr. 25(7), 758–765 (2012).
- Carpentier A, Chauvaud S, Fabiani J et al. Reconstructive surgery of mitral valve incompetence: ten-year appraisal. J. Thorac. Cardiovasc. Surg. 79(3), 338–348 (1980).
- Maffessanti F, Marsan NA, Tamborini G et al. Quantitative analysis of mitral valve apparatus in mitral valve prolapse before and after annuloplasty: a three-dimensional intraoperative transesophageal study. J. Am. Soc. Echocardiogr. 24(4), 405–413 (2011).
- Tamborini G, Muratori M, Maltagliati A et al. Pre-operative transthoracic real-time three-dimensional echocardiography in patients undergoing mitral valve repair: accuracy in cases with simple vs. complex prolapse lesions. Eur. J. Echocardiogr. 11(9), 778–785 (2010).
- Müller S, Müller L, Laufer G et al. Comparison of three-dimensional imaging to transesophageal echocardiography for preoperative evaluation in mitral valve prolapse. Am. J. Cardiol. 98(2), 243–248 (2006).
- Gripari P, Tamborini G, Barbier P et al. Real-time three-dimensional transoesophageal echocardiography: a new intraoperative feasible and useful technology in cardiac surgery. Int. J. Cardiovasc. Imaging 26(6), 651–660 (2006).
- Sallustri A, Becker AE, van Herwerden L, Vletter WB, Ten Cate FJ, Roelandt JR. Three-dimensional echocardiography of normal and pathologic mitralvalve: a comparison with two-dimensional transesophageal echocardiography. J. Am. Coll. Cardiol. 27(6), 502–510 (1996).
- Sharma R, Mann J, Drummond L, Livesey SA, Simpson IA. The evaluation of real-time 3-dimensional transthoracic echocardiography for the preoperative functional assessment of patients with mitral valve prolapse: acomparison with 2-dimensional transesophageal echocardiography. J. Am. Soc. Echocardiogr. 20(8), 934–940 (2007).
- Grewal J, Mankad S, Freeman WK et al. Real-time three-dimensional transesophageal echocardiography in the intraoperative assessment of mitral valve disease. J. Am. Soc. Echocardiogr. 22(1), 34–41 (2009).
- La Canna G, Arendar I, Maisano F et al. Real-time three-dimensional transesophageal echocardiography for assessment of mitral valve functional anatomy in patients with prolapse-related regurgitation. Am. J. Cardiol. 107(9), 1365–1374 (2011).
- Watanabe N, Ogasawara Y, Yamaura Y et al. Mitral annulus flattens in ischemic mitral regurgitation: geometric differences between inferior and anterior myocardial anfarction: a real-time 3-dimensional echocardiographic study. Circulation 112(9 Suppl.), I458–I462 (2005).
- Watanabe N, Ogasawara Y, Yamaura Y et al. Quantitation of mitral valve tenting in ischemic mitral regurgitation by transthoracic real-time three-dimensional echocardiography. J. Am. Coll. Cardiol. 45(5), 763–769 (2005).
- Saito K, Okura H, Watanabe N et al. Influence of chronic tethering of the mitral valve on mitral leaflet size and coaptation in functional mitral regurgitation. JACC Cardiovasc. Imaging 5(4), 337–345 (2012).
- Otsuji Y, Handschumacher MD, Schwammenthal E et al. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation 96(6), 1999–2008 (1997).
- Kaplan SR, Bashein G, Sheehan FH et al. Three-dimensional echocardiographic assessment of annular shape changes in the normal and regurgitant mitral valve. Am. Heart J. 139(3), 378–387 (2000).
- Veronesi F, Corsi C, Sugeng L et al. Quantification of mitral apparatus dynamics in functional and ischemic mitral regurgitation using real-time 3-dimensional echocardiography. J. Am. Soc. Echocardiogr. 21(4), 347–354 (2008).
- Ben Zekry S, Lang RM, Sugeng L et al. Mitral annulus dynamics early after valve repair: preliminary observations of the effect of resectional versus non-resectional approaches. J. Am. Soc. Echocardiogr. 24(11), 1233–1242 (2011).
- Levine RA, Handschumacher MD, Sanfilippo A et al. Three-dimensional echocardiographic reconstruction of the mitrla valve, with implications for the diagnosis of mitral valve prolapse. Circulation 80(3), 589–598 (1989).
- Caiani EG, Fusini L, Veronesi F et al. Quantification of mitral annulus dynamic morphology in patients with mitral valve prolapse undergoing repair and annuloplasty during a 6-month follow-up. Eur. J. Echocardiogr. 12(5), 375–383 (2011).
- Veronesi F, Caiani EG, Sugeng L et al. Effect of mitral valve repair on mitral–aortic coupling: areal-time three-dimensional transesophageal echocardiography study. J. Am. Soc. Echocardiogr. 25(5), 524–531 (2012).
