Review Article - Interventional Cardiology (2013) Volume 5, Issue 1
Utilizing devices from interventional radiology in congenital heart disease
- Corresponding Author:
- Daniel S Levi
Mattel Children’s Hospital at University of California, Los Angeles
Pediatric Cardiology, B2-427 MDCC 10833 Le Conte Avenue
Los Angeles, CA 90095-1743, W310 206 3478, USA
E-mail: dlevi@mednet.ucla.edu
Abstract
Keywords
interventional pediatric congenital cardiology, radiology devices, catheterization, stent, coil, microcatheter, embolization retrieval
In 1966, Rashkind and Miller performed the first balloon atrial septostomy, and in the intervening years, intracardiac therapeutic catheterization for congenital heart disease in both children and adults has risen to become an essential part of current management [1]. The prominence of the field is mirrored in, and has been fueled by, the array of medical devices available, current examples of which include endovascular stents, balloon catheters, atrial and ventricular septal defect occluders and prosthetic heart valves.
One issue facing the field is the lack of devices specifically built for congenital cardiac use, pediatric or otherwise. This problem sprouts from the same forces which afflict pediatric and congenital cardiology in general, namely the small sample size and the patient heterogeneity, which can make obtaining safety data and regulatory approval for a device prohibitively difficult. Concerted efforts are being launched towards alleviating these barriers. For example, in the USA, the US FDA includes a Humanitarian Device Exemption pathway, which approves devices utilized in treating rare conditions with frequencies of less than 4000 cases per year if probable benefit can be asserted [2]. In addition, US legislation passed in 2007, entitled the ‘Pediatric Medical Device Improvement and Safety Act’, was designed to encourage data-sharing registries and to create financial incentives for medical manufacturers in an effort to spur device development [3].
Nevertheless, despite these initiatives, only seven devices have been approved/labeled specifically for children since the Humanitarian Device Exemption pathway began [3], and a recent retrospective survey of transcatheter interventions at a tertiary care center in the USA revealed that, for certain devices, up to 99% of the usage was off-label, out of necessity [4]. In the USA, off-label use is clearly an important and legal part of contemporary congenital pediatric care [5]. One field from which the pediatric interventional cardiologists frequently appropriate devices for their needs is the field of interventional radiology. This manuscript will review the technologies, materials and devices of interventional radiology, and their potential applications in pediatric cardiology.
Stents
▪ Stent grafts
The primary devices missing from the armamentarium of transcatheter treatment of congenital heart disease are covered stents appropriately sized for use in children and adolescents. Covered stents are incredibly useful in the treatment of pulmonary artery stenosis, aortic coarctations, and especially for treatment of ruptures or contained dissections of vessels. Although covered stents designed specifically for congenital heart disease in the pediatric population are not yet available, there are many covered stents approved for use in interventional radiology and vascular surgery that can be of great use to congenital interventionalists. Knowledge of these devices is paramount and even life-saving for all doctors who routinely treat this population. Covered stents – also known as stent grafts, endoluminal grafts or endografts – are originally intended for the endovascular repair of aortic or peripheral vascular lesions, including aneurysms, stenoses, dissections and fistulas demonstrates an array of stent grafts in various sizes and configurations [6]. Stent grafts can either be self-expanding or balloon expandable.
An example of such a graft is the Endologix Powerlink® stent-graft (Endologix, CA, USA), a flexible self-expanding covered stent created from a cobalt–chrome alloy and covered with expanded polytetrafluoroethylene (PTFE; W L Gore & Associates, Inc., AZ, USA) [7]. The stent is marketed for use in endovascular abdominal aortic aneurysm repair. The nonbifurcated stents come in diameters ranging from 16–28 mm, with lengths from 55–88 mm, requiring 17–19F introducer systems [8]. While the large sheath sizes preclude use in smaller children, these and similar covered stents find application in Fontan patients with either stenoses in their Fontan or with multiple atrial-level Fontan fenestrations.
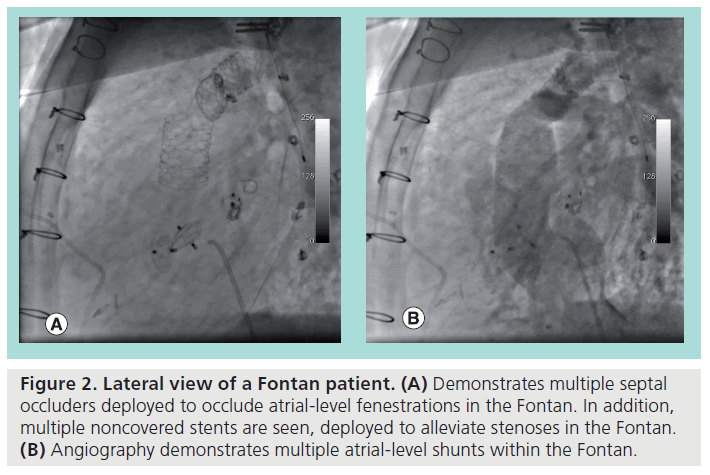
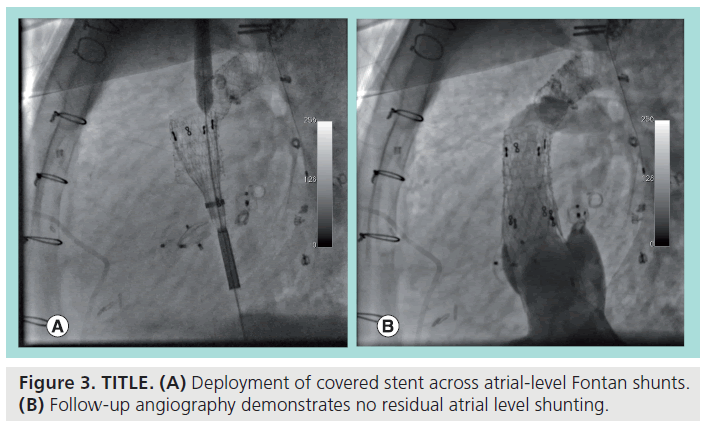
Figure 2 demonstrates an example of the latter case, with the figure showing a lateral view during cardiac catheterization of a Fontan patient with residual atrial-level shunting due to Fontan fenestrations, despite evident prior attempts at occluding the fenestrations with septal occluders. In an attempt to optimize hemodynamics and minimize right-to-left shunting, covered stents were chosen to occlude the fenestrations. With prior angiography identifying the locations of the shunts, the self-expanding covered stent was advanced via a femoral vein, and unsheathed at the desired position. Figure 3A shows the stent in the process of being deployed, with follow-up angiography (Figure 3B) demonstrating total occlusion of residual shunts in the Fontan.
Figure 2: Lateral view of a Fontan patient. (A) Demonstrates multiple septal occluders deployed to occlude atrial-level fenestrations in the Fontan. In addition, multiple noncovered stents are seen, deployed to alleviate stenoses in the Fontan. (B) Angiography demonstrates multiple atrial-level shunts within the Fontan.
Figure 3: TITLE. (A) Deployment of covered stent across atrial-level Fontan shunts. (B) Follow-up angiography demonstrates no residual atrial level shunting.
Smaller caliber covered stents also find a role in single-ventricle patients. In single-ventricle patients with severe progressive cyanosis who have undergone a cavopulmonary shunt operation, an axillary or brachial arteriovenous fistula (AVF) is a palliative option for augmenting pulmonary bloodflow [9–11]. The axillary AVF procedure involves surgically creating a sideto- side anastomosis of the axillary artery and vein, with subsequent ligation of the distal vein [9]. The procedure can be used as an end-stage palliation in a failed Fontan patient, as a bridge to transplantation [12], or as a temporizing procedure until Fontan completion [10], and carries with it the additional benefit of providing the putative ‘hepatic factor’ thought to be protective against the formation of pulmonary arteriovenous malformations [10]. Should the patient become eligible for Fontan completion, the AVF may be closed in a transcatheter fashion with a covered stent [10].
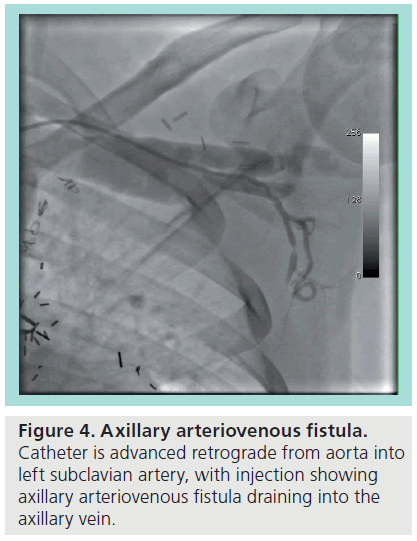
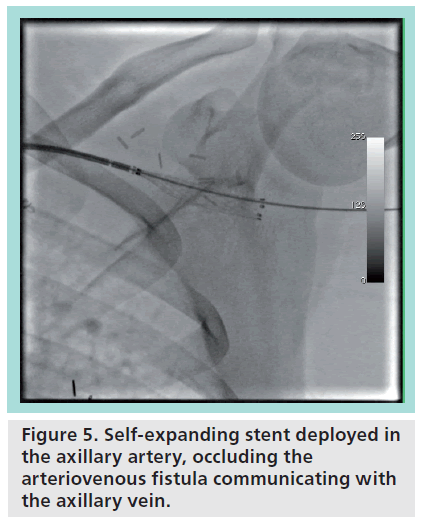
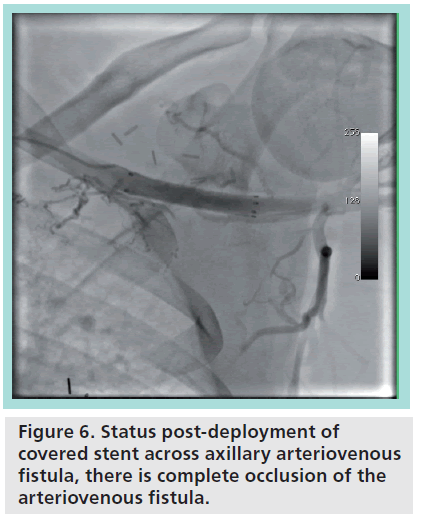
Figure 4 demonstrates a patient with an axillary AVF determined to be suitable for closure. The catheter is advanced retrograde from the aorta into the subclavian artery, with the tip at the level of the AVF, and with subsequent contrast injection revealing the fistula draining into the axillary vein. Figure 5 shows the positioning of the wire through the subclavian artery and the unsheathing and deployment of a small caliber self-expanding stent at the level of the axillary AVF. Follow-up angiography (Figure 6) demonstrated total occlusion of the fistula.
Stent grafts also find utility in the treatment of aortic coarctation. Bare-metal stents have long been an established method of treating aortic coarctation [13]. Stenting offers advantages over balloon angioplasty, including decreased risk of recurrence in native coarctations, lesser need for oversizing of balloons, increased radial support for the vessel wall and the ability to enlarge the stent at a later time [13]. Risks associated with the deployment of bare-metal stents include intimal tears, dissection and aneurysm formation [13]. In the cases of angiographically evident dissections or aneurysms, where emergent surgical intervention is not warranted, rapid placement of covered stents can provide immediate treatment. Furthermore, simply using covered stents in place of bare-metal stents can preempt or ameliorate these complications altogether. Numerous options for stents are available in this setting, including creating selffabricated covered stents [14], or commercially available stents, including the iCAST™, the Advanta™ V12 Covered Stent (both Atrium, NH, USA), and the GORE® EXCLUDER® AAA Endoprosthesis (W L Gore & Associates, Inc.).
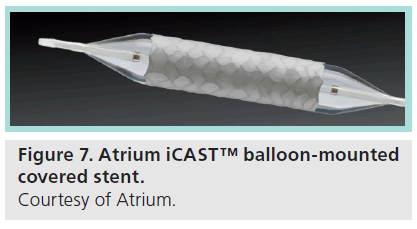
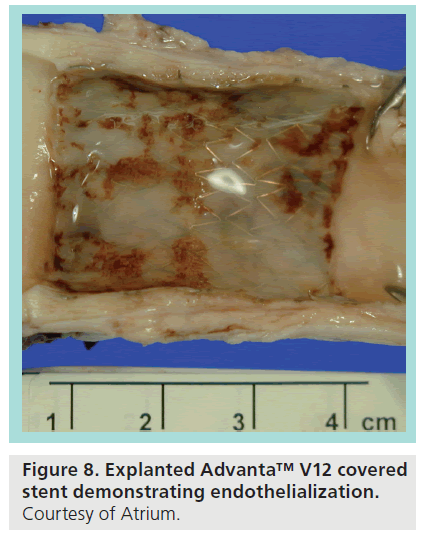
The iCAST stent (Figure 7) is a PTFE-covered, premounted, balloon expandable, stainless steel stent marketed in the USA for the treatment of tracheobronchial strictures [15]. The stent requires either a 6 or 7F introducer sheath, and is available in 5–10 mm diameters. One limitation is that, while longer lengths of stent are capable of postdilation up to 12 mm, beyond that the PTFE lining is prone to tearing. One consequence of this is that, while the stent is useful for emergency management of a rupture or dissection, the inability to dilate beyond 12 mm limits its implantation in pediatric situations, where patient growth will necessitate future postdilation. The V12 stent is of similar design to the iCAST stent, and is approved for the use (though not in the USA) of restoring patency in the iliac and renal vessels [16], and is available in sizes ranging from 5–12 mm, with the large diameter variant available in 12, 14 and 16 mm diameter sizes. Clinical trials are currently underway in the USA to evaluate the V12’s use in coarctation of the aorta. One significant advantage the Large Diameter V12 offers over the iCAST stent is the ability to postdilate up to 20 mm [17], potentially advantageous in the situation described earlier where stent enlargement is required as the patient grows. Similar to other stents, the Advanta is endothelialized over time, with Figure 8 demonstrating an explanted Advanta V12 stent and complete endothelialization of the stent.
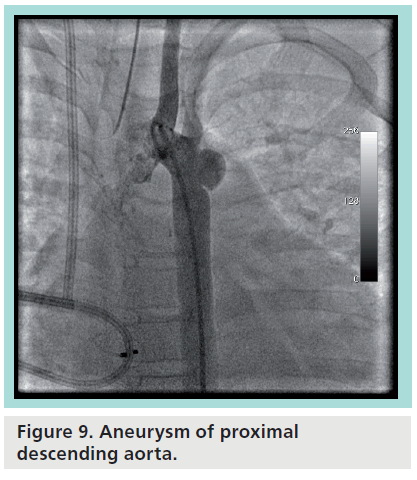
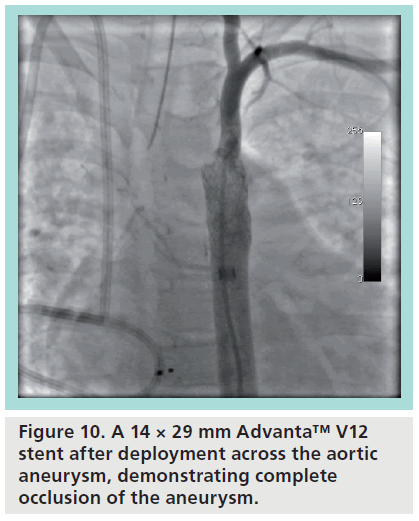
Figure 9 demonstrates a prototypical usage case with the Advanta V12 stent. The patient is a 7-year-old male with a prior history of an aortic coarctation status postrepair at an early age and status post-ASD closure with an Amplatzer® Septal Occluder (St Jude Medical, MN, USA), who went on to develop severe hepatopulmonary syndrome and severe secondary pulmonary hypertension. While being considered for liver transplantation, it was felt that a stable aortic aneurysm at the site of the prior coarctation repair (Figure 9) would need to be addressed prior to transplant listing. A transcatheter intervention was felt to be the best option for the patient. After obtaining FDA and institutional review board approval for compassionate use of the device, a 14 × 29 mm Advanta V12 premounted stent was chosen and advanced into position across the aneurysm. Figure 10 shows the result after deployment, with angiography showing exclusion of the aneurysm and the left subclavian artery free from jailing. The patient was subsequently deemed eligible for, and received a liver transplant, and has continued to more than 1 year removed from the intervention.
Microcatheters
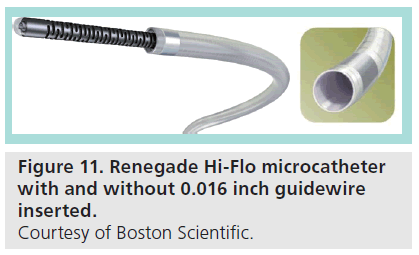
Microcatheters are small caliber catheters designed to coaxially track over a guidewire, while themselves inserted into a directional guiding catheter, in order to facilitate access into small, distal or tortuous vessels. They are typically designed with materials to decrease pushing resistance and improve trackability, while being of suitable caliber to allow for interventions and diagnostic measures, such as contrast injection. Microcatheters find application most commonly in the fields of peripheral and neurovascular intervention, but usage scenarios in congenital cardiology are plentiful. In adult patients, microcatheters allow navigation into small caliber or tortuous vessels where conventional catheters cannot follow. In pediatrics specifically, microcatheters’ small caliber (often <3 F) allows for catheterization in neonates, premature or otherwise, with minimum trauma to the diminutive vessels. For example, microcatheters have already been described over 15 years ago in crossing the pulmonary valve in infants with critical pulmonary valve stenosis [18]. More recently, Brown et al. described their extensive single center experience with microcatheters for a diverse array of congenital cardiac cases including stenting, angioplasty, embolization, and, in one instance, in the traversing of the lattice of a septal occlusion device to place intradevice embolization coils [19]. Examples of commercially available microcatheters include the Echelon™ (Covidien, ev3, MA, USA), Progreat™ (Terumo, NJ, USA), and Renegade® Hi-Flo (Boston Scientific Natick, MA, USA) microcatheters.
The Renegade Hi-Flo shares features typical of this class of microcatheter (Figure 11). It is a fiber-braided catheter with a 0.027 inch inner lumen, a radiolucent tip, coated with a hydrophilic, lubricious polymer [20]. The catheter requires a >2.7F introducer and comes in lengths ranging from 105–135 mm. It is designed to coaxially track over a guidewire, and the catheter itself can be coaxially inserted into any guiding catheter capable of accepting an 0.038 inch guidewire. It is intended and marketed for general intravascular use, including neuro, peripheral and coronary vasculature. The catheter is rated for 800 psi of infusion pressure, suitable for most injections.
