Case Report - Diabetes Management (2018) Volume 8, Issue 3
A physician-patient’s perspective on lowering glycemic variability – Part II: the role of exercise
- *Corresponding Author:
- Elsamma Chacko
Connecticut Valley Hospital
1000 Silver Street, Middletown, CT 06457, USA
E-mail: elsammac@msn.com
Abstract
A physician with long-standing type 2 diabetes and impaired awareness of hypoglycemia sought to lower the hypoglycemia risk using continuous glucose monitoring to optimize the medications-meals-exercise triad. As part of the lifestyle modification the patient made a remarkable observation: a glycogen depleting exercise, pre-meal or post-meal, followed by a mid-postprandial walk was superior to the post-meal walk alone for improving glycaemia. She found four exercise options that were useful and doable for lowering glycemic variability.
Keywords
Hypoglycemia, breakfast-centered low-carb meals, pre-breakfast walk, mid-postprandial walk, balanced meals
Abbreviations
IAH – Impaired Awareness of Hypoglycemia, CGM – Continuous Glucose Monitor, AE – Aerobic Exercise, RE – Resistance Exercise, HIIE – High Intensity Interval Exercise, Stair Ex – Stair Exercise, FBG – Fasting Blood Glucose, PPG – Postprandial Glucose.
Introduction
Lowering glycemic variability is thought to be more beneficial than lowering HbA1c or fasting blood glucose toward decreasing diabetes complications [1]. Lowering glycemic variability also means less hyperglycemia or hypoglycemia. The net, real-time response blood glucose levels have to medications, meals and exercise activities can be complex. The glucose response is dictated by the interplay of a large number of variables, including the state of diabetes, type and dosage of medications, meal timing and meal composition, and timing, intensity, duration and sequence of exercise. It is challenging to sort out the effects of exercise on glucose response under free-living conditions. Although an impressive body of results have accumulated on the immediate and short term effects of various exercise modalities [2], improvements in HbA1c, fasting glucose and lipids have been marginal or inconsistent [3,4] and data on the prevention of hypoglycemia are not reassuring [5]. Not surprisingly, translational efforts are exceedingly slow. In the absence of reliable results from largescale studies, there is no official guidance from diabetes organizations on, for example, exercise duration or timing. On the other hand, most of the factors affecting glycaemia can be kept constant when a single subject explores a variety of exercise options with continuous glucose monitor’s (CGM) help. This paper reports how a physician-patient used exercise to lower glycemic variability. Part I reports how meal timing and meal composition influenced glycaemia benefit. This patient living with T2D for 19 years also had impaired awareness of hypoglycemia (IAH) [6,7]. After a second seizure episode while driving to work, ~2½ h after a resistance exercise, her endocrinologist recommended CGM to adjust the medication-meal-exercise triad towards reducing hypoglycemia risk. She was on metformin 1gm twice a day and glargine insulin 36 units a day when she had the first seizure episode which came after not eating for eight h on a busy day. Insulin dose came down to 18 units when she started a breakfast-centered low-carb balanced meal plan which called for eating every 3-4 h [8]. After the second seizure episode the patient would eat within two h after RE. The insulin dose came down further to 7 units when dulaglutide, 0.75 mg/week was added to the med regimen. Keeping the same med regimen and meal plan she explored different exercise options.