- Mirabel M, Iung B, Baron G et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur. Heart J. 28(11), 1358–1365 (2007).
- Chiam PT, Ruiz CE. Percutaneous transcatheter mitral valve repair: a classification of the technology. JACC Cardiovasc. Interv. 4(1), 1–13 (2011).
- Maisano F, Torracca L, Oppizzi M et al. The edge-to-edge technique: a simplified method to correct mitral insufficiency. Eur. J. Cardiothorac. Surg. 13(3), 240–246 (1998).
- Feldman T, Wasserman HS, Herrmann HC et al. Percutaneous mitral valve repair using the edge-to-edge technique: six-month results of the EVEREST Phase I clinical trial. J. Am. Coll. Cardiol. 46(11), 2134–2140 (2005).
- Foster E, Wasserman HS, Gray W et al. Quantitative assessment of severity of mitral regurgitation by serial echocardiography in a multicenter clinical trial of percutaneous mitral valve repair. Am. J. Cardiol. 100(10), 1577–1583 (2007).
- Mauri L, Garg P, Massaro JM et al. The EVEREST II trial: design and rationale for a randomized study of the evalve mitraclip system compared with mitral valve surgery for mitral regurgitation. Am. Heart J. 160(1), 23–29 (2010).
- Mauri L, Foster E, Glower DD et al. Four-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J. Am. Coll. Cardiol. 62(4), 317–328 (2013).
- Feldman T, Forster E, Glower DD et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 364(15), 1395–1406 (2011).
- Whitlow PL, Feldman T, Pedersen WR et al. Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) high risk study. J. Am. Coll. Cardiol. 59(2), 130–139 (2012).
- Tamborini G, Gripari P, Muratori M et al. Head-to-head comparison of two-dimensional and three-dimensional transthoracic and transesophageal echocardiography in patients selection for MitraClip implantation. Eur. Heart J. Cardiovasc. Imaging 13(Suppl.), i38 (2012).
- Biner S, Perk G, Kar S et al. Utility of combined two-dimensional and three-dimensional transesophageal imaging for catheter-based mitral valve clip repair of mitral regurgitation. J. Am. Soc. Echocardiogr. 24(6), 611–617 (2011).
- Armstrong EJ, Rogers JH, Swan CH et al. Echocardiographic predictors of single versus dual MitraClip device implantation and long-term reduction of mitral regurgitation after percutaneous repair. Catheter. Cardiovasc. Interv. 82(4), 673–679 (2013).
- Schmidt FP, Von Bardeleben RS, Nikolai P et al. Immediate effect of the MitraClip procedure on mitral ring geometry in primary and secondary mitral regurgitation. Eur. Heart J. Cardiovasc. Imaging 14(9), 851–857 (2013).
- Altiok E, Hamada S, Brehmer K et al. Analysis of procedural effects of percutaneous edge-to-edge mitral valve repair by 2D and 3D echocardiography. Circ. Cardiovasc. Imaging 5(6), 748–755 (2012).
- Scandura S, Ussia GP, Capranzano P et al. Left cardiac chambers reverse remodeling after percutaneous mitral valve repair with the MitraClip system. J. Am. Soc. Echocardiogr. 25(10), 1099–1105 (2012).
- Volker R, Knap M, Franzen O et al. Echocardiographic and clinical outcomes of MitraClip therapy in patients not amenable to surgery. J. Am. Coll. Cardiol. 58(21), 2190–2195 (2011).
- Grewal J, Suri R, Mankad S et al. Mitral annular dynamics in myxomatous valve disease: new insights with real-time three dimensional echocardiography. Circulation 121(12), 1423–1431 (2010).
- Chandra S, Salgo IS, Sugeng L et al. Characterization of degenerative mitral valve disease using morphologic analysis of real-time three-dimensional echocardiographic images. Circ. Cardiovasc. Imaging 4(1), 24–32 (2011).
- Little SH, Ben Zekry S, Lawrie GM, Zoghbi WA. Dynamic annular geometry and function in patients with mitral regurgitation: insight from three-dimensional annular tracking. J. Am. Soc. Echocardiogr. 23(8), 872–879 (2010).
- Thavendiranathan P, Phelan D, Thomas JD, Flamm SD, Marwick TH. Quantitative assessment of mitral regurgitation validation of new methods. J. Am. Coll. Cardiol. 60(16), 1470–1483 (2012).
- Marsan NA, Westenberg JJ, Ypenburg C et al. Quantification of functional mitral regurgitation by real-time 3D echocardiography. JACC Cardiovasc. Imaging 2(11), 1245–1252 (2009).
- Shanks M, Siebelink HM, Delgado V et al. Quantitative assessment of mitral regurgitation: comparison between three-dimensional transesophageal echocardiography and magnetic resonance imaging. Circ. Cardiovasc. Imaging 3(6), 694–700 (2010).
- Thavendiranathan P, Liu S, Datta S et al. Automated quantification of mitral inflow and aortic outflow stroke volumes by three-dimensional real-time volume color-flow Doppler transthoracic echocardiography: comparison with pulsed-wave Doppler and cardiac magnetic resonance imaging. J. Am. Soc. Echocardiogr. 25(1), 56–65 (2012).
- Maffessanti F, Lang RM, Corsi C, Mor-Avi V, Caiani EG. Feasibility of left ventricular shape analysis from transthoracic real-time 3D echocardiographic images. Ultrasound Med. Biol. 35(12), 1953–1962 (2009).
- Maffessanti F, Caiani EG, Tamborini G et al. Serial changes in left ventricular shape following early mitral valve repair. Am. J. Cardiol. 106(6), 836–842 (2010).
- Salgo IS, Gorman JHI, Gorman RC et al. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation 106(6), 711–717 (2002).
- Ryan LP, Jackson BM, Eperjesi TJ et al. A methodology for assessing human mitral leaflet curvature using real-time 3-dimensional echocardiography. J. Thorac. Cardiovasc. Surg. 136(3), 726–734 (2008).
- Sotaquira M, Fusini L, Lang RM, Caiani EG. Nearly-automated quantification of mitral annulus and leaflet morphology from transesophageal real-time 3D echocardiography. Comput. Cardiol. 38, 145–148 (2012).
- Xu C, Brinster CJ, Jassar AS et al. A novel approach to in vivo mitral valve stress analysis. Am. J. Physiol. Heart Circ. Physiol. 299(6), H1790–H1794 (2010).
- Mansi T, Voigt I, Georgescu B et al. An integrated framework for finite-element modeling of mitral valve biomechanics from medical images: application to MitralClip intervention planning. Med. Image Anal. 16(7), 1330–1346 (2012).
- Pouch AM, Xu C, Yushkevich PA et al. Semi-automated mitral valve morphometry and computational stress analysis using 3D ultrasound. J. Biomech. 45(5), 903–907 (2012).
- Morris MF, Maleszewski JJ, Suri RM et al. CT and MR imaging of the mitral valve: radiologic-pathologic correlation. Radiographics 30(6), 1603–1620 (2010).
- Hundley WG, Li HF, Willard JE et al. Magnetic resonance imaging assessment of the severity of mitral regurgitation. Comparison with invasive techniques. Circulation 92(5), 1151–1158 (1995).
- Maffessanti F, Gripari P, Pontone G et al. Three-dimensional dynamic assessment of tricuspid and mitral annuli using cardiovascular magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 14(10), 986–995 (2013).
- Han Y, Peters DC, Salton CJ et al. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc. Imaging 1(3), 294–303 (2008).
- Gabriel RS, Kerr AJ, Raffel OC, Stewart RA, Cowan BR, Occleshaw CJ. Mapping of mitral regurgitant defects by cardiovascular magnetic resonance in moderate or severe mitral regurgitation secondary to mitral valve prolapse. J. Cardiovasc. Magn. Reson. 10, 16 (2008).
- Guo YK, Yang ZG, Ning G et al. Isolated mitral regurgitation: quantitative assessment with 64-section multidetector CT – comparison with MR imaging and echocardiography. Radiology 252(2), 369–376 (2009).
- Shah RG, Novaro GM, Blandon RJ, Wilkinson L, Asher CR, Kirsch J. Mitral valve prolapse: evaluation with ECG-gated cardiac CT angiography. AJR Am. J. Roentgenol. 194(3), 579–584 (2010).
- García-Orta R, Moreno E, Vidal M et al. Three-dimensional versus two-dimensional transesophageal echocardiography in mitral valve repair. J. Am. Soc. Echocardiogr. 20(1), 4–12 (2007).
- Sugeng L, Coon P, Weinert L et al. Use of real-time 3-dimensional transthoracic echcoardiography in the evaluation of mitral valve disease. J. Am. Soc. Echocardiogr. 19(4), 413–421 (2006).
▪▪ Up-to-date guidelines focused on the management of valvular heart disease, with interesting clinical and surgical perspectives.
▪▪ Comprehensive and influential recommendations on how to acquire, analyze and display cardiac structures using 3D echocardiography.
▪ The additional diagnostic value of real-time 3D versus 2D transesophageal echocardiography was investigated in a large series of patients. The results demonstrated not only the higher accuracy of 3D versus 2D echocardiography, but also the capability to recognize dominant and secondary mitral valve lesions.
▪ First study investigating the intraprocedural effects of percutaneous mitral valve repair on the morphology of the mitral valve apparatus using 3D transesophageal echocardiography.
▪▪ Comprehensive and detailed overview of the novel modalities for the assessment of mitral regurgitation severity, paying special attention to the role of real-time 3D echocardiography.