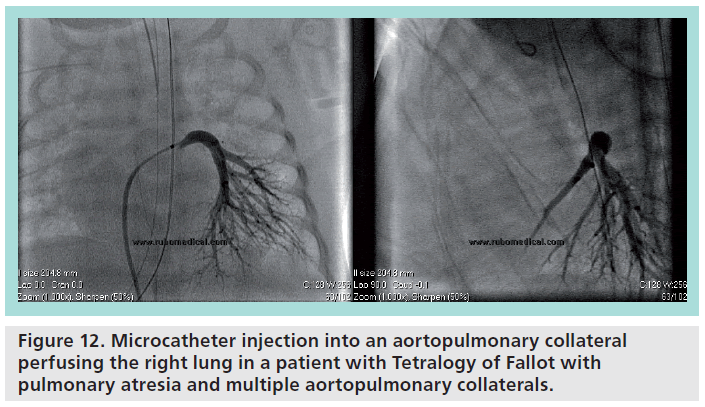
Figure 12 represents a prototypical usage case for microcatheters. The patient is a 1-month old female with Tetralogy of Fallot (ToF) with pulmonary atresia (PA) with multiple aortopulmonary collaterals. Prior cardiac MRI/magentic resonance angiography had determined the origins of the major collateral vessels. However, given the remaining questions regarding the presence or absence of pulmonary arteries, the pulmonary vascular bed of each collateral vessel, distal pressures in the collateral vessels, and any areas of overlapping perfusion in the lung fields, the patient was taken for cardiac catheterization. A 4 F Judkins right 1.5 catheter was advanced retrograde from the femoral artery, with a coaxially inserted Renegade Hi-Flo microcatheter, and a 0.014 coronary guidewire. Using the Judkins right catheter as a directional guiding catheter, the combination of the microcatheter and guidewire were used to selectively gain access to the collateral vessels, some of which would have been prohibitively difficult for other catheters to track into. In addition, the Renegade allowed pressure transduction to identify which vessels were hypertensive or stenotic, and allowed contrast injection to clearly delineate the vascular bed of each vessel.
Figure 12: Microcatheter injection into an aortopulmonary collateral perfusing the right lung in a patient with Tetralogy of Fallot with pulmonary atresia and multiple aortopulmonary collaterals.
Coils
Since the advent of the Gianturco coil in 1975 [21], transcatheter embolization of vascular structures has become a commonplace pediatric cardiac intervention. Contemporary embolization of congenital cardiac lesions began in 1992 with Cambier’s original description of the percutaneous closure of a small patent ductus arteriosus (PDA) using coil embolization [22], and coiling has since expanded to other uses. For example, congenital cyanotic heart disease is a well-known risk factor for systemic- or aortopulmonary (AP) collateral vessels. In an effort to decrease shunting and to minimize intracardiac blood return during subsequent cardiac surgery, these collaterals are frequent targets for embolization. In addition, in anatomies with increased venous pressure, such as the bidirectional superior cavopulmonary anastomosis, there is a propensity to develop decompressing systemic venous collaterals to lower pressure venous circuits. As these venovenous collaterals can be progressive and cause profound cyanosis, they are also often targets for embolization.
Coils have undergone significant evolution since their initial design, and are now discriminated by a number of key features. Coils typically range in wire diameters from 0.018 to 0.052 inches with varying lengths and varying coil or helical diameters. Coils of appropriate caliber – typically 0.018 inches – can be delivered via microcatheters and are termed microcoils. Coils can be additionally tufted with thrombogenic material. Original designs used highly antigenic natural wool fibers to promote thrombosis; however, this has now been replaced with nonantigenic synthetic fibers, such as Dacron®. Despite their advancement, many coils remain off-label for use in congenital heart disease.
▪ Pushable coils
Pushable coils represent the initial development of coils, and still remain the simplest coil design. They are loose, unattached coils delivered through a catheter via pusher guidewires, and as such, they are limited by their lack of controlled delivery and the inability to retrieve and reposition the coil, leading to a potentially increased risk of malposition and nontarget embolization. Though methods exist for making pushable coils retrievable and redeployable, they can increase the technical complexity of the procedure. The general delivery technique is via an appropriately-sized catheter and a pusher wire of equal caliber to the coil, to prevent the wire from overriding the coil within the catheter. With the excepting of microcoils, the soft, flexible end of a guidewire can be used as the pusher wire to deliver the coils [23]. Microcoils typically require either a specialized coil pusher or delivery via 1–2 ml saline flush [24]. Angiograms should be performed to place the catheter in the desired position, proximal to the vessel to be occluded. For microcoils, conventional techniques are used to place the guiding catheter and delivery microcatheter in a good position for embolization. The coil is then advanced via the introducer into a catheter, and extruded via the pusher wire (or possibly saline flush in the case of microcoils) out of the catheter. In general, the goal is to use appropriately-sized coils in the necessary number to produce as dense and cross-sectional an occlusion of the vessel as possible, to promote both the mechanical occlusion and intimal injury that are the precursors to thrombus formation and, ultimately, occlusion [24].
▪ Gianturco coil
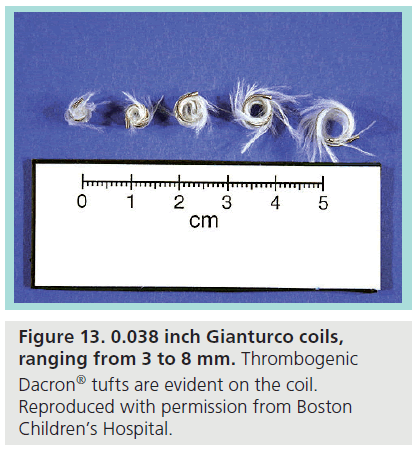
The contemporary Gianturco coil (Figure 13) is a Dacron-tufted, stainless steel coil with a wire diameter up to 0.052 inches and available in a range of coil diameters. The Gianturco coil has found widespread use in congenital cardiology in the closure of small-to-medium PDAs, primarily in the young, but even in geriatric patients [25]. Despite the inability to reposition the device, multicenter registry data has demonstrated that Gianturco coils have a complication and efficacy rate comparable to, or better than, detachable devices, including the Amplatzer™ Duct Occluder (St Jude Medical) [26]. Despite this efficacy, the Gianturco coil is being increasingly surplanted for use in PDA closure by the Amplatzer Duct Occluder and the Flipper coil. One of the known and more serious complications of the Gianturco in PDA closure is postprocedural hemolysis, which is thought to relate to residual ductal shunting [27–29]. Besides PDAs, Gianturco coils have been used to close other vascular structures such as Blalock–Taussig shunts [30].
▪ Cook Tornado® coil
The Tornado® coil (Cook Medical, IN, USA) is a similar pusher coil, though less familiar to congenital interventionalists, as it is intended for use in the embolization of arteriovenous malformations and other vascular malformations, and has found extensive use in neuroradiological treatment. The Tornado is a platinum coil laced with synthetic fibers and is available in 0.018, 0.035 and 0.038 inch wire diameters [31]. The diameter of the helices tapers, and the coil is available with either the small or large end delivered first. One advantage the Tornado offers over the Gianturco coil is its conditional safety in MRI scanners up to 3T. The platinum is softer and flopper than the stainless steel coils of the Gianturco, potentially a disadvantage if there is difficulty in retaining position. The 0.018 inch coils are typically used in 3F catheters, while the 0.035- and 0.038-inch versions are used in 5F and 6F catheters. With regards to congenital heart disease, the Tornado has successfully been used in PDA closure, [32] using a bioptome for controlled delivery, with the chief benefit being future MRI compatibility. One novel use of the Tornado is its use in conjunction with an Amplatzer Vascular Plug II (St Jude Medical) to facilitate transcatheter occlusion of the sequester vessel in Scimitar syndrome in lieu of surgical correction [33].
Detachable coils
While pusher coils have enjoyed great success in certain applications, the ability to have a controlled delivery and release dramatically increases the opportunity for a safe outcome. To ameliorate the limitations of pusher-style coils, various forms of detachable coil technologies have been developed. Methods for facilitating detachment include mechanical means, such as interlocking coupling mechanisms or electrolytic processes, where the metallic bond to the pusher guidewire is broken by a low-voltage electrical charge. One detachable coil already familiar with pediatric cardiac interventionalists is the MReye Flipper® Detachable Embolization Coil (Cook Medical) a 5 F, 0.035 inch diameter, fibered, mechanically coupled coil created from a nickel– chromium super alloy, intended for embolization in the peripheral arterial and venous vasculature [34]. There are extensive data on the coil in the pediatric cardiac setting, namely supporting its efficacy in PDA closure [26], and as such, given the familiarity that the field already has with this device, this article will not delve further into its characteristics.
▪ Guglielmi detachable coil
The Guglielmi detachable coil (Target Therapeutics, Boston Scientific, MA, USA) Figure 14 is an electrolytically detachable platinum coil designed for neurovascular embolization of aneurysms. It has been used successfully in congenital cardiac interventions for the treatment of coronary artery fistulae [35]. Limitations of the device include cost and the absence of thrombogenic fibers.
Figure 14: Guglielmi detachable coil. Reproduced with permission from [101].
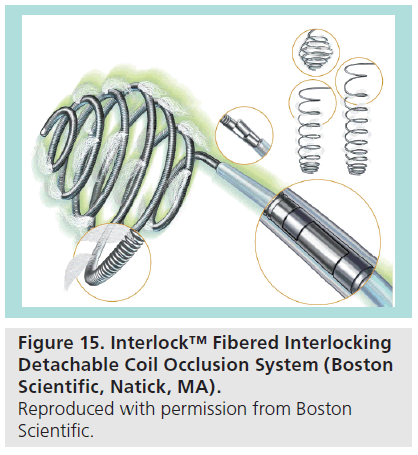
▪ Interlock™ fibered interlocking detachable coil occlusion system.
