Review Article - International Journal of Clinical Rheumatology (2021) Volume 16, Issue 1
Ultrasound-guided caudal epidural steroid injection for sciatica: simplicity, efficacy and safety
- Corresponding Author:
- Charles Sougué
Department of Internal Medicine
Sourô Sanou University Hospital, Burkina Faso
E-mail: souguecharles@gmail.com
Abstract
The possibility of precisely locating the sacral hiatus, which is greatly facilitated by echography, has rekindled interest in epidural infiltrations via this route, particularly as rare but serious complications have been reported for other epidural injection techniques, such as those using the foraminal route or particulate products. Products injected via the sacral hiatus have been shown to ascend efficiently to the lumbar spine. In recalcitrant sciatica, epidural injections of corticosteroids via the three principal routes (sacral hiatus, interlaminar, foraminal) have similar mean efficacies. Injection via the sacral hiatus, or caudal injection, has similar complications to interlaminar and foraminal injections, but these complications appear to be much rarer by this route, and avoidable if several indispensable technical precautions are applied: five-step asepsis, echographic control to check for the absence of pilonidal cysts, avoidance of canal catheterization, avoidance of the injection of air, allergenic products, anesthetic products or particulate products, and slow injection. The sacral hiatus route can be used to ensure epidural infiltration without a risk of dural leak, in patients on antiplatelet treatment, even after surgery. Further studies are required to increase the efficacy and safety of this technique and to evaluate the benefits of injecting therapeutic agents other than corticosteroids via this route.
Keywords
glucocorticoid ● caudal injection ● epidural ● sacral hiatus ● sciatica ● ultrasound
Introduction
Caudal Injection (CI) via the Sacral Hiatus (SH) is a very old technique, first described by Sicard and Cathelin [1,2] in 1901, at about the same time as epidural and intradural injections [3,4]. The product injected was not a glucocorticoid, as these drugs were unknown at the time, but cocaine, and results were reported for peroperative analgesia and sciatica. Even at the time, it was the adverse effects of epidural and intradural injections, such as headaches, nausea and vomiting over several days that drove the search for another distal approach to the epidural space without secondary effects. Experiments involving the injection of dyes into the dorsal sacrococcygeal ligament in dogs showed that this approach was simple, respected the subarachnoid spaces, with no leak of cerebrospinal fluid, and that the injected project effectively reached the lumbar and dorsal regions in large quantities, sometimes even reaching the cervical area. Studies on human cadavers confirmed the results obtained in dogs, with effective epidural diffusion along the vertebral column. The venous diffusion of the product was demonstrated with colored wax. The first series of nine patients with sciatica received injections of cocaine diluted in 5 to 15 ml of saline [2]. Four patients were cured and a marked improvement was observed in another two patients, but the results were only transient in patients with tabes dorsalis. The only adverse effect report was a transient numbness of the scrotum. The technique was precisely described and recommended as an alternative to epidural injections for the indications of sciatica, pain during childbirth, cancer pain, and postoperative pain.
Nevertheless, during the course of the 20th century, epidural injections were essentially favored, with three in every four bibliographic references still providing strong support for interlaminar epidural injections, and a decrease in the number of publications on SH infiltration over the last five years (610 over the last year, versus 701 to 834 per year over five-year periods covering the last 30 years). The reasons for this imbalance are unclear. It does not seem to be due to any doubts about the analgesic efficacy of SH infiltration, the subject of most papers on this technique, as large series since the 1960s have highlighted the analgesic efficacy of this technique up to the dorsal region and the absence of adverse effects [5]. Despite the location of the injection site, in the buttock region, infectious complications have been reported only very rarely. Failures due to a lack of precision of CI based on anatomic landmarks provide a possible explanation (failure rates of 26% reported by Stitz [6] and 32% by Barham [7]). A randomized comparison of CI with use of the direct epidural route based on simple anatomic landmarks, with subsequent checking by epidurography reported precisions of 64% and 93%, respectively, figures very much against the use of CI [8]. However, Naidoo considered that X rays would be sufficient to improve this precision provided that the intervention was performed by an experienced surgeon [9]. Similarly, echography has an efficiency close to 100%, except in cases of morbid obesity [10]. This review aims to highlight the value of CI via the SH performed with a guidance technique, and their favorable benefit/risk ratio.