▪ Exercise timing
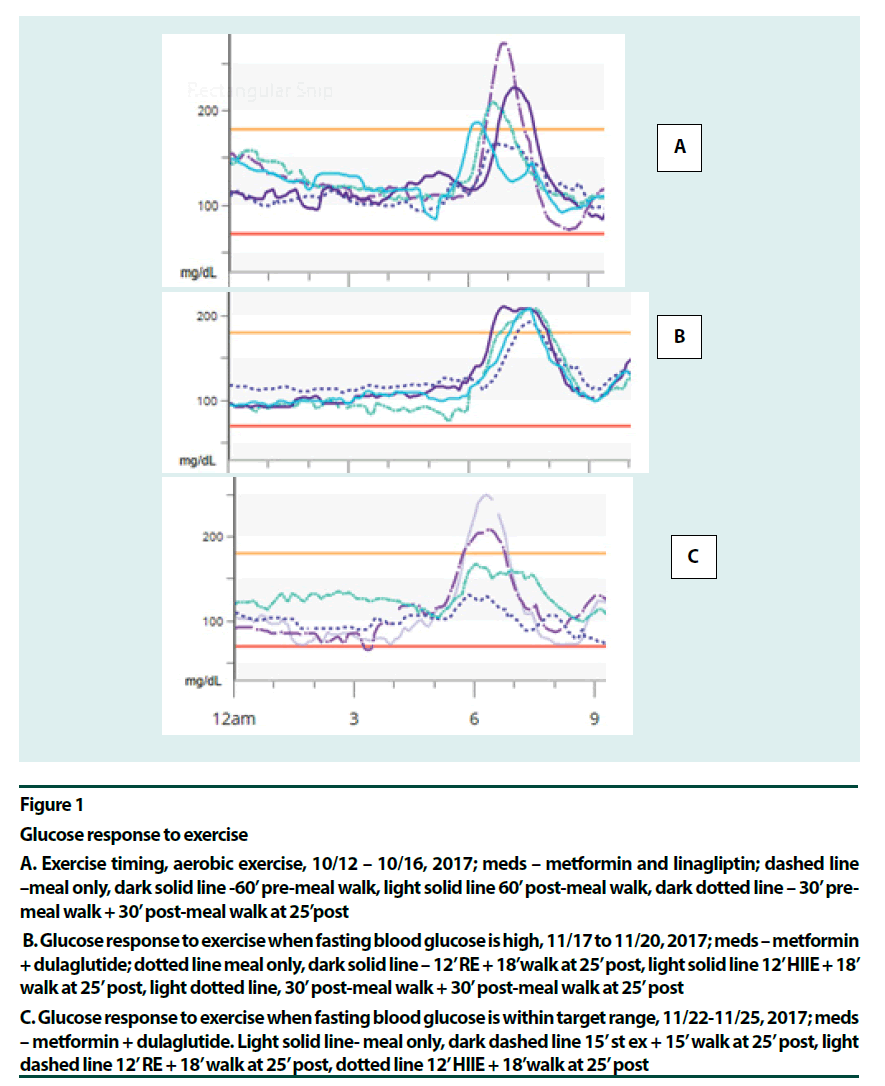
Although an uneasy consensus favoring moderate mid-postprandial exercise as better suited for glycaemia benefits exists among researchers [2,9-16], pre-meal exercise has its advantages: little risk for hypoglycemia [14], enhanced insulin sensitivity [17], and improved muscle glycogen content and GLUT-4 protein levels [18]. The downside of pre-meal exercise is elevated postprandial glucose (PPG), [9- 11,19]. A recent systematic review concluded that >45 min of aerobic exercise (AE) post-meal offered consistent glycaemia benefits [15]. Also, the review identified resistance training as an effective modality in this regard. The patient found a 30-min pre-meal walk followed by another 30 minutes of post-meal walk blunted the post-meal glucose surge better than a 60 min pre-meal or post-meal walk (Figure 1A and B). The post-meal walk might have cleared some of the extra blood glucose arrived from the liver. Split exercise at lunch time was comparable to mid-postprandial exercise in improving glycaemia and oxidative stress [20], although there was less hyperglycemia after the meal. Also, the patient confirmed that the best point to start the exercise for blunting the glucose surge has been 25-30 min past the start of the meal [21- 35]. (Most of the studies starting the exercise at 30 min post-meal was done in patients with type 1 diabetes [32,33] or in normal people [27,34].
Figure 1
Glucose response to exercise
A. Exercise timing, aerobic exercise, 10/12 – 10/16, 2017; meds – metformin and linagliptin; dashed line –meal only, dark solid line -60’ pre-meal walk, light solid line 60’ post-meal walk, dark dotted line – 30’ pre-meal walk + 30’ post-meal walk at 25’post
B. Glucose response to exercise when fasting blood glucose is high, 11/17 to 11/20, 2017; meds – metformin + dulaglutide; dotted line meal only, dark solid line – 12’ RE + 18’walk at 25’ post, light solid line 12’ HIIE + 18’ walk at 25’ post, light dotted line, 30’ post-meal walk + 30’ post-meal walk at 25’ post
C. Glucose response to exercise when fasting blood glucose is within target range, 11/22-11/25, 2017; meds – metformin + dulaglutide. Light solid line- meal only, dark dashed line 15’ st ex + 15’ walk at 25’ post, light dashed line 12’ RE + 18’ walk at 25’ post, dotted line 12’ HIIE + 18’walk at 25’ post
They did not make the cut to be included in the systematic review on post-meal exercise in type 2 diabetes [15].
▪ Exercise intensity and duration
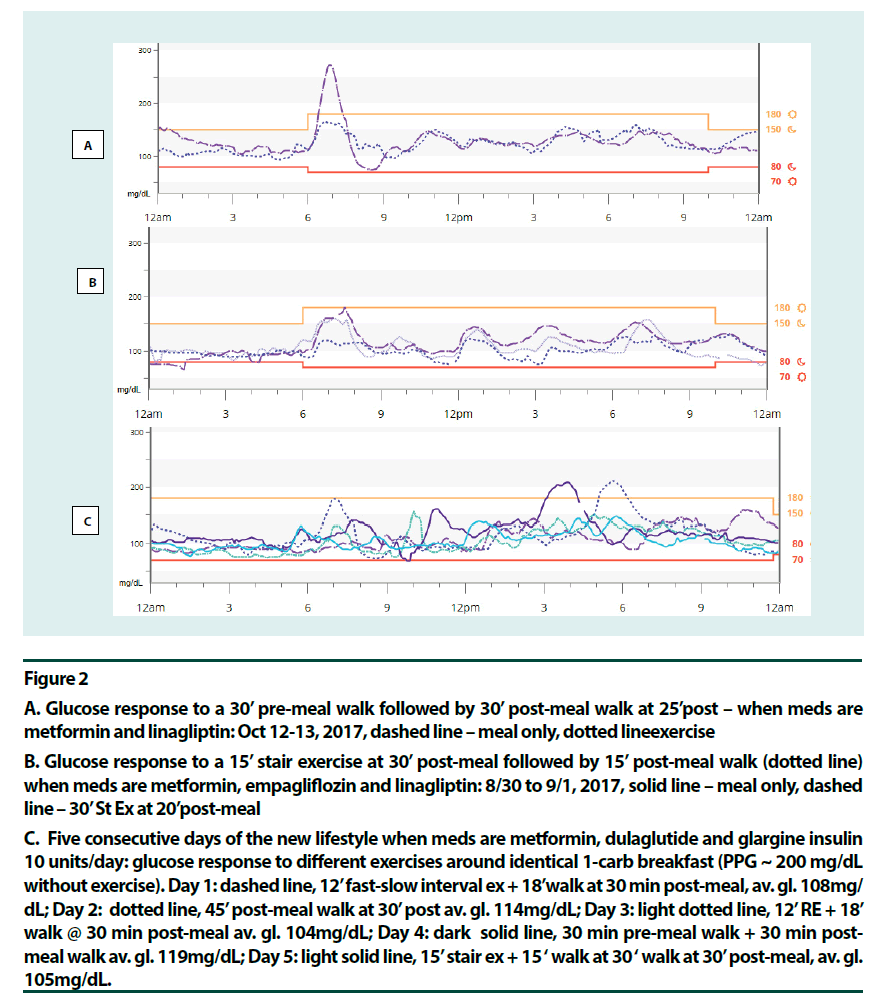
Various exercise modalities were explored based on existing data. Most long-duration premeal exercises, aerobic exercise (AE) [14,15], resistance exercise (RE) [22] and fast-slow highintensity interval exercise (HIIE) [23] offered glycaemia benefits. Pre-meal exercises that did not help were fast/rest HIIE [24] and high intensity continuous exercise [17]. These two studies showed post-exertion glucose elevation. In the mid-postprandial period, HIIE for up to 10 min duration was beneficial [25,26]. RE for up to 45 min did well [22]. Moderate aerobic activity, however, could offer stable glucose levels for over 2 h [14]. As intensity increased glucose levels started to go up in 75 min at 60% VO2max [14], in 20 min at intensity 71% VO2max [27] and in <10 min at intensity 80% VO2max [28]. It seemed that moving from moderate aerobic to resistance to interval exercise to high intensity continuous exercise, as the anaerobic component increased, hepatic glucose production and glucose turnover increased, and duration needed to be shortened accordingly [23,25,27,28]. The recent HIIE study by Hatamoto and colleagues [25] seemed to concur: long duration post-meal or fast-rest pre-meal exercises did not work; intermittent short duration exercise pre or post did. (Figures 1C & 2A–C) point to high intensity post-meal exercise options that were effective for this patient: a short (~15 min) bout of stair climbing (Stair Ex) or 12 min of HIIE or RE before a short post-meal walk seemed helpful. Here medication, exercise (contraction mediated) and glycogen repletion were working together to increase glucose output.