The Interlock ™ coil (Boston Scientific) is another mechanically-detachable coil, which has found application in congenital cardiology. It is an interlocking, 0.018–0.035 inch platinumfibered coil, intended for embolic use in the peripheral vasculature [36]. The device is conditionally safe in MRIs up to 1.5 T. The Interlock coil has been described in an array of congenital cardiac cases, including AP collaterals, venovenous collaterals, PDAs and coronary artery fistulae [37–39]
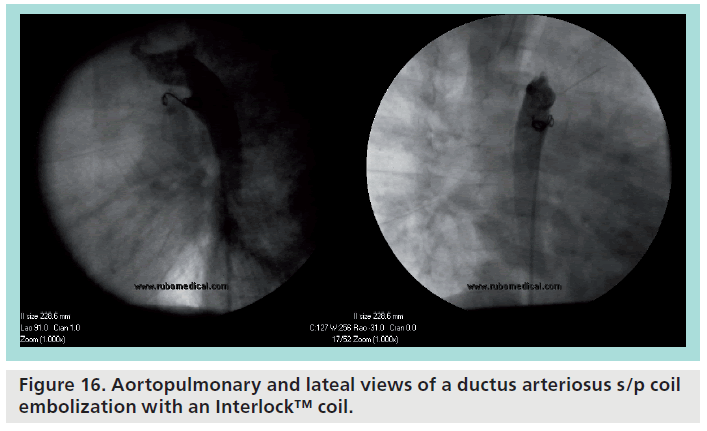
Figure 16 demonstrates a typical usage of an Interlock coil. The patient is an asymptomatic 17-month male with a history of a PDA and mild pulmonary stenosis. Angiography demonstrated a PDA with a 3.3 mm aortic ampulla narrowing to a small insertion on the pulmonary side. A 0.014 inch wire and an Angled Glidecath® (Terumo) was used to cross the PDA from the aortic side. The coil was advanced and allowed to coil on the pulmonary side, with the remaining coils deployed on the aortic side, and subsequently released. Postprocedure angiography demonstrated a good position and complete occlusion of the PDA.
Alternative embotherapies
Beyond coils, particulate agents have also been identified and utilized as key embolic agents. Particulates include microspheres, liquid-phase agents, gel foams and glues, and find common usage for the management of arteriovenous malformations, hypervascularized tumors and symptomatic uterine fibroids. [40,41]. Particulates work by occluding the capillary and precapillary vessels of the target structure, and are suitable only in situations where the particulate matter will embed itself in the terminus of the desired vessel, and not in any situation where the potential exists for the particulates to return to the circulation and cause nontarget embolic phenomena.
▪ Gelfoam
Gelfoam (Pfizer, NY, USA) is a biological substance prepared from purified pork skin gelatin, and was first used in the intravascular setting in 1964 in a case report describing embolization of a cavernous carotid fistula [42]. Gelfoam has hemostatic properties, the mechanism of which is not clearly understood, but is thought to be related to physical occlusion rather than alteration of the coagulation cascade [43]. Embolization usage of Gelfoam is off-label, but within the standard-of-care [44]. Gelfoam is primarily a temporary embolization agent, and recanalization can be expected within a few weeks [44]. This property makes Gelfoam suitable for acute hemorrhagic events, but less well suited for congenital cardiac catheterization, where its application has been limited.
▪ Polyvinyl alcohol
Polyvinyl alcohol (PVA) particulates are created from rasping and sieving a sheet of PVA into particles of set sizes [44]. PVA works by adhering to and occluding vessels. The chief limitation of PVA is the irregular size and shape of the actual particles, which can cause both aggregation in vessels more proximally than intended and migration into more distal capillary beds [45]. This behavior makes the occlusion level of the particles difficult to control in a targeted fashion, and thus usage of PVA has been largely superseded by spherical embolic agents (microspheres).
▪ Microspheres
Microspheres are spherical nonresorbable particles, which have the characteristics of being highly biocompatible and demonstrating good uniformity of particle size and shape [46]. Embolic microspheres usually range in size from 100–1200 μm. Current industry standards package microspheres in sizes that span about a 200 μm range: 100–300 μm, 300–500 μm, 500– 700 μm, 700–900 μm and 900–1200 μm [46], with the appropriate size chosen based on the diameter of the targeted vessel. AP collateral vessels are usually embolized with 900 μm or greater microspheres. Most microspheres are designed to be diluted with a 50:50 (by volume) mixture of saline and contrast, prior to injection through a catheter. Dilution prevents clustering of the microspheres, with the concern being that clustering will prevent the spheres from reaching their target vessel. Dilution ratios differ based on the size of the microspheres. Given the increased density of microspheres/ml of smaller diameter microspheres, a higher dilution ratio is necessary [47]. For large microspheres, a dilution ratio of 1 part microspheres to 10 parts contrast/saline is sufficient. For smaller sized microspheres, in the range of 100–300 μm, a ratio of 1:100 and above, can be warranted. Intermittent agitation of the suspension is necessary to prevent sedimentation. Efficacy of the embolization after injection can be determined by angiography; however, given that current generation microspheres are neither radiopaque nor visible by MRI, there is no way to directly assess where the microspheres have physically migrated to.
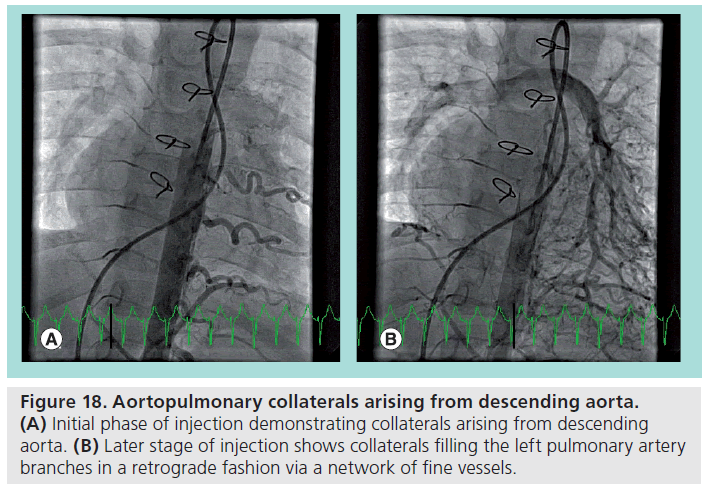
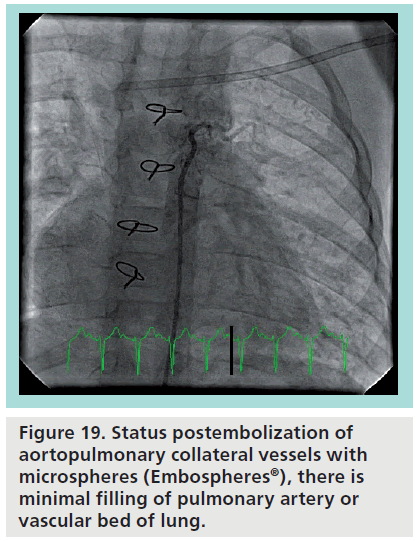
Embospheres® (Embosphere, Biosphere Medical, MA, USA) (Figure 17) were the first calibrated microspheres, initially approved for use in Europe in 1997 and subsequently approved in the USA in 2000 [47]. Embospheres are microspheres made of a trisacryl polymer, and are coated with gelatin to facilitate cell adhesion and growth. Embospheres are available in 40–120 μm, 100–300 μm, 300–500 μm, 500–700 μm, 700–900 μm and 900–1200 μm sizes. The catheter size necessary for injection depends on the size of the spheres chosen. Given the preference to use ≥900 μm (0.035 inches) spheres for AP collaterals, it can initially be reasoned that a catheter delivering these spheres should have a minimum of a 0.035 inch inner diameter. However, Embospheres are able to compress by up to 33%, which allows some leeway in catheter choice, and for the 700–900 μm sizes, a catheter with a 0.027 inch inner diameter suffices [48]. From a technical standpoint, all equipment in contact with Embospheres must be rigorously segregated from other equipment to prevent accidental embolizations. Injections should be slow and controlled, to prevent kickback and/or reflux of the microspheres into nontarget sites. Contraindications to Embosphere usage includes likely onset of vasospasm, hemorrhage or severe atheromatous disease. Other contraindications include high vascular resistance of the target vessel, which may preclude Embospheres from migrating in the intended antegrade direction, or large caliber AVM or collateral vessel pathways which may allow passage of Embospheres to unintended locations. Known complications are largely related to nontarget embolism, such as pulmonary embolism and stroke. However, even with on-target embolization, there exists the possibility of postembolization syndrome, where the embolized tissue becomes ischemic and swells, potentially resulting in ischemia in adjacent tissue simply by mass effect [49]. Figure 18 demonstrates a typical usage of microspheres. The patient is a complex congenital heart disease patient, who demonstrated significant collateral vessels arising from the descending aorta. Late phase of contrast injection shows filling of the left pulmonary artery in a retrograde fashion via a network of fine collateral vessels. Given the fine nature of the terminal collateral vessels, particulates, specifically Embospheres, were chosen as the embolization strategy. Each collateral vessel was selectively cannulated with a directional catheter and 900–1200 μm Embospheres were carefully injected into each vessel. Follow-up angiography (Figure 19) demonstrated near-total occlusion of collateral vessels.
Figure 18: Aortopulmonary collaterals arising from descending aorta. (A) Initial phase of injection demonstrating collaterals arising from descending aorta. (B) Later stage of injection shows collaterals filling the left pulmonary artery branches in a retrograde fashion via a network of fine vessels.
Glue & liquid embolics
▪ Ethylene vinyl alcohol copolymer
Ethylene vinyl alcohol copolymer (EVCO; Onyx) is a nonadhesive, liquid material dissolved in dimethyl sulfoxide (DMSO). Once the suspension is injected into the bloodstream, the DMSO is rapidly diffuses away, and the residual EVCO rapidly polymerizes and solidifies into occlusive material. EVCO was developed in Japan for use in neurovascular arteriovenous malformations [50]. It is marketed in the USA under the name Onyx® Liquid Embolic System (LES) (ev3), and comes in two formulations: Onyx 18 (6% EVCO) and Onyx 34 (8% EVCO), each suspended with micronized tantalum powder for radiopacity. Onyx 18 has a lower viscosity than Onyx 34, and is more easily able to penetrate smaller and deeper structures as a result. Final solidification takes place in 5 min regardless of concentration used [51].
Onyx comes in a 1.5 ml vial and is packaged with a 1.5 ml vial of DMSO. Special DMSOcompatible catheters must be used for the intervention (e.g., Marathon™, Rebar® or UltraFlow™ HPC catheters [ev3]) [51]. Immediatly prior to use, Onyx should be continuously mixed to create a suspension of the tantalum to allow for fluoroscopic visualization. The catheter is advanced to the desired location, catheter dead space is filled with DMSO to inhibit premature polymerization, and then the Onyx is injected into the target vessel [44], at a recommended rate of 0.16 ml/min [49]. After injection, a few seconds should be allowed to elapse prior to catheter retrieval, as premature catheter withdrawl may lead to fragmentation of the embolus, into nontarget vessels. Contraindications to Onyx usage include vasospasm severe enough to stop bloodf low in the target vessel. In addition, in high flow arteriovenous malformations (>200 ml/min) or vessels greater than 3 mm, adjunctive coil usage is recommended prior to Onyx injection. Onyx has not been tested in patients younger than 18 years old, and is not intended for use in premature infants (<1500 g) or in patients with significant kidney or liver impairment. No specific congenital cardiac use of Onyx is documented in the literature, though it has been used successfully in this institution for the embolization of AP collateral vessels in pediatric patients. Chief advantages of Onyx include its nonadhesive properties, which reduce complications such as the catheter adhering to the occlusive mass. Catheter entrapment can still occur however, and recommendations for freeing a catheter include repeated applications of gentle traction (manifest as 3–4 cm of stretch of the catheter) and/or gentle traction followed by a quick wrist-snap motion of the catheter [52]. Should a catheter prove irretrievable, rather than risk greater traction and the concomitant risk of rupture and hemorrhage, official recommendations are to cut the shaft of the microcatheter at the vascular access point and leave the catheter in the vascular system. One further advantage of Onyx is, unlike adhesives, an Onyx injection can be halted midprocedure to allow for repositioning or angiography. Importantly, no longer than 2 min should elapse prior to re-injection as this may be sufficient time for the catheter to occlude. Visualization of Onyx during injection is crucial, as otherwise there is the risk of nontarget embolization or there is concern for catheter occlusion. Catheter occlusion can lead to overpressurization and catheter rupture, so no more than thumb pressure is recommended when injecting, and any increase in resistance should immediately prompt stopping the injection. Disadvantages of Onyx include the need for DMSO-compatible catheters, and DMSO’s own toxicity, namely that rapid injection of DMSO can cause vasospasm and necrosis. The safe injection rate of DMSO is <0.3 ml injected over 40 s [44].
▪ N-butyl cyanoacrylate
N-butyl cyanoacrylate (n-BCA) is a synthetic glue available by many trade names, and used in many medical settings including, topically for surgical incision closure, endoscopically for gastric varices and intravascularly for presurgical neurovascular aneurysm embolization among others. Cyanoacrylates intended for intravascular use are low-viscosity liquid monomers which rapidly polymerize when exposed to the anionic environment of the blood [53], and being inherently radiolucent, are suffused with powdered metals, commonly tantalum, to promote radiopacity. The polymerization rate is dependent on the concentration of the n-BCA in the solvent [44], with the solvents in use being either glacial acetic acid or Lipiodol (Guerbet, Paris, France), the latter in ratios of 1:1 or 1:4 parts n-BCA to Lipiodol [53]. Special precautions must be taken with catheterization equipment when using n-BCA. Polypropylene syringes should be used, as n-BCA can destroy polycarbonate syringes, and nonionic solution (e.g., dextrose-water [D5W]) should be used for all flushes [53]. Delivery should be via a microcatheter attached to two syringes via a three-way stopcock, one being a 1 ml syringe carrying the n-BCA, the other of which is a 3 ml syringe carrying D5W. The n-BCA should be injected, followed immediately by the D5W bolus to propel the n-BCA out of the catheter and into the target. After delivery, the tip of the catheter should be withdrawn to prevent adhering to the glue embolus. After embolization, a glue cast of the occluded vessel will remain. No significant endovascular use of n-BCA in congenital cardiac conditions has been published, though potential applications include any vessels needing occlusion. Obtaining an interventional radiologist’s guidance should be paramount if considering using n-BCA.
Retrieval devices
Although uncommon, unintentional device or foreign body embolism is a dreaded and possibly emergent complication, and as such it is incumbent on the interventionalist to be familiar with the range of retrieval options available. Since the first percutaneous retrieval of an intravascular foreign body in 1962 – an ad hoc affair involving the use of bronchoscopy forceps [54] – retrieval procedures and the associated specialized retrieval devices have expanded in number and scope. Retrieved objects have included broken catheters, guidewires, stents, embolization coils [55] and septal occlusion devices. Contemporary percutaneous retrieval devices came into being in 1991 with the advent of the Nitinol gooseneck snare [56], prior to which urological baskets and forceps were the primary mode of endovascular retrieval [57]. Current examples of snare technology include the Amplatz GooseNeck® snare (ev3) and the EN Snare® device (Merit, UT, USA). These snares are likely to be familiar to the pediatric or congenital interventionalist and further discussion of them will be forsaken in favor of discussing devices more specific to the interventional radiologist.
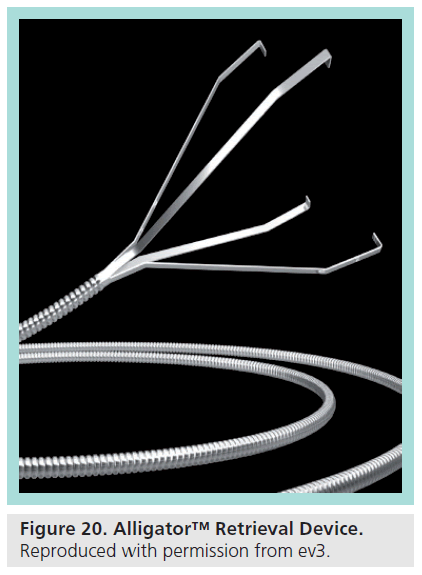
▪ Alligator™ retrieval device
The Alligator™ Retrieval Device (ARD; ev3) is an endovascular foreign object retrieval device intended for use in the peripheral and neurovasculature. It is made of a set of four grasping jaws attached to a flexible wire (Figure 20). The jaws of the ARD are open when not otherwise constrained, and collapse when inside the lumen of a catheter. The ARD is available in millimeter increments from 2 to 5 mm and is designed to be deployed via a 3F microcatheter (minimum inner diameter of 0.021 inches).
To use the device, the approximate vessel size in which the foreign body is embedded must be ascertained, and from that measurement the appropriately-sized ARD is chosen. A microcatheter is then guided to a position several millimeters proximal to the object to be retrieved. The ARD is withdrawn into the provided introducer until the grasping jaws are in their collapsed state, and, via a rotating hemostatic valve, the ARD is advanced into the microcatheter until it reaches the tip. The microcatheter is gently retracted, unsheathing the grasping jaws of the ARD. After the grasping jaws have fully expanded, the ARD is advanced beyond the proximal edge of the object. At this point, the microcatheter is advanced over the ARD until the radiopaque tip passes the base of the grasping jaws, which clamps the jaws onto the object. Now, as a unit, the object, the microcatheter, and the ARD, are withdrawn into the guiding catheter and outside the patient [58]. Caution must be taken to not torque the ARD, as this can cause the jaws to become entangled upon themselves.
Though intended and successfully used for retrieving foreign objects [59], the ARD has been used successfully for purposes of mechanical thrombectomy in acute stroke [60,61]. No literature addressing its use in congenital cardiology has been published, but similar to its use in the neurovasculature, the ARD could potentially be used in the congenital cardiac setting for mechanical thrombectomy or foreign body retrieval. As the ARD is contraindicated for use in retrieving foreign bodies which are overgrown by, or enmeshed in, tissue, acute situations where a thrombus or foreign body has recently embolized represent the ARD’s best chance at success. Possible scenarios include concern for thrombus after catheter device implantation where anticoagulation has been unsuccessful, or postsurgically, in cases where the patient would not be a candidate for re-operation. Complications of ARD usage are not dissimilar to other catheter-based interventions, and include vessel perforation or dissection, hemorrhage and thromboemboli.
▪ Merci®
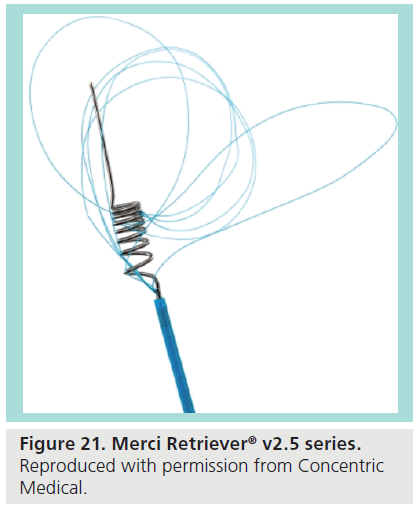
The Merci® is a minimally invasive catheter-based system designed to retrieve and remove clots in patients experiencing acute ischemic stroke. The system is intended for use in patients presenting within 3–8 h after onset of stroke symptoms, or for stroke patients who are otherwise ineligible for thrombolytic therapy. The device is FDA approved and demonstrates good rates of recanalization and neurologic outcomes [62]. The device is also indicated for foreign body retrieval in neuro, peripheral, and coronary vasculature. The retrieval system comprises the Merci Retriever®, the Merci® Balloon Guide Catheter and the Merci® Microcatheter [63]. The Merci Balloon Guide Catheter is an 8 or 9F end-hole catheter with a balloon at its distal tip [64] and the microcatheter is a 2.4F microcatheter. The Merci Retriever itself is a flexible Nitinol wire which conforms to a corkscrew shape, with later generations including filaments to increase clot retention (Figure 21).
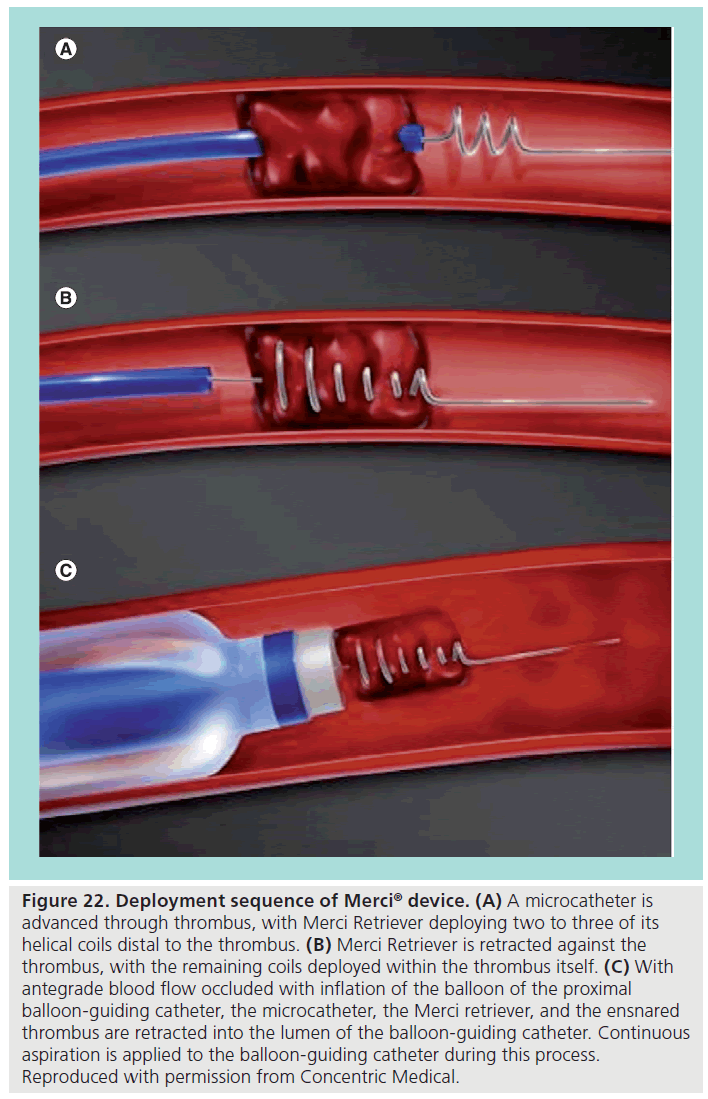
The procedure involves using the guiding balloon catheter to direct the microcatheter across the thrombus. From there, the Merci Retriever is passed through the microcatheter and two or three helices are allowed to deploy distal to the thrombus (Figure 22A). The Merci Retriever is retracted until the loops contact the thrombus, and the remaining loops are deployed within the thrombus itself (Figure 22B). The balloon of the guiding catheter is inflated to prevent antegrade bloodflow in an effort to prevent clot fragmentation, and then five clockwise rotations are applied to the Merci Retriever to further ensconce the thrombus. At this point, with continuous aspiration applied to the guiding catheter, the microcatheter, the Merci Retriever, and the ensnared thrombus are withdrawn together into the lumen of the guiding catheter (Figure 22C).
Figure 22: Deployment sequence of Merci® device. (A) A microcatheter is advanced through thrombus, with Merci Retriever deploying two to three of its helical coils distal to the thrombus. (B) Merci Retriever is retracted against the thrombus, with the remaining coils deployed within the thrombus itself. (C) With antegrade blood flow occluded with inflation of the balloon of the proximal balloon-guiding catheter, the microcatheter, the Merci retriever, and the ensnared thrombus are retracted into the lumen of the balloon-guiding catheter. Continuous aspiration is applied to the balloon-guiding catheter during this process. Reproduced with permission from Concentric Medical.
The Merci has already gone through a few iterations. The first-generation Merci X5 and X6 devices are only helical Nitinol wire loops [65]. The second-generation L4, L5, and L6 devices added filaments to increase clot retention, and third-generation devices (V series) have loops with differing tensions. Further adjuvant devices include a 4.3 and a 5.2F distal access catheter to advance further than the balloon-guiding catheter allows, to obtain a stable foothold closer to the clot. While the Merci device has been used over 10,000-times worldwide [65], there is no literature regarding its use in a cardiac setting. Similar to the ARD, possible indications for use would be an acute thrombus or foreign body embolization, especially one where tortuosity would make the presence of a directional catheter and microcatheter system advantageous over conventional retrieval devices. Known complications of the Merci are typical of any vascular intervention, and official recommendations to minimize vessel trauma include performing no more than six passes of the Merci Retriever through any given vessel. Complications specific to the Merci are related to the possibility of fracturing the Retreiver. This risk can be ameliorated by limiting the total number of Merci Retriever rotations to no more than five (counting both clockwise and counter-clockwise rotations), and by keeping the microcatheter in a buttressing position as close as possible to the Merci Retriever’s loops [66]. Should the Merci Retriever become entrapped in the vasculature, the microcatheter should be advanced distally while retracting the Merci Retriever into the microcatheter, and subsequently retracting both as a unit into the guiding catheter. Contraindications for Merci usage in the recent Multi MERCI trial chiefly related to minimizing bleeding risk, and included an international normalized ratio >3, a partial thromboplastin time >2-times normal, known bleeding diathesis or coagulation factor deficiency, thrombocytopenia with platelets <30,000, or hypertension with systolic pressure >185 or diastolic pressure >110 [67].
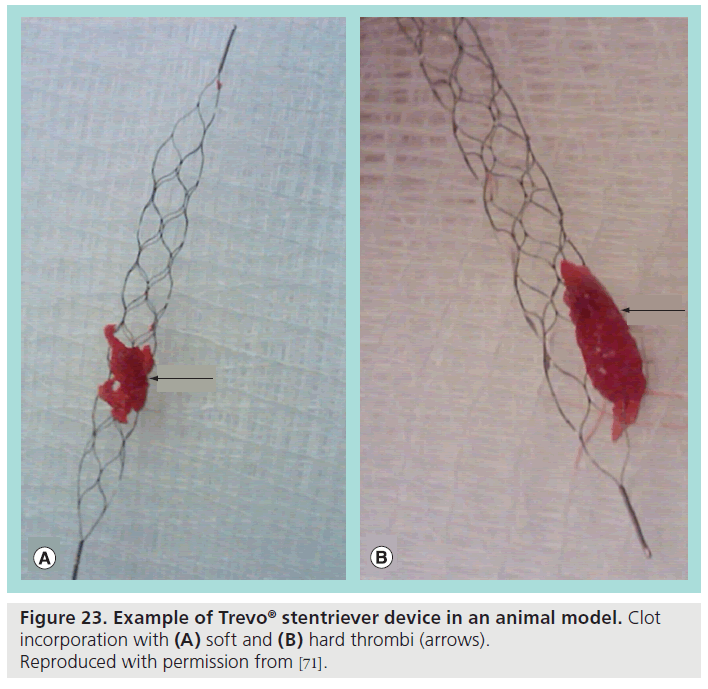
▪ Retrievable stents
Retrievable stents, commonly referred to as Stentrievers™ (Concentric Medical Inc., CA, USA), were born out of the idea that in an acute stroke, stenting would be a rapid way to reacquire perfusion to the distal cerebral vasculature. In practice, however, placement of an intracranial stent is compromised by the need to be on dual antiplatelet therapy to prevent shunt thrombosis [68] in a patient population already at risk for hemorrhagic complications. Stentrievers bypass this problem by being retrievable. After having been deployed in the vasculature, the Stentrievers are withdrawn, and in the process of doing so also perform direct mechanical thrombectomy (Figure 23). A guiding catheter, typically a balloon-guiding catheter, and a microcatheter are used to gain position near the area of thrombus, and then the Stentriever is deployed directly across the thrombus. The Stentriever is left in place for a period of time, typically ranging from 3 to 10 min, with the thought being that when warmed to body temperature, the Nitinol in the Stentriever increases its radial force [68], thus increasingly enmeshing the stent in the thrombus. Then, while applying vigorous aspiration to the guiding catheter with at least a 60 ml syrine, the Stentriever is then retracted into the guiding catheter, along with any retained pieces of clot. This technique has been shown in limited studies to obtain complete recanalization within two passes in up to 77% of patients [69], with a typical procedure time of only 40 min. Stentrievers enjoy widespread use in European markets, but are still undergoing FDA trials in the USA. Current examples include the Trevo® (Concentric), and the Solitaire F™ (ev3). No literature exists regarding usage in congenital cardiac lesions, though potential indications would be similar to other mechanical thrombectomy devices including acute thrombus or foreign body embolization. Known complications are comparable to any vascular interventional device. If the retriever is difficult to withdraw into the microcatheter, one technique is to affix a locking extension wire to the proximal end of the retriever wire, to allow exchanging the microcatheter currently in place for a larger caliber catheter. Once the retriever is resheathed into this larger catheter, the entire unit can be withdrawn into the guiding catheter, and removed as usual. A single Trevo is designed to be reused up to three-times, barring evidence of damage after use, again with the restriction that use is limited to no more than six-times on a given vessel [70].
Figure 23: Example of Trevo® stentriever device in an animal model. Clot incorporation with (A) soft and (B) hard thrombi (arrows). Reproduced with permission from [71].
Conclusion
There is a multitude of devices and technologies from interventional radiology which can be used in congenital cardiac intervention. Successful use and widespread adoption will hinge on familiarity with the devices. When using unfamiliar technologies, collaboration with colleagues from interventional radiology, neurointerventional radiology, and vascular surgery will be crucial to ensure good outcomes.
Future perspective
Devices from interventional radiology currently in the nascent phases of adoption by the congenital cardiac interventionalists will gain much more widespread usage as more results are published and as physician comfort and knowledge grows. The number of devices in both interventional radiology and congenital cardiac intervention will grow and device diversity will increase the fruition of efforts from the FDA and NIH to spur pediatric congenital cardiac device development. There can be the expectation that devices will increasingly target niche applications in each field.
Financial & competing interests disclosure
DS Levi is a consultant with PFM Medical and with Atrium Medical. He is also on the advisory board of Neurosigma Vascular. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Stent grafts
▪ Stent grafts are flexible self-expanding or balloon-expandable covered stents, currently marketed for use in situations, such as endovascular abdominal aortic aneurysm repair.
▪ Appropriately-sized stent grafts represent a key deficiency in the congenital interventionalist’s device portfolio.
▪ Congenital cardiac uses of stent grafts include occlusion of fenestrations in Fontan circuits, exclusions of aneurysms and dissections, and closure of fistulous connections.
▪ Examples include the iCAST™, the Advanta™ V12 Covered Stent and the GORE® EXCLUDER® AAA Endoprosthesis.
▪ Large introducer sheath sizes may preclude use in small children.
Microcatheters
▪ Microcatheters are small caliber catheters designed to coaxially track over a guidewire, while themselves inserted into a directional guiding catheter, in order to facilitate access into small, distal or tortuous vessels.
▪ Microcatheters are very useful in congenital heart disease in accessing collateral or otherwise diminutive vessels.
▪ Microcatheters are suitable for angiography and intervention, including coil embolization and stenting.
▪ Examples include Echelon™, Progreat™ Microcatheters and Renegade® Hi-Flo Microcatheters.
Coils
▪ Pushable coils are delivered through a catheter via pusher guidewires. The pushable Gianturco coil finds extensive use in the closure of small-to-medium sized patent ductus arteriosus, though newer devices including the Flipper Coil and the Amplatzer™ Septal Occluder are claiming increasing market share of this usage.
▪ Cook Tornado® coils are softer platinum pusher coils, which are safe for MRI use.
▪ Detachable coils use detachment mechanisms, either mechanical or electrolytic, to allow a controlled release of the coil.
▪ The Guglielmi detachable coil is an electrolytically detachable coil used successfully in treating coronary artery fistulae. Usage is limited by expense and lack of thrombogenic fibers.
▪ Interlock™ Fibered coils are mechanically detachable coils which have been used in aortopulmonary (AP) collaterals, venovenous collaterals, patent ductus arteriosus and coronary artery fistulae.
Alternative embotherapies
▪ There is common need in congenital cardiology to occlude collateral vessels. Such vessels include AP collateral vessels in the cyanotic patient, and veno-venous collaterals in Glenn or Fontan patients who are reliant on increased passive venous pressures for antegrade flow.
▪ Particulates are embolic therapies designed to occlude vessels, with the occlusion level dependant on the size of the particle chosen.
▪ Polyvinyl alcohol and Gelfoam are older classes of particulates, which can behave unpredictably. They have been largely superseded for intravascular use by other technologies.
▪ Microspheres are spherical nonresorbable particles, which are highly biocompatible and demonstrate good uniformity of particle size and shape. For example, 900–1200 μm sized Embospheres® can be used to embolize AP collaterals. Care must be taken to avoid nontarget embolization.
▪ Ethylene vinyl alcohol copolymer is a nonadhesive liquid embolic dissolved in dimethyl sulfoxide, which, when injected into the bloodstream, polymerizes into a solid occlusive mass. It has also been used to embolize AP collaterals. Its use requires special dimethyl sulfoxide-compatible catheters.
Retrieval devices
▪ The Alligator™ Retrieval Device comprises passively deployed jaws attached to a wire, deployed via a microcatheter. It is suitable for retrieving emboli in vessels up to 5 mm.
▪ The Merci® Retrieval system is a corkscrew-shaped Nitinol wire meant to ensnare and retrieve a thrombus.
▪ Retrievable stents, often referred to by their trademarked name ‘Stentrievers’, are stents temporarily deployed across a thrombus, and then retracted, performing a mechanical thrombectomy on the enmeshed thrombus.
References
- Allen HD, Driscoll DJ, Shaddy RE, Fel es TF. Moss and Adams’ Heart Disease in Infan s, Children, and Adolescents (7th Edition). AllenHD, Driscoll DJ, Shaddy RE, Feltes TF (Eds). Lippincott Williams & Wilkins, Philadelphia, P , USA (2007).
- US FDA. Food nd Drug dministration Website. 2006. www.fda.gov/downloads/ RegulatoryInformation/Guidances/ UCM127067.pdf (Accessed 16 July 2012)
- Almond CS. The FDA review process for cardiac medical devices in children: a review for clinicians. Prog. Pediatr. Cardiol. 33(2), 105–109 (2012).
- Sutherell JS, Hirsch R, Beekman RH 3rd. Pediatric interventional cardiology in the united states is dependent on the off-label use of medical devices. Congenit. Heart Dis. 5(1), 2–7 (2010).
- Federal Food, Drug, And Cosmetic Act § 906. (2002).
- Rutherford RB. Open versus endovascular stent graft repair for abdominal aortic aneurysms: an historical view. Semin.Vasc. Surg. 25(1), 39–48 (2012).
- Endologix. Intuitrak System Brochure. www.endologix.com/fls/IntuiTrak_System_ Brochure MM0153_Rev_01.pdf Accessed 16 July 2012)
- Endologix. The PowerLink System. www.endologix.com/fls/MM0002%20 Rev%20D.pdf (Accessed 16 July 2012)
- Magee A, Sim E, Benson LN, Williams WG, Trusler GA, Freedom RM. Augmentation of pulmonary blood flow with an axillary arteriovenous fistula after a cavopulmonary shunt. J. Thorac. Cardiovasc. Surg. 111(1), 176–180 (1996).
- McElhinney DB, Marshall AC, Lang P, Lock JE, Mayer JE. Creation of a brachial arteriovenous fistula for treatment of pulmonary arteriovenous malformations after cavopulmonary anastomosis. Ann. Thorac. Surg. 80(5), 1604–1609 (2005).
- Quinonez LG, Brown ML, Dearani JA, Burkhart HM, Puga FJ. Axillary arteriovenous fistula for the palliation of complex cyanotic congenital heart disease: is it an effective tool? J. Thorac. Cardiovasc. Surg. 141(1), 188–192 (2011).
- Hickey EJ, Alghamdi AA, Elmi M et al. Systemic arteriovenous fistulae for end-stage cyanosis after cavopulmonary connection: a useful bridge to transplantation. J. Thorac. Cardiovasc. Surg. 139(1), 128–134 (2010).
- Golden AB, Hellenbrand WE. Coarctation of the aorta: stenting in children and adults. Catheter Cardiovasc. Interv. 69(2), 289–299(2007).
- Kenny D, Cao QL, Kavinsky C, Hijazi ZM. Innovative resource utilization to fashion individualized covered stents in the setting of aortic coarctation. Catheter Cardiovasc. Interv.78(3), 413–418 (2011).
- Atrium. Atrium iCAST™ Covered Stent Product Brochure. www.atriummed.com/en/interventional/ Documents/iCASTDatasheet003620F.pdf (Accessed July 24 2012).
- Atrium. Atrium V12 Covered Stent. www. atriummed.com/en/interventional/v12.asp (Accessed July 23 2012).
- Atrium. V12 Large Diameter Covered Stent. 2012 www.atriummed.com/en/interventional/ v12LD.asp (Accessed 31 July 2012)
- Zellers TM, Moake L, Wright J. Use of the Terumo SP catheter system for crossing the pulmonary valve in infants with critical pulmonary valve stenosis. Am. J. Cardiol. 76(14), 1082–1084 (1995).
- Brown SC, Boshoff DE, Mertens L, Gewillig Use of a microcatheter in a telescopic system to reach difficult targets in complex congenital heart disease . Catheter Cardiovasc. Interven. 73(5), 676–681 (2009).
- Boston Scientific. Boston Scientific: Products: Renegade® HI-FLO™. Microcatheter. www.bostonscientific.com/templatedata/ imports/collateral/Radiology/spec _ renhiflo_01_us.pdf (Accessed 24 July 2012)
- Gianturco C, Anderson J, Wallace D. Mechanical devices for arterial occlusion. Am.Radiol. 124(3), 428–435 (1975).
- Cambier P, Kirby W, Wortham D et al. Percutaneous closure of small (less than 2.5 mm) patent ductus arteriosus using coil embolization. Am. J. Cardiol. 69(8), 815–816 (1992).
- Binkert CA. Embolization tools and techniques. Appl. Radiol. 31(8), 55–64 (2002).
- Kessel DO, Lee MJ, Watkinson AF, Ray CE. Transcatheter Embolization and Therapy.Kessel DO (Ed.). Springer, New York, NY, USA (2010).
- Aoyagi S, Chihara S, Fukunaga S, Mori R, Suda K. Transcatheter coil embolization for patent ductus arteriosus in the elderly: report of a case and review of the published work. Geriatr. Gerontol. Int. 9(3), 329–332 (2009).
- Brunetti MA, Ringel R, Owada C et al. Percutaneous closure of patent ductus arteriosus: a multiinstitutional registry comparing multiple devices. Catheter Cardiovasc. Interv. 76(5), 696–702 (2010).
- Uzun O, Veldtman GR, Dickinson DF, Parsons JM, Blackburn MEC, Gibbs JL. Haemolysis following implantation of duct occlusion coils. Heart 81(2), 160–161 (1999).
- Ali Khan M, al Yousef S, Mullins C, Sawyer
- Experience with 205 procedures of transcatheter closure of ductus arteriosus in 182 patients, with special reference to residual shunts and long-term follow-up. J. Thorac. Cardiovasc. Surg. 104(6), 1721–1727 (1992).