Anatomy of the sacral hiatus
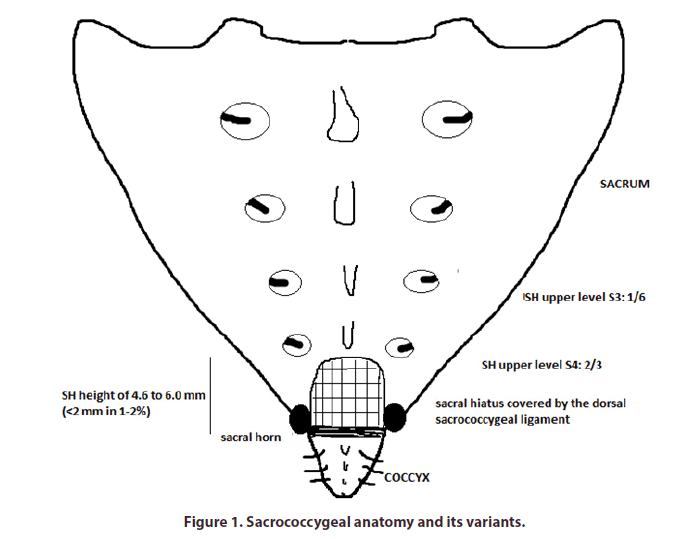
The sacrococcygeal symphysis can be identified at the top of the intergluteal cleft thanks to two terminal protuberances of the lower part of the sacrum, the vestiges of the S5 zygapophyses, and the disappearance at this level of the blades and spines [11]. These protuberances are often palpable, but are much more difficult to find in obese patients. Three fine layers protect the SH (in order, from the exterior to the interior): the skin, subcutaneous tissue of variable thickness, and the dorsal sacrococcygeal ligament (Figure 1). This ligament may display fine calcification in some cases, but this does not prevent access to the SH. The dimensions of the SH are highly variable, with an anterior-posterior diameter of 4.6 to 6.1 mm [12]. However, in a small proportion (a few percent) of cases it may be very small (<2 mm in diameter) or even, in exceptional cases, completely closed. Its upper part is generally located at the S4 level (in two thirds of cases), but it may reach S3 in 15% of cases and, in 1% of cases, there may be no fusion of the posterior blade over the entire sacrum.
Figure 1: Sacrococcygeal anatomy and its variants.
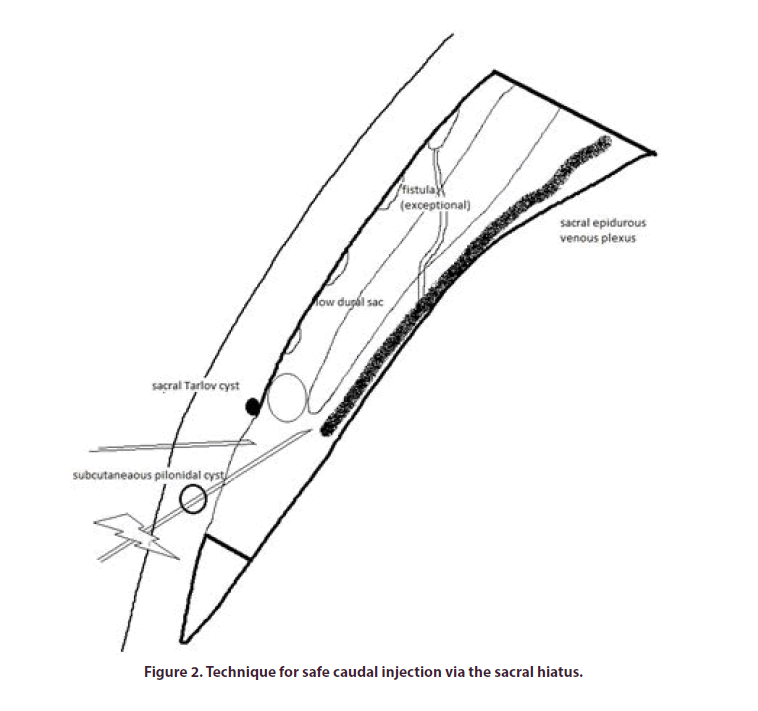
The canal includes the dural sac and the vascular and nervous system elements (Figure 2). The dural sac generally ends at S1-S2, but it may descend lower, to S3, or lower still, in 1 to 5% of cases. This leaves a nonnegligible margin between the top of the SH and the dural sac, of 32 ± 12 mm according to measurements on cadavers, still leaving at least 4 mm above the upper part of the SH in the most extreme 1% of cases [13].
A recent MRI study of 1000 images reported a mean measured distance between the dural sac and the upper part of the SH of 44.6 ± 11.8 mm [14]. This study reported a low dural sac, located below S3, in 4.9% of cases, and located below S4 in 0.1% of cases [15]. Sacral Tarlov cysts are the other principal risk factor for dural puncture. These cysts are frequent in the sacrum (1 to 5%) and 40% occur below S3. In the MRI study cited above, 13 Tarlov cysts were observed in 1000 patients on MRI, and only two of these cysts reached the lower part of S3 [15]. The roots of the nerves exit via the sacral foramina at the level of the dural sac at S1 and S2, and below this sac at S3, S4 and S5. A filum terminale from the dura mater extends under the dural sac and attaches to the coccyx. The sacral arteries are lateral and anterior to the sacrum, and are located in front of the sacral plexus. There is no artery in the sacral canal, other than very small perinervous arterioles in the dural sac towards the top (an injection via the sacral hiatus may therefore be considered equivalent to a foraminal injection). By contrast, veins originating in the presacral venous plexus are present in the deep and upper parts of the sacral canal. There is no nerve plexus at the level of the coccyx. By contrast, vascular abnormalities, such as arterial/ venous fistulas, have been described for the sacral dura mater vessels. They are almost certainly very rare, but could also explain possible neurological complications of vascular origin at this level [16].
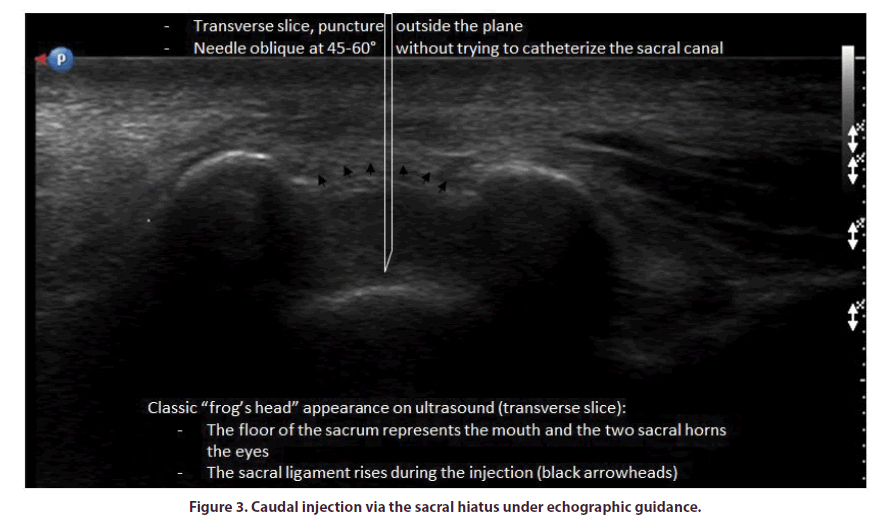
The SH can be explored by sacral X ray in profile or by CT-scan, to locate the injection space, but these techniques do not visualize the dural sac or vascular structures. Furthermore, MRI is the examination of choice for the exploration of recalcitrant sciatica with a view to treatment by SH infiltration, with imaging down to the sacrococcygeal level to observe the dural sac and any Tarlov cysts located at this level. Nevertheless, only arteriography of the sacral artery can reveal possible vascular abnormalities, but this approach is not possible in routine practice. Echography readily identifies the SH, with the characteristic sacral horns, the dorsal sacrococcygeal ligament linking them and the floor of the sacrum, with the sacral canal lying between them. The characteristic image calls to mind a caricature of a frog’s head from the front and a tadpole in profile. Echography can also be used to visualize the entire subcutaneous space, and to eliminate the possibility of a pilonidal cyst developed from an in-growing hair, which could act as a portal of entry for infection if crossed during the injection.
Taking all these anatomic considerations into account, we can deduce that the type of imaging that should be preferred for the exploration of sciatica before injection via the SH is MRI with slices descending right down to the SH to determine the precise extent of the dural sac and to detect any sacral Tarlov cysts that may be present. The sacral canal should not be catheterized and it is important to check that there is no reflux of blood or of cerebrospinal fluid.
Technique for caudal injection via the sacral hiatus The patient is placed in the lateral decubitus position with the knees flexed or in the ventral decubitus position with a cushion beneath the pelvis to provide access to the upper part of the intergluteal cleft [10]. Adhesive paper can be used to draw the skin of the buttocks towards the exterior, so as to open up the upper part of intergluteal cleft. Palpation can be used to identify the sacral horns in non-obese patients, together with the small depression between them. Asepsis should be achieved through a five-step disinfection protocol given the potentially septic nature of the site of puncture in the upper part of the intergluteal cleft. Mild cutaneous and subcutaneous local anesthesia during the injection can be achieved by the subcutaneous injection of 1 or 2 ml of lidocaine. We avoid injecting anesthetic into the canal or under the ligament. Transient anesthesia of the cauda equina is certainly possible and benign, but can be troublesome during an ambulatory procedure. The sensation of the passage of the needle through the dorsal sacrococcygeal ligament resembles that of the passage of the epidural space in lumbar injections. There should be no blood reflux. With the injection of 20 ml of saline, it is possible to reach L5 to S1 in 96% of cases [10]. However, the volume of the sacral canal varies considerably between individuals and the patient may experience a sensation of swelling during the filling of the canal and a painful sensation of excess pressure when the canal is full. In our experience, an injection of lidocaine into the canal has little effect on this painful sensation of excess pressure. It is possible to inject particulate corticosteroids, because the risks of vascular injection are avoided by not catheterizing the sacral canal, but we prefer to use soluble products, such as dexamethasone, given the thrombogenic effects reported for particulate products [17]. Finally, the injection of saline should be slow and progressive, and may even be stopped if the pain is too strong.
In the absence of a means of identifying the correct injection site, adjuvant techniques have been reported, such as the “whoosh” or “swoosh” test, in which 2 ml of air is injected and its passage through the canal is detected by auscultation of the dorsolumbar region, or the perception of sacral swelling after the injection of saline [12]. However, the sensitivities reported for these techniques rarely exceed 80%, and specificity is also poor. The mean failure rate of 25 to 35% for injection into the sacral canal with the use of simple anatomical landmarks can be considered sufficient justification for the use of guidance techniques.
The SH can be identified by CT-scan or X ray before the injection. This identification is simple and the correct localization of the intracanal injection site can be demonstrated by the injection of an iodated product [12]. Vascular injection frequently occurs (3 to 14%) and justifies, as we have seen, injections in which the needle follows a trajectory that is essentially vertical, but slightly oblique towards the top [12]. The characteristic images obtained are the “smoke in the chimney” image in profile, with the ascension of the product in the sacral canal, and the “Christmas tree” image in profile, with opacification of the sheaths of the sacral roots leaving the sacral foramina on either side. However, this reference technique requires X-ray equipment and the injection of a contrast agent (both of which entail costs) and radioprotection.
In our practice, we prefer to use echography, which allows greater flexibility, and avoids the need for exposure to X rays and the injection of a contrast agent [10]. It is easy to visualize the SH, between the sacral horns, on transverse ultrasound images. The sacral canal can also be visualized on the longitudinal view, making it possible to observe the needle (Figure 3). However, it is important to avoid catheterizing the sacral canal, through an injection along the axis of this canal. This approach is possible and best achieved with transverse non-planar echographic location, with the needle following a vertical trajectory that is slightly oblique at its upper end, without the risk of sacral canal catheterization. Some operators use Doppler scans to demonstrate that intracanal injection, but the “pumping sign” reveals the lifting of the dorsal sacrococcygeal ligament during the injection, and this is specific for the filling of the sacral canal. Echoguidance has the advantages of a rapid learning curve, a gain of time (112 seconds versus 222 for X-ray guidance according to Hazra [18]), an absence of irradiation and contrast product and the feasibility of performance in a simple consultation room.
Efficacy of caudal injections via the sacral hiatus We will limit ourselves here to a review of the randomized studies performed on this topic. Seven studies, including more than 700 patients in total, have been performed on sciatica [19-25] (Table 1). Five of these seven studies compared a corticosteroid with an anesthetic, and two compared the injection of corticosteroid with that of saline. Five of the seven studies involved CI via the SH without guidance. Five used particulate products (triamcinolone acetonide, methylprednisolone, betamethasone) and two used soluble dexamethasone. The doses in prednisone equivalent also varied, ranging between 50 and 100 mg. Six of the seven studies reported positive results in the short term, after three, four or six weeks, depending on the study, with a difference between the control and glucocorticoid groups of 11 to 33/100 for scores on a pain scale, and of 15/100 for scores on the Oswestry questionnaire. In the longer term, after three to 12 months, the results remained positive in four of the seven studies. No serious complications were reported.
Table 1. Characteristics and results of randomized studies evaluating glucocorticoid caudal injections via the SH by comparison with a control group, for sciatica.
| Author | Disease | Type of infiltration | Guidance | Type of corticosteroid | N per group | Short-term results | Long-term results | Complications |
|---|---|---|---|---|---|---|---|---|
| Bush Spine 1991 | Sciatica | CI vs. control | X ray | Triamcinolone (80 mg) ± procaine | 12/11 | VAS at week 4* T -22.5 Pro -4,2 |
VAS at week 52 AT -24.3 Pro -19,6 |
0 |
| Manchikanti Pain Phys 2008 |
Sciatica | CI vs. control | X ray | Methylprednisolone (40 mg) and dexamethasone (6 mg) ± lidocaine | 42/42 | - | VAS/ODI at weeks 12, 26, 52 Ns |
0 |
| Sayegh Spine 2009 | Lower back pain + sciatica | CI vs. control | Anatomical | Betamethasone (7 mg) ± lidocaine | 63/60 | ODI at weeks 1/4* BM -26.4 / -29.8 Lido -8.6 / -15.0 |
ODI at weeks S26/52* BM -32.7/-33.6 Lido -24.9/-25.0 |
0 |
| Murakibhavi Evid Based Spine Care J 2011 |
Sciatica | CI vs. control | Anatomical | Triamcinolone (40 mg) ± lidocaine | 50/50 | VAS at week 3 * T -33.9 Lido -0.6 |
Cure at week 26* T 86% Lido 24% |
0 |
| Iversen BMJ 2011 |
Sciatica | CI vs. control vs. sham-treated | Anatomical | Triamcinolone (40 mg) ± saline | 40/39/37 | VAS at week 6 Ns |
VAS at weeks 12 and S52 Ns |
0 |
| Datta Med J Armed Forces India 2011 |
Sciatica | CI vs. control | Anatomical | Triamcinolone (80 mg) vs. methylprednisolone (80 mg) vs. dexamethasone (50 mg) ± bupivacaine | 40/40/42/41 | VAS at week 3* T -11 MP -11 DM -9 B -4 |
VAS at week 12* T -26 MP -25 DM -21 B -10 |
0 |
| Nandi J Clin Diagn Res 2017 |
Sciatica | CI vs. control | Anatomical | Methylprednisolone (80 mg) ± saline | 46/47 | VAS at week 4* MP -29.6 SP -11.7 |
VAS at week 12* MP -34.3 SP -21.7 |
0 |
VAS: Visual Analog Scale ; ODI: Oswestry Disability Index ; *: significant ; ns: not significant
Only one study has been published for lumbar stenosis, in which 6 mg of betamethasone was compared with lidocaine, with no significant effect on pain at 3, 6 or 12 weeks [26]. The two studies evaluating the efficacy of corticosteroid CI relative to anesthetic injected via the SH in patients with lower back pain reported negative results in the short, medium and long term, although both were of limited methodological interest due to the exclusion of lower back pain of interapophysis origin in one study, and a lack of double-blinding relative to oral NSAID in the other [27,28]. One study comparing betamethasone with lidocaine for postoperative lower back pain gave negative results at 3, 6 and 12 months [29]. In the short term (12 hours), two studies found that an injection of dexamethasone via the SH was superior to an injection of ropivacaine via the SH, and, in one of these studies, it was also superior to the injection of dexamethasone via the intravenous route [30,31].
For comparisons of CI injections with injections via the foraminal and interlaminar routes for the treatment of sciatica, we can identify three studies comparing all three routes of injection (interlaminar, CI and foraminal) [32-34] and two comparing the SH route with the foraminal route [35,36]. Only one of these studies reported results in favor of the foraminal route, at one and six months. The results of injections via the caudal and lumbar interlaminar routes were similar in each case. Two meta-analyses recently compared the foraminal and sacrococcygeal routes and reported an absence of significant difference at 2, 12, 24 and 52 weeks [37,38].
None of these studies was of particularly high methodological quality and they were highly heterogeneous in terms of the technique used (with or without guidance), inclusion criteria, corticosteroid used, timing of evaluations and principal outcome measure. It is not, therefore, possible to draw any formal conclusions, and even a global meta-analysis would be difficult to interpret. We can simply express an overall opinion that the efficacy of corticosteroid CI via the HS seems to be similar to that of other epidural or for aminal infiltrations for sciatica, with a decrease in pain relative to the control group of about 10 to 30 points on a 100-point Visual Analog Scale (VAS), and that rigorous double-blind studies are required to confirm this overall impression.
Complications
Benign complications are frequent following the injection of corticosteroids (headaches, hypertension, loss of glycemic balance in patients with diabetes, flushing, redness and swelling of the face, insomnia) or lumbar puncture (reflex syncope, pain). A threeday episode of hiccups was reported in one case after lumbar epidural injection [39], and anosmia possibly related to a glucocorticoid CI via the SH was reported in another [40]. Serious complications remain exceptional. Anaphylactic shock in response to the injection of contrast product was reported in one case, without sequelae [41]. We have seen that air can be injected to provide evidence of CI into the sacral canal. However, this technique should not be used in practice because two complications associated with it have been reported: air embolism of the portal vein and an epidural air bubble compressing the S1 root [42,43]. Cerebrospinal fluid leakage was reported in two studies, after catheterization of the sacral canal up to S3 with a large-caliber needle (17G) in one case [44], and related to a low dural sac descending to S3-S4 in the other [45]. Vascular injections may occur. Manchikanti reported a frequency of vascular reflux of 0.5%, 3.1% and 7.9% for SH, epidural and transforaminal injections, respectively, in a cohort of 10 000 injections [46]. Vascular injection may occur despite correct epidurography, use of a blunt-ended Tuohy needle and an absence of detectable reflux during puncture. Doo noted that a CI under direct echographic guidance just behind the dorsal sacrococcygeal ligament was not associated with intravascular injection, whereas CI into the sacral canal with a 1 cm needle resulted in opacification of the local vascular network in 24% of cases [47]. A lumbar epidural hematoma (from L2 to S1 on MRA) was reported in a patient on cilostazol (antiplatelet factor and vasodilator) after the X ray- guided CI via the SH and into the canal, with a fine needle (22G), of a combination of dexamethasone, lidocaine and iodine [48]. The symptoms occurred six hours after the injection and the patient was able to undergo surgery without sequelae. All these observations indicate that the sacral canal should not be catheterized. Catheterization of the sacral canal was a characteristic common to all these cases of complications and is not in itself useful, as seen in the description of the technique. Arachnoiditis has been reported four days after the intervention; it was treated with antibiotics, with sequelae affecting the L5 root [49]. Spondylodiscitis has been reported after cortisone infiltration via the SH. It was treated with antibiotics, without sequelae [50]. We should not forget the proximity of the anus and the difficulty of disinfecting the intergluteal cleft, which should be performed attentively, in five steps. A non-exteriorized asymptomatic subcutaneous pilonidal cyst can serve as a point of entry for infection, observed during echographic control. An injection of particulate prednisolone and lidocaine via the SH was complicated by medullary infarction in one case [51]. Subsequent MRI showed an absence of disc herniation, but preexisting extensive myelitis and spinal cord attachment. Finally, two other serious complications with cauda equina syndrome symptoms and complete paralysis have recently been reported [52,53]. A patient with lumbar stenosis received an injection of triamcinolone associated with bupivacaine and hyaluronic acid, with catheterization of the sacral canal. A control MRI scan was rapidly performed, but was non-informative. The patient developed complete paraplegia in the second hour after injection. MRI showed only severe stenosis. Despite early surgery, this patient still displays a marked partial deficit one year later. Another patient was reported to have suffered medullary infarction and myelitis after the injection of 100 mg prednisolone with 30 ml of saline and lidocaine [54]. Subsequent MRI found no evidence of disc herniation, but did identify a spinal cord attachment syndrome. The patient displayed persistent complete lower limb paralysis and incontinence. Slow injection of the product might (for this and potentially also for other injection sites) make it possible to decrease the risk of these rare adverse events still further.
Nevertheless, if strict recommendations are followed (Table 2) the risk of severity remains very small for these rare complications. It is difficult to make a rigorous comparison with other techniques based on interlaminar or foraminal injections given the rarity of these incidents, but the ambulatory CI technique appears to have a particularly favorable benefit/risk ratio (Table 3).
Table 2. Technical guidelines for avoiding the rare severe complications reported after caudal injection via the SH.
| Five-stage asepsis |
|---|
| Echographic control to check for the absence of subcutaneous pilonidal cysts |
| Contraindicated for patients on anticoagulants (but not for those on antiplatelet agents alone) |
| Avoid canal catheterization: remain under the sacrum in the SH, with vertical puncture at a slightly oblique angle |
| Do not inject air, allergenic products, anesthetics or particulate products |
Table 3. Comparison of the three principal techniques for epidural injection.
| Caudal injection | Interlaminar epidural | Lumbar foraminal | |
|---|---|---|---|
| Technical means | Echography or X ray | X ray | X ray or CT scan |
| Injection site | At a distance from the epidural space and the conflict | Epidural space, at the same level as the conflict | Unilateral and close to the conflict |
| Products injected | Soluble corticosteroids, but not contraindicated for particulate products Large volume (> 20 ml) |
Soluble corticosteroid, not contraindicated for particulate products Small volume (1-2 ml) |
Soluble corticosteroids only Small volume (1-2 ml) |
| Contraindications | Anticoagulants (possible after surgery) |
Anticoagulants and antiplatelet agents Prior surgery |
Anticoagulants and antiplatelet agents Prior surgery |
| Precautions and difficulties | « 5-step » asepsis | Obesity Scoliosis and osteoarthritis Risk of dural rupture |
Obesity Scoliosis Risk of vascular injection |
| Efficacy | Similar to interlaminar injection | In the short and medium term | Possibly slightly better |
| Complications | Exceptional | Very rare | Rare |
Conclusions
Caudal injections have been performed for almost as long as interlaminar epidural injections, but they fell out of favor due to the difficulty identifying the hiatus, particularly in obese patients. They have returned to the fore thanks to echography, which has removed this obstacle, and due to the rare neurological complications reported for other injection techniques (especially for aminal) trying to reach sites as close as possible to the presumed “conflict” (whereas the radicular pathogenesis is undoubtedly attributable at least as much, if not more so, to the stretching of small vessels present at the surface of the roots, due to their acquired adhesion to diverse structures, including the discs) (54). The cases of irreversible paralysis reported have involved, in particular, the use of particulate products associated with a thrombogenic risk, and injections during spinal surgery. It appears that, provided a certain number of precautions are followed, involving careful asepsis, echographic guidance and non-catheterization of the sacral canal, CI via the SH are reliable, as effective as other types of injections and can be performed without risk of dural rupture or injection into a large periradicular artery or vein. New comparative studies with high levels of methodological rigor nevertheless remain indispensable, to improve both the efficacy and safety of this technique relative to the other procedures, and to assess the value of this route of administration for therapeutic agents other than glucocorticoids.
Conflict of interest
All authors declare that they have no competing interest.
References
- Cathelin MF. A new route of spinal injection. Method of epidural injections by the sacral canal method. Applications to humans. C. R. Soc. Biol. Paris. 53, 452 (1901).
- Sicard JA. Sacrococcygeal extradural drug injections. C. R. Soc. Biol. Paris. 53, 396-398 (1901).
- Corning JL. Local anaesthesia, 1886, New York, Appleton and Company Eds, pp,85-92.
- Bier AKG. Experiments on the cocainization of the spinal cord. Deutsche. Zeitschrift. fur. Chirurgie. 51(3-4), 361-369 (1899).
- Gunther RE, Harer WB Jr. Long-acting single-injection caudal anesthesia; 1,208 obstetrical deliveries with mepivacaine. Calif. Med. 105, 424-428 (1966).
- Stitz MY, Sommer HM. Accuracy of blind versus fluoroscopically guided caudal epidural injection. Spine. 24(13), 1371-1376 (1999).
- Barham G, Hilton A. Caudal epidurals: the accuracy of blind needle placement and the value of a confirmatory epidurogram. Eur. Spine. J. 19(9), 1479-1483 (2010).
- Price CM, Rogers PD, Prosser AS et al. Comparison of the caudal and lumbar approaches to the epidural space. Ann. Rheum. Dis. 59(11), 879-882 (2000).
- Naidoo K, Alazzawi S, Montgomery A. The use of contrast in caudal epidural injections under image intensifier guidance: is it necessary? Clin. Orthop. Surg. 9(2), 190-192 (2017).
- Blanchais A, Le Goff B, Guillot P et al. Feasibility and safety of ultrasound-guided caudal epidural glucocorticoid injections. Joint. Bone. Spine. 77(5), 440-444 (2010).
- Porzionato A, Macchi V, Parenti A et al. Surgical anatomy of the sacral hiatus for caudal access to the spinal canal. Acta. Neurochir. Suppl. 108, 1-3 (2011).
- Kao SC, Lin CS. Caudal epidural block: an updated review of anatomy and techniques. BioMed. Research. International. 2017, 1-5 (2017).
- Aggarwal A. Anatomic consideration of caudal epidural space: a cadaver study. Clin. Anat. 22(6), 730–737 (2009).
- Senoglu N, Senoglu M, Ozkan F et al. The level of termination of the dural sac by MRI and its clinical relevance in caudal epidural block in adults. Surg. Radiol. Anat. 35(7), 579–584 (2013).
- Crighton IM, Barry BP, Hobbs GJ. A study of the anatomy of the caudal space using magnetic resonance imaging. Br. J. Anaesth. 78(4), 391–395 (1997).
- Kim S, Choi Y, Park J et al. Acute paraplegia after lumbar steroid injection in patients with spinal dural arteriovenous fistulas: case reports. Ann. Rehabil. Med. 40(5), 949-954 (2016).
- Laemmel E, Segal N, Mirshahi M et al. Deleterious effects of intra-arterial administration of particulate steroids on microvascular perfusion in a mouse model. Radiology. 279(3), 731-740 (2016).
- Hazra AK, Bhattacharya D, Mukherjee S et al. Ultrasound versus fluoroscopy-guided caudal epidural steroid injection for the treatment of chronic low back pain with radiculopathy: A randomised, controlled clinical trial. Indian. J. Anaesth. 60(6), 388-392 (2016).
- Bush K, Hillier S. A controlled study of caudal epidural injections of triamcinolone plus procaine for the management of intractable sciatica. Spine. 16(5), 572-575 (1991).
- Datta R, Upadhyay KK. A randomized clinical trial of three different steroid agents for treatment of low backache through the caudal route. Med. J. Armed. Forces. India. 67(1), 25-33 (2011).
- Iversen T, Solberg TK, Romner B et al. Effect of caudal epidural steroid or saline injection in chronic lumbar radiculopathy: multicentre, blinded, randomised controlled trial. BMJ. 343, d5278 (2011).
- Manchikanti L, Singh V, Cash KA et al. Effect of fluoroscopically guided caudal epidural steroid or local anesthetic injections in the treatment of lumbar disc herniation and radiculitis: a randomized, controlled, double blind trial with a two-year follow-up. Pain. Physician. 15, 273-286 (2012).
- Murakibhavi VG, Khemka AG. Caudal epidural steroid injection: a randomized controlled trial. Evid. Based. Spine. Care. J. 2, 19-26 (2011).
- Nandi J, Chowdhery A. A randomized controlled clinical trial to determine the effectiveness of caudal epidural steroid injection in lumbosacral sciatica. J. Clin. Diagn. Res. 11, RC04-RC08 (2017).
- Sayegh FE, Kenanidis EI, Papavasiliou KA et al. