Review Article - Imaging in Medicine (2015) Volume 7, Issue 2
What is multiparametric-MRI of the prostate and why do we need it?
Tristan Barrett*
Department of Radiology, Addenbrooke’s Hospital and the University of Cambridge, Cambridge, CB2 0QQ, UK
- Corresponding Author:
- Tristan Barrett
Department of Radiology
Addenbrooke’s Hospital and the University of Cambridge
Cambridge, CB2 0QQ, UK
Tel: +44 1223 245151
E-mail: tb507@medschl.cam.ac.uk
Abstract
Post-Prostate cancer is the second leading cause of cancer death in men. Prostate-specific antigen (PSA) testing has led to an over-diagnosis of relatively indolent disease, which has been further compounded by the limitations of traditional diagnosis by transrectal ultrasound-guided biopsy. Improvements in MRI technique and, in particular, functional imaging have enabled the radiologist to play a key role in the risk stratification and management of patients, with a drive towards utilizing MRI early in the diagnostic pathway. However, the technique remains challenging both in the acquisition of images and in their interpretation, highlighting the need for the recent push towards greater standardisation. The benefits and limitations of MRI are discussed, along with future directions in the field.
Keywords
Post-prostate cancer, multiparametric MRI, pre-biopsy MRI
Introduction
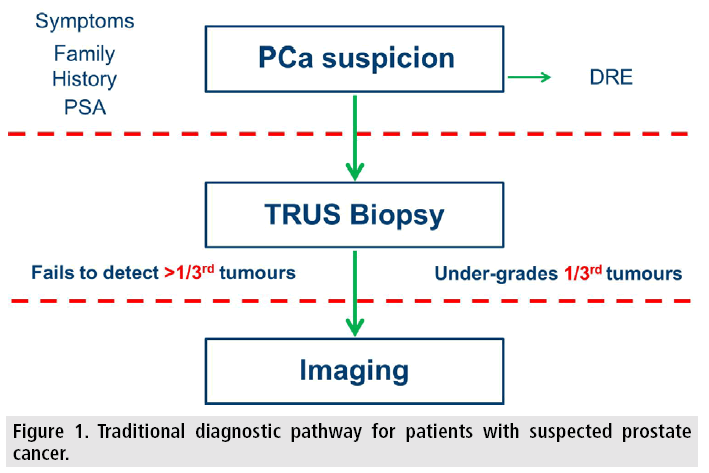
Prostate cancer is the second leading cause of cancer death in men, with the incidence expected to double by 2030 mainly due to the ageing global population [1]. Prostate-specific antigen (PSA) testing has had a dramatic effect of the type of patient treated for prostate cancer. Prior to approval in the late 1980s most men presented with high risk disease, within 15 years this had shifted with the majority presenting with low risk, organ confined disease [2]. Given the typically indolent nature of low risk disease, this brings a danger that we may inadvertently over-treat clinically insignificant cancers that would otherwise not have resulted in morbidity to the patient [3]. There is further concern that current urological practice may serve to exacerbate the problem by repeat PSA testing, lowering thresholds for biopsy, taking more cores at biopsy, and repeating a biopsy after initial negative results [4]. Conversely, we risk under-treating more aggressive disease due to limitations of the current standard diagnostic test for confirming prostate cancer. Transrectal ultrasound (TRUS) guided biopsy guides the needle to the prostate but not to the cancer and as such is prone to systematic sampling errors. The technique particularly under samples the anterior prostate, the midline portion or the gland, and the extreme apex. As a result, the technique misses cancer in up to half of cases and has consistently been shown to underestimate the aggressiveness of disease in a third of cases [5,6].
There are currently no blood or urinebased biomarkers that can reliably detect the presence of a high-grade aggressive tumour in the prostate, and realistically imaging offers the greatest potential means of differentiating indolent disease from the more aggressive, lethal cancers. Fortunately improvements in MRI techniques and in particular functional imaging have enabled the radiologist to play a key role in the risk stratification and management of patients. The key issues remain standardisation of the MRI acquisition and interpretation and considerations of whom to image and when to image in the clinical pathway.