▪ Exercise frequency and sequence
There is general agreement that AE may be done every day [29] and high intensity exercise no more than 3 times a week [30]. The preferred sequence is high intensity exercise before any aerobic activity for glycaemia benefit and muscle conditioning [30,31].
▪ The new lifestyle
Figure 2C shows the glucose profiles for five consecutive days of the new lifestyle. The breakfast itself remained identical at 1 carb through the 5 days. Here, high-intensity exercises were done on days 1, 3 and 5 at 30 min post-meal (fast-slow HIIE, RE and Stair Ex, respectively) and a brief walk followed. Days 2 and 4 were AE days (45 min walk at 30 min post-meal and 30 min pre-meal walk plus 30 min post-meal walk, respectively). All four glycogen depleting activities followed by post-meal walks were more effective in lowering PPG peak of the breakfast, than the post-meal walk alone. Glycemic variability remained low: only twice did PPG peak exceed 200 mg/dL during these five days. Daily average glucose was 104 to 108 mg/dL when glycogen depleting activities were done but slightly higher with AE, 114-119 mg/dL. Hypoglycemia risk was minimized. A surprising observation was that there was no glycaemia benefit from exercise when fasting blood glucose (FBG) was high (Figure 1B). This was right after a week-long vacation. This effect most likely, resulted from low insulin levels in this longterm patient and more fat content in the liver. As soon as the FBG was corrected using strict carbohydrate control and a long pre-breakfast walk [21] glycaemia benefits from exercise was regained (Figure 1C). The patient observed that her state of diabetes had been dynamic.
Figure 2
A. Glucose response to a 30’ pre-meal walk followed by 30’ post-meal walk at 25’post – when meds are metformin and linagliptin: Oct 12-13, 2017, dashed line – meal only, dotted lineexercise
B. Glucose response to a 15’ stair exercise at 30’ post-meal followed by 15’ post-meal walk (dotted line) when meds are metformin, empagliflozin and linagliptin: 8/30 to 9/1, 2017, solid line – meal only, dashed line – 30’ St Ex at 20’post-meal
C. Five consecutive days of the new lifestyle when meds are metformin, dulaglutide and glargine insulin 10 units/day: glucose response to different exercises around identical 1-carb breakfast (PPG ~ 200 mg/dL without exercise). Day 1: dashed line, 12’ fast-slow interval ex + 18’walk at 30 min post-meal, av. gl. 108mg/ dL; Day 2: dotted line, 45’ post-meal walk at 30’ post av. gl. 114mg/dL; Day 3: light dotted line, 12’ RE + 18’ walk @ 30 min post-meal av. gl. 104mg/dL; Day 4: dark solid line, 30 min pre-meal walk + 30 min post-meal walk av. gl. 119mg/dL; Day 5: light solid line, 15’ stair ex + 15 ‘ walk at 30 ‘ walk at 30’ post-meal, av. gl. 105mg/dL.
Out of the 2 lifestyle modifications the patient could do, healthy meals and exercise, meal plan sets the stage: staying on the personalized meal plan improved fasting glucose, glycaemia benefit from exercise followed, insulin dose might come down and state of diabetes improved week after week. On the contrary, when the patient went off the track state of diabetes deteriorated. For high-intensity exercises, long duration pre-meal and short duration post-meal offered glycaemia benefits. This patient preferred low-carb balanced meals (75 to 90 gms/day) to very low carb ketogenic diet (<20 gm/day) [36]. The structured exercise options around breakfast as described here were safe and practical with her busy schedule compared with the intermittent physical activity reported in recent studies [37]. It was crucial to individualize the medication-meals- exercise triad.
Conclusion
Adding a glycogen-depleting activity before the mid-postprandial walk lowered the glucose surge more efficiently than a post-meal walk alone. The four glycogen depleting exercise options described here are doable for lowering glycaemic variability. The observations described here are glimpses gained by a single individual with long-standing T2D and they require further testing by conventional studies for accelerated translation.
Acknowledgement
The authors thank Jorge Munoz, RN, APRN for assistance with preparing the figures used in this report.
Author’s Contribution
Elsamma Chacko: Literature search, study design, data collection, data interpretation; put together the first draft. Christine Signore: Endocrinologist caring for the patient, devised the treatment plan, ordered medications, CGM and lab tests, data interpretation and extensive review of the various drafts of the paper.
References
- Ceriello A, Kilpatrick E. Glycemic variability: both sides of the story. Diabetes. Care. 36(2), S272–275 (2013).
- Haxhi J, di Palumbo A, Sacchetti M. Exercising for metabolic control: is timing important? Ann. Nutr. Metab. 62(1), 14–25 (2013).
- Cassidy S, Thoma C, Houghton D et al. High-intensity interval training: a review of its impact on glucose control and cardiometabolic health. Diabetologia 60(1), 7-23 (2017).
- Strasser B, Pesta D. Resistance Training for Diabetes Prevention and Therapy: Experimental Findings and Molecular Mechanisms. Biomed. Res. Int. 805217, (2013).
- Iqbal A, Heller SR. The role of structured education in the management of hypoglycaemia. Diabetologia. 61(4), 751–760 (2018).
- Maran A, Pavan P, Bonsembiante B. Continuous glucose monitoring reveals delayed nocturnal hypoglycaemia after intermittent high-intensity exercise in non-trained patients with type 1 diabetes. Diabetes. Technol. Ther. 12(10), 763–768 (2010).
- Zoungas S, Patel A, Chalmers J et al. Severe hypoglycaemia and risks of vascular events and death. The. New. Engl. J. Med. 363, 1410–1418 (2010).
- Chacko E, Awruch P, Swartz E. Breakfast-centered meal plan for people with diabetes: a modest cohort study under free-living conditions. Diabetes. Manag. 8(1), 32–37 (2018).
- Derave W, Mertens A, Muls E et al. Effects of post-absorptive and postprandial exercise on glucoregulation in metabolic syndrome. Obesity 15(3), 704–711 (2007).
- Colberg S, Zarrabi L, Bennington L et al. Post-prandial walking is better for lowering the 6-8,14,15]glycemic effect of dinner than pre-dinner exercise in type 2 diabetic individuals. J. Am. Med. Dir. Assoc. 10, 394–397 (2009).
- Di Pietro L, Gribok A, Stevens M et al. Three 15-min bouts of moderate post-meal walking significantly improves 24-h glycemic control in older people at risk for impaired glucose tolerance. Diabetes. Care. 36(10), 3262–3268 (2013).
- Erickson M, Little J, Gay J et al. Post meal exercise blunts postprandial glucose excursions in people on Metformin therapy. J. Appl. Physiol. 123, 444 – 450 (2017).
- Van Dijk J, Venema M, Van Mechelen W et al. Effect of moderate-intensity exercise versus activities of daily living on 24-hourblood glucose homeostasis in Male patients with type 2 diabetes. Diabetes. Care. 36(11), 3448–3453 (2013).
- Kirwan J, O'Gorman D, Cyr-Cambell D et al. Effects of a moderate glycemic meal on exercise duration and substrate utilization. Med. Sci. Sports. Exerc. 33(9), 1517– 1523 (2001).
- Borror A, Zieff G, Battaglini C et al. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med. 48(6), 1479–1491 (2018).
- Shambrook P, Kingsley M, Wundersitz D et al. Glucose response to exercise in the post‐prandial period is independent of exercise intensity. Scand. J. Med. Sci. Sports. 28(3), 939–946 (2018).
- Kjaer M, Hollenbeck C, Frey-Hewitt B et al. Glucoregulation and hormonal responses to maximal exercise in non-insulin-dependent diabetes. J. Appl. Physiol. 68(5), 2067–2074 (1990).
- Nybo L, Pedersen K, Christensen B et al. Impact of carbohydrate supplementation during endurance training on glycogen storage and performance. Acta. Physiol. 197(2), 117–127 (2009).
- Francois M, Baldi J, Manning P et al. Exercise snacks' before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia. 57 (7), 1437–1445 (2014).
- Haxhi1 J, Leto G, Di Palumbo A et al. Exercise at lunchtime: effect on glycemic control and oxidative stress in middle aged men with type 2 diabetes. Eur. J. Appl. Physiol. 116(3), 573–582(2016).
- Borer K, Wuorinen E, Lukos J et al. Two bouts of exercise before meals, but not after meals, lower fasting blood glucose. Med. Sci. Sports. Exerc. 41(8), 1606–1614 (2009).
- Heden T, Winn N, Mari A et al. Post-dinner resistance exercise improves postprandial risk factors more effectively than pre-dinner resistance exercise in patients with type 2 diabetes. J. Appl. Physiol. 118(5), 624–34 (2014).
- Terada T, Wilson B, Myette-Cote et al. Targeting specific interstitial parameters with high-intensity interval exercise and fasted-state exercise in type 2 diabetes. Metabolism 65(5), 599–608 (2016).
- Harmer A, Chisholm D, McKenna M et al. High-intensity training improves plasma glucose and acid-base regulation during intermittent maximal exercise in type 1 diabetes. Diabetes. Care. 30(5), 1269–1271 (2007).
- Hatamoto Y, Yoshimura E, Yamada Y et al. Effect of exercise timing on elevated postprandial glucose levels. J. Appl. Physiol. 123(2), 278–284 (2017).
- Gillen J, Percival M, Ludzki A Tarnopolsky MA et al. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity. 21(11), 2249–2255 (2013).
- Marmy-Conus N, Fabris S, Proietto J et al. Preexercise glucose ingestion and glucose kinetics during exercise. J. Appl. Physiol. 81(2), 853–857 (1996).
- Achten J, Jeukendrup A. Effects of pre-exercise ingestion of carbohydrate on glycaemic and insulinaemic responses during subsequent exercise at differing intensities. Eur. J. Appl. Physiol. 88, 466– 471 (2013).
- Van Dijk J, Tummers K, Stehouwer C et al. Exercise therapy in type 2 diabetes. Is daily exercise required to optimize glycaemic control. Diabetes. Care. 35(5), 948–954 (2012).
- Cadore E, Izquierdo M. How to simultaneously optimize muscle strength, power functional capacity and cardiovascular gains in the elderly: an update. Age. 35(6), 2329–2344 (2013).
- Yardley J, Kenny G, Perkins B et al. Effects of performing resistance exercise before versus after aerobic exercise on glycaemia in type 1 diabetes. Diabetes. Care. 35(4), 669–675 (2012).
- Nelson J, Poussier P, Marliss E et al. Metabolic response of normal man and insulin-infused diabetics to postprandial exercise. Am J Physiol 242(5), E309– E316 (1982).
- Caron D, Poussier P, Marliss E et al. The effect of poastprandial execise on meal-related glucose intolerance in insulin-dependent diabetic individuals. Diabetes. Care. 5(4), 364–369 (1982).
- Shin Y, Jung H, Ryu J et al. Effects of pre-exercise meal on plasma growth hormone response and fat oxidation during walking. Prev. Nutr. Food. Sci. 18(3), 175–180 (2013).
- Chacko E. A time for exercise: the exercise window. J. Appl. Physiol. 122(1), 206–209 (2007).
- Yancy W, Foy M, Chalecki A et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. 2, 34 (2005).
- Duvivier B, Schaper N, Hesselink M et al. Breaking sitting with light activities vs. structured exercise: a randomized crossover study demonstrating benefits for glycemic control and insulin sensitivity in type 2 diabetes. Diabetologia. 60(3), 490–498 (2016).