- Liang CD, Ko SF, Huang CF et al. Immediate echocardiographic surveillance after transcatheter closure of a patent ductus arteriosus: a feasible method to assess residual shunt. Pediatr. Neonatol. 51(1), 52–56 (2010).
- Santiago JJ, Pedra CAC, Arnoni D et al. Percutaneous closure of Blalock-Taussig shunts using Gianturco coils. Rev. Esp.Cardiol. 61(12), 1342–1345 (2008).
- Cook. Tornado® Embolization Coils Instructions for Use. www.cookmedical.com/ readFile?fileName=T_CE_TEM_REV1 (Accessed 31 July 2012)
- Suda K, Matsumura M. Transcatheter occlusion of patent ductus arteriosus using tornado platinum coils. Pediatr. Int. 45(1), 45–48 (2003).
- Weems C, Peuster M, Trivedi K. One plug may not be enough: a novel technique for the occlusion of high-flow vascular connections: combined AGA vascular plug II and coil occlusion of a sequester artery in a patient with Scimitar syndrome. Catheter Cardiovasc. Interv. 78(5), 687–691 (2011).
- Cook Medical. Cook Medical Pe iphe al Intervention. www.cookmedical.com/di/content/mmedia/ PI-BM - MRF-EN -200710.pdf (Accessed 24 July 2012)
- Munawar M, Siswanto BB, Harimurti GM, Nguyen TN. T anscatheter closure of coronary a te y fistula using Guglielmi detachable coil. J. Ge iatr. Cardiol. 9(1), 11–16 (2012).
- B st n Scientific. Interlock™ Fibered IDC Occlusi n System. www.b st nscientific.com/templatedata/ imports/collateral/Radiology/spec_in erlock 01 us.pdf (Accessed 24 July 2012)
- Seltzer S, Aboulhosn J, Levi D. Use of in erlock fibered detachable coils for occlusion of collaterals, coronary artery fistulae, and patent ductus arteriosus. Catheter Cardiovasc. Intervent. 74(5), 770–776 (2009).
- Sato Y, Ogino H, Hara M et al. Embolization of collateral vessels using mechanically detachable coils in young children with congenital heart disease. Cardiovasc. Intervent. Radiol. 26(6), 528–533 (2003).
- Sim JY, Alejos JC, Moore JW. Techniques and applications of transcatheter embolization procedures in pediatric cardiology. J. Interv. Cardiol. 16(5), 425–448 (2003).
- Ikeda K, Kumada H, Saitoh S, Arase Y, Chayama K. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma: an analysis by the Cox proportional hazard model. Cancer 68, 2150–2154 (1991).
- Pelage JP, Dref OL, Beregi JP et al. Limited uterine artery embolization with tris-acryl gelatin microspheres for uterine fibroids. J. Vasc. Interv. Radiol. 14, 15–20 (2003).
- Speakman TJ. Internal Occlusion of a Carotid-Cavernous Fistula. J. Neurosurg. 21(4), 303–305 (1964).
- Pfizer. Gelfoam absorbable gelatin powder. http://labeling.pfizer.com/ShowLabeling. aspx?id=574 (Accessed 31 July 2012)
- Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Semin. Intervent. Radiol.25(3), 204–215 (2008).
- Osuga K, Maeda N, Higashihara H et al. Current status of embolic agents for liver tumor embolization. Int. J. Clin. Oncol. 17(4), 306–315. (2012).
- Laurent A, Beaujeux R, Wassef M, Rufenacht D, Boschetti E, Merland JJ. Trisacryl gelatin microspheres or therapeutic embolization, I: devel pment and in vitro evaluation. AJNR Am. J. Neur radiol. 17(3), 533–540 (1996).
- Laurent A. Microspheres and nonspherical particles f r embolization. Tech. Vasc. Interv. Radi l. 10(4), 248–256 (2007).
- Bi Sphere Medical. Embosphere Microspheres Catheter Compatibility Chart. www.biospheremed.com/publication_files/ MR01–005RevD.PDF (Accessed 29 July 2012)
- Biosphere Medical. Embosphere Microsphere Instructions for Use. www.biospheremed.com/publication_files/ Embosphere-syringeZ1758revF06–1225–1207–12.pdf (Accessed 10 October 2012)
- Taki W, Yonekawa Y, Iwata H, Uno A, Yamashita K, Amemiya H. A new liquid material for embolization of arteriovenous malformations. AJNR Am. J. Neuroradiol. 11(1), 163–168 (1990).
- Coviden. Onyx® LES. www.ev3.net/neuro/us/liquid-embolics/onyx-liquid-embolic-system.htm (Accessed 31 July 2012)
- Coviden. Onyx® LES. www.ev3.net/assets/007/5766.pdf (Accessed 10 October 2012)
- Pollak JS, Robert I. White J. The use of cyanoacrylate adhesives. J. Vasc. Interv. Radiol. 12(8), 907–913 (2001).
- Thomas J, Sinclair-Smith B, Bloomfield D, Davachi A. Non-surgical retrieval of a broken segment of steel spring guide from the right atrium and inferior vena cava. Circulation 30, 106–108 (1964).
- Gabelmann A, Kramer S, Gorich J. percutaneous retrieval of lost or misplaced intravascular objects. AJR Am. J. Roentgenol. 176(6), 1509–1513 (2001).
- Joseph W. Yedlicka J, Carlson JE et al. Nitinol gooseneck snare for removal of foreign bodies: experimental study and clinical evaluation. Radiology 178(3), 691–693 (1991).
- Dondelinger RF, Lepoutre B, Kurdziel JC. Percutaneous vascular foreign body retrieval: experience of an 11-year period. Eur.Radiol. 12(1), 4–10 (1991).
- ev3. Alligator™ Retrieval Device. www.ev3.net/neuro/us/retrieval-devices/ device-retrieval-product.htm (Accessed 31 July 2012)
- Henkes H, Lowens S, Preiss H, Reinartz J, Miloslavski E, Kühne D. A new device for endovascular coil retrieval from intracranial vessels: alligator retrieval device. AJNR Am. Neuroradiol. 27(2), 327–329 (2006).
- Hussain MS, Kelly ME, Moskowitz SI et al. Mechanical thrombectomy for acute stroke with the alligator retrieval device. Stroke 40(12), 3784–3788 (2009) .
- Kerber CW, Wanke I, Bernard J Jr, Woo HH, Liu MW, Nelson PK. Rapid intracranial clot removal with a new device: the alligator retriever. AJNR Am. J. Neuroradiol. 28(5), 860–863 (2007).
- Smith WS, Sung G, Starkman S et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 36(7), 1432–1438 (2005).
- Concentric Medical. Concentric Medical. The Merci Retrieval System. Product Backgrounder. www.concentric-medical.com/product-backgrounder (Accessed 31 July 2012)
- Gobin YP, Starkman S, Duckwiler GR et al. MERCI 1: a Phase 1 study of mechanical embolus removal in cerebral ischemia. S roke 35(12), 2848–2854 (2004).
- Nguyen TN, Babikian VL, Romero R et al. Intra-arterial treatment methods in acute stroke therapy. Front. Neurol. 2(9), 1–8 (2011).
- Concentric Medical. Merci Retrieval System – Instructions for Usage.www.concentric-medical.com/resources/ PK50570_015_F_IFU_Merci_Retriever%20_ with_filaments_Extendable_%202010_09. pdf (Accessed 10 October 2012)
- Smith WS, Sung G, Saver J et al. Mechanical Thrombectomy for acute ischemic stroke: final results of the multi MERCI trial. Circulation 39(4), 1205–1212 (2008).
- Pereira VM, Narata AP, Gonzalez AM, Sztajzel R, Lovblad KO. Use of stentrievers in acute stroke: tips, tricks, and current results. Tech. Vasc. Interv. Radiol. 15(1), 68–77(2012).
- Dávalos A, Pereira VM, Chap t R, B nafé A, Andersson T, Gralla J. Ret ospective multicenter study of solitai e f revascularization in the t eatment of acute ischemic stroke. Stroke 43(10), 2699–2705 (2012). Concent ic Medical. Trevo® ro Retriever Inst uctions for Usage.www.concent ic -medical.com/resources/ PK50670 015 B IFU _ Retriever_ Extendable L ng Insertion_Tool_2010_09. pdf (Accessed 12 October 2012)
- Nogueira RG, Levy EI, Gounis M, Siddiqui AH. The Trevo device preclinical data of a novel stroke thrombectomy device in two different animal models of arterial thrombo-occlusive disease. J. NeuroIntervent. Surg. 4(4), 295–300 (2012).
- American Academy of Pediatrics. AAP Website. www.aap.org/en-us/advocacy-and-policy/ federal-advocacy/Pages/Pediatric-Device-Legislation.aspx (Accessed 16 July 2012)
- Rose SC. Commentary: mechanical devices for arterial occlusion and therapeutic vascular occlusion utilizing steel coil technique: clinical applications. AJR Am. J. Roentgenol. 192(2), 321–324 (2009).
- Quinonez L, Brown M, Dearani J et al. Axillary arteriovenous fistula for the palliati n of complex cyanotic congenital heart disease: is it an effective tool? J. Thorac. Cardi vasc. Surg. 141(1), 188–192 (2011).
- Andrews R, Tulloh R, Anderson D. Coil occlusion of systemic venous collaterals in hypoplastic left heart syndrome. Heart 88(2), 167–169 (2002).
- Grifka RG, Fenrich AL, Tapio JB. Transcatheter closure of patent ductus arteriosus and aorto-pulmonary vessels using non-ferromagnetic Inconel MReye embolization coils. Catheter Cardiovasc. Interv. 72(5), 691–695 (2008).
- Radiology information resource for patients. www.radiologyinfo.org
▪ Website