What is “multiparametric” MRI?
Multiparametric (mp) MRI of the prostate is essentially any functional form of imaging used to supplement standard anatomical T1 and T2- weighted imaging. The functional sequences of choice are dynamic contrast-enhanced (DCE) MRI and diffusion-weighted imaging (DWI), including the calculation of apparent diffusion co-efficient (ADC) maps. Another technique, MR spectroscopy has recently fallen out of favour. To a certain extent the more sequences the better: it has been shown that inclusion of all three of these functional parameters achieves a positive predictive value for cancer of 98%, compared to the detection rate of 68% for T2W MRI alone [7]. However, spectroscopy is challenging, often requiring significant postprocessing and input from MR physicists. The low sensitivity (16%) makes spectroscopy poor for lesion detection, and although its excellent specificity (100%) can improve lesion characterisation, the overall benefit is comparatively small [13], in particular relative to the step-wise increase in costs incurred [8].
Why is prostate MRI challenging?
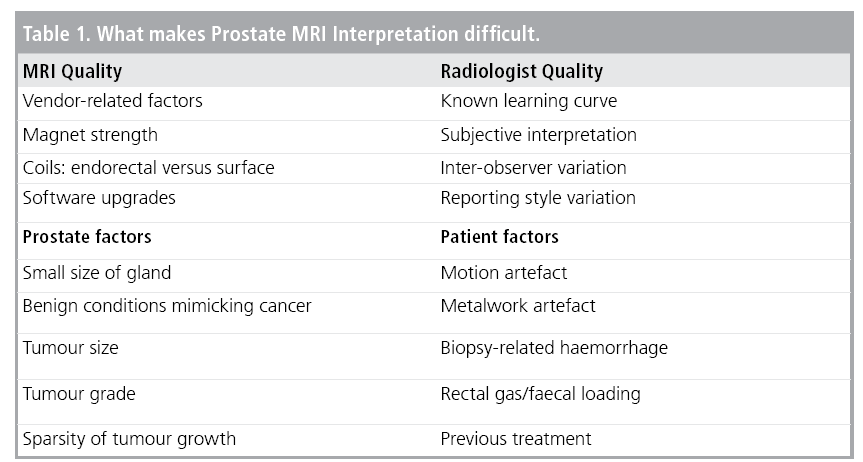
One of the difficulties with MRI in general and perhaps even more so for prostate MRI is the heterogeneity of imaging quality between centres. This is multifactorial 87 and is dependent on the magnet strength, coils (number of elements, endorectal versus surface coil), software upgrades and protocols employed. These factors along with sequences specific parameters (e.g., choice of b-values) can make inter-centre comparison challenging for quantifiable functional measurements derived from DWI and DCE-MRI. Another variable to consider is the experience of the radiologist. There is a known learning curve for prostate MRI [9,10], and radiologists need to regularly audited their outcomes in order to maintain standards [11]. Anecdotally 100-150 studies should be second reported to achieve an appropriate level. With this in mind, the European Society of Urogenital Radiology (ESUR) in 2012 published the Prostate Imaging Reporting and Data System (PI-RADS), aimed at standardising the acquisition, interpretation and reporting of prostate MRI [12]. This was subsequently updated in collaboration with the American College of Radiology (ACR), with PI-RADS version 2 being made available online in early 2015 [13,14]. Other factors that are harder to standardise are tumour-specific factors including sparse growth patterns [15], and patient-related artefact due to hip metalwork, prior biopsy or rectal loading (TABLE 1).
How good is mpMRI?
MRI cannot detect all prostate tumours, and has poor sensitivity for low volume Gleason 3+3 disease. Ironically, this latter point could be argued as an advantage because these indolent tumours are the very ones in which there is a concern of “over-diagnosis”. In fact, in the context of selecting patients with presumed low volume, low grade disease for active surveillance, the lack of a lesion on MRI is a good prognostic finding for this very reason [16,17]. Predictably, the larger a tumour and the higher its grade, the more likely it is to be seen on MRI. As a general rule, lesions with a predominant Gleason pattern 3 need to have a volume of ≥0.5 cm3 (~ a 10 mm diameter sphere) and those of predominant Gleason ≥ 4 a
volume ≥ 0.2 cm3 (~ a 7 mm diameter sphere) to be consistently identified [11,18,19]. Clearly this is also dependent on the technical factors mentioned above; a recent meta115 analysis suggests MRI has a pooled sensitivity and specificity of 74% and 88% respectively for detecting tumours [20]. However, a degree of caution should be applied when interpreting published studies. Almost invariably they are retrospective in nature and typically, in order to account for the patient-related factors mentioned in TABLE 1, such patients are excluded from analysis, which is not reflective of a real-life reporting list. Many studies use the definitive histology provided by radical prostatectomy for validation in order to overcome the inherent sampling error of biopsy techniques. However, such cohorts will bring a selection bias with a relative under-representation of low grade Gleason 3+3 and of high grade ≥ Gleason 4+5 disease; this may in particular limit any correlations of parameters to tumour grade. In addition, such studies are often from expert centres with the advantage of state-of-the-art equipment, optimised protocols, and with highly experienced sub-specialised radiologists interpreting the images.
When to perform mpMRI?
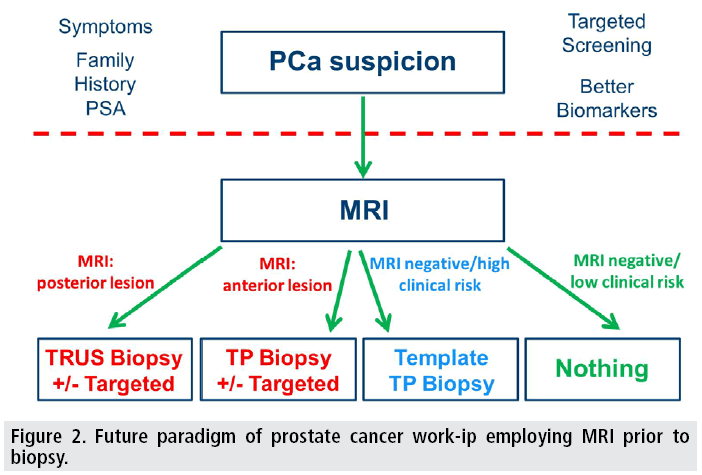
In the UK, the recently updated clinical guidance on prostate cancer recommends a greater role for MRI, but stops short of recommending MRI pre-biopsy [21]. Prostate cancer is unique amongst solid organ tumours in that it is predominantly 134 diagnosed by an indirect, non-targeted method (FIGURE 1). In the case of any other non-haematogenous malignancy a suspicion of cancer leads to an imaging test (radiological or otherwise) and a subsequent biopsy targeted to the area of abnormality. Performing MRI prior to biopsy has the added advantage of avoiding biopsy-related haemorrhage which can hinder interpretation [22] and performing MRI earlier in the clinical pathway may help improve time-to-treat pathways [23,24]. The counter argument is that healthcare cost implications of performing MRI pre biopsy outweigh the diagnostic benefits as the majority of patients undergoing TRUS biopsy have a negative result. However, this assumes that the biopsy is accurate and moreover will be accepted by clinicians with no further investigations requested. The UK guidance recommends MRI even for patients with a negative biopsy provided this is warranted on clinical suspicion. This implies that nearly all patients requiring a TRUS biopsy will qualify for an MRI at some stage in their clinical pathway and, theoretically, shifting the MRI time-point alone will be almost cost neutral. Although there are clear cost implications for MRI pre148 biospy, there are potential savings including avoiding biopsy under certain circumstances and using a targeted approach to mitigate the repeat negative-TRUS biopsy cycle some patients previously endured prior to a final diagnosis. Furthermore, there is evidence that mpMRI directed biopsy can increase the detection of significant disease and markedly reduce the detection of clinically insignificant indolent cancer in biopsy-naive patients [25]. The number of centres performing MRI prebiopsy is increasing and we are rapidly reaching the point where MRI will be used pre-biopsy to triage patients as to the type of biopsy they will undergo (imaged-guided versus cognitive targeting) and its approach (transperineal versus transrectal), or to avoiding biopsy altogether (FIGURE 2).
Figure 2: Future paradigm of prostate cancer work-ip employing MRI prior to biopsy.
Future directions
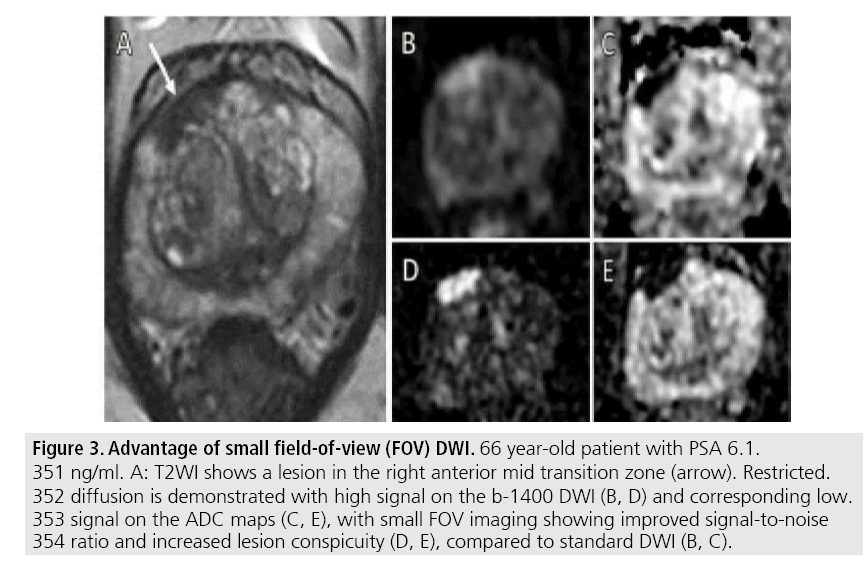
It is difficult to envisage any future step-wise “game changing” MR improvements akin to the introduction of DWI, which had the added advantage of being a short sequence without exogenous contrast. Small improvements will be achieved by technological advances in coil design and possibly by use of stronger magnets. Refinements to the existing techniques such as use of higher b-values and small field-of-view DWI (FIGURE 3) have already shown promise [26,27], and further progress is to be expected. It is unlikely that a new imaging modality will replace MRI as it would need to reach a very high level of diagnostic accuracy. The role of MRI in management pathways will be further clarified, including the almost inevitable move towards MRI pre-biopsy. There will be an expansion of the role of MRI in active surveillance (AS) programmes, where MRI has already become established at enrolment, capitalising on the high (>90%) negative predictive value of MRI for the presence of significant disease [10,16,28]. Increasingly MRI will be used in the follow-up of the “organ-sparing” treatments of AS and the myriad of focal therapy options now being trialled. MRI can potentially limit the number of follow-up biopsies required for the former [29] and aid the detection of recurrent/residual disease at an early stage for the latter [30].
Figure 3: Advantage of small field-of-view (FOV) DWI. 66 year-old patient with PSA 6.1. 351 ng/ml. A: T2WI shows a lesion in the right anterior mid transition zone (arrow). Restricted. 352 diffusion is demonstrated with high signal on the b-1400 DWI (B, D) and corresponding low. 353 signal on the ADC maps (C, E), with small FOV imaging showing improved signal-to-noise 354 ratio and increased lesion conspicuity (D, E), compared to standard DWI (B, C).
MRI may also have a more prominent role to play in other areas of prostate cancer work-up such as staging advanced disease and detecting recurrent disease. Accurate local staging with MRI intuitively improves on clinical nomograms, previously used alongside digital-rectal examination to predict extra-prostatic disease. It may be that this can help avoid investigations in patients accurately ascribed to intermediate risk disease, with the health care savings redirected to move specific tests than bone scintigraphy in the work-up of high risk disease. In advanced and recurrent disease, there is currently interest in the use of whole body MRI [31] and 68Gallium- PMSA-PET [32,33]; increasingly there is likely to be a move from PET-CT towards PET-MRI, regardless of the tracer employed.
Conclusion
Multiparametric MRI incorporating functional imaging has led to a paradigm shift in how prostate cancer is diagnosed and increasingly in how it is followed-up. MRI is moving early in the pathway and with lesion detection now key, optimisation of MR protocols and sub192 specialist reporting has become essential.
Acknowledgements
The author acknowledges research support from National Institute of Health Research Cambridge Biomedical Research Centre, Cancer Research UK, Cancer Research UK and the Engineering and Physical Sciences Research Council Imaging Centre in Cambridge and Manchester and the Cambridge Experimental Cancer Medicine Centre.
References
- Maddams J, Utley M, Møller H. Projections of cancer prevalence in the United Kingdom, 2010-2040. Br. J. Cancer. 107, 1195-1202 (2012).
- Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986- 2005. J. Natl. Cancer. Inst. 101, 1325-1329 (2009).
- Klotz L. Prostate cancer overdiagnosis and overtreatment. Curr. Opin. Endocrinol. Diabetes. Obes. 20, 204-209 (2013).
- Porten SP, Whitson JM, Cowan JE et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J. Clin. Oncol. 29, 2795-2800 (2011).
- Lecornet E, Ahmed HU, Hu Y et al. The accuracy of different biopsy strategies for the detection of clinically important prostate cancer: a computer simulation. J. Urol. 188, 974-980 (2012).
- Kvåle R, Møller B, Wahlqvist R, et al. Concordance between Gleason scores of needle biopsies and radical prostatectomy specimens: A population-based study. BJU. Int. 103: 1647- 1654 (2009).
- Turkbey B, Mani H, Shah V et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: Histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J. Urol. 186, 1818-1824 (2011).
- Mowatt G, Scotland G, Boachie C et al. The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: A systematic review and economic evaluation. Health. Technol. Assess. 17, 1-281 (2013).
- Latchamsetty KC, Borden LS Jr, Porter CR et al. Experience improves staging accuracy of endorectal magnetic resonance imaging in prostate cancer: What is the learning curve? Can J Urol 14: 3429-3434 (2007).
- Gaziev G, Wadhwa K, Barrett T et al. Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI-transrectal ultrasonography (TRUS) fusion-guided transperineal prostate biopsies as a validation tool. BJU. Int. (2014).
- Kirkham AP, Haslam P, Keanie JY et al. Prostate MRI: Who, when, and how? Report from a UK consensus meeting. Clin. Radiol 68, 1016-1023 (2013).
- Barentsz JO, Richenberg J, Clements R et al. ESUR prostate MR guidelines 2012. Eur. Radiol. 22, 746-757 (2012).
- Weinreb JC, Barentsz JO, Choyke PL et al. PIRADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur. Urol. (2015).
- Barrett T, Turkbey B, Choyke PL. PI-RADS version 2: What you need to know. Clin. Radiol. 70, 1165-1176 (2015).
- Langer DL, van der Kwast TH, Evans AJ et al. Intermixed normal tissue within prostate cancer: effect on MR imaging measurements of apparent diffusion coefficient and T2-sparse versus dense cancers. Radiology. 249, 900-908 (2008).
- Lee DH, Koo KC, Lee SH et al. Low-risk prostate cancer patients without visible tumor (T1c) on multiparametric MRI could qualify for active surveillance candidate even if they did not meet inclusion criteria of active surveillance protocol. Jpn. J. Clin. Oncol. 43, 553-558 (2013).
- Dianat SS, Carter HB, Pienta KJ et al. Magnetic resonance-invisible versus magnetic resonance-visible prostate cancer in active surveillance: A preliminary report on disease outcomes. Urology. 85, 147-153 (2015).
- Turkbey B, Albert PS, Kurdziel K et al. Imaging localized prostate cancer: Current approaches and new developments. AJR. Am. J. Roentgenol. 192, 1471-1480 (2009).
- Lemaitre L, Puech P, Poncelet E et al. Dynamic contrast-enhanced MRI of anterior prostate cancer: Morphometric assessment and correlation with radical prostatectomy findings. Eur. Radiol. 19, 470-480 (2009).
- de Rooij M, Hamoen EH, Fütterer JJ et al. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR. Am. J. Roentgenol. 202, 343-351 (2014).
- Barrett T, Vargas HA, Akin O et al. Value of the hemorrhage exclusion sign on T1- weighted prostate MR images for the detection of prostate cancer. Radiology. 263, 751-757 (2012).
- Ahmed HU, Kirkham A, Arya M et al. Is it time to consider a role for MRI before prostate biopsy? Nat. Rev. Clin. Oncol. 6, 197-206 (2009).
- Padhani AR, Petralia G, Sanguedolce F. Magnetic Resonance Imaging before Prostate Biopsy: Time to Talk. Eur. Urol. (2015).
- Padhani AR, Petralia G, Sanguedolce F Magnetic Resonance Imaging Before Prostate Biopsy: Time to Talk. Eur. Urol. (2015).
- Schoots IG, Roobol MJ, Nieboer D et al. Magnetic Resonance Imaging-targeted Biopsy May Enhance the Diagnostic Accuracy of Significant Prostate Cancer Detection Compared to Standard Transrectal Ultrasoundguided Biopsy: A Systematic Review and Metaanalysis. Eur. Urol. 68, 438-450 (2015).
- Ueno Y, Kitajima K, Sugimura K et al. Ultrahigh b-value diffusion-weighted MRI for the detection of prostate cancer with 3-T MRI. J. Magn. Reson. Imaging. 38, 154-160 (2013).
- Korn N, Kurhanewicz J, Banerjee S et al. Reduced-FOV excitation decreases susceptibility artifact in diffusion-weighted MRI with endorectal coil for prostate cancer detection. Magn. Reson. Imaging. 33, 56-62 (2015).
- Abd-Alazeez M, Ahmed HU, Arya M et al. Can multiparametric magnetic resonance imaging predict upgrading of transrectal ultrasound biopsy results at more definitive histology? Urol. Oncol. 32, 741-747 (2014).
- Schoots IG, Petrides N, Giganti F et al. Magnetic resonance imaging in active surveillance of prostate cancer: A systematic review. Eur. Urol. 67, 627-636 (2015).
- Barrett T, Davidson SR, Wilson BC et al. Dynamic contrast enhanced MRI as a predictor of vascular-targeted photodynamic focal ablation therapy outcome in prostate cancer post-failed external beam radiation therapy. Can. Urol. Assoc. J. 8, E708-714 (2014).
- Padhani AR, Makris A, Gall P et al. Therapy monitoring of skeletal metastases with wholebody diffusion MRI. J. Magn. Reson. Imaging. 39, 1049-1078 (2014).
- Afshar-Oromieh A, Zechmann CM, Malcher A et al. Comparison of PET imaging with a (68) Ga-labelled PSMA ligand and (18)F-cholinebased PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 41, 11-20 (2014).
- Demirkol MO, Acar O, Ucar B et al. Prostate-specific membrane antigen-based imaging in prostate cancer: Impact on clinical decision making process. Prostate. 75, 748-757 (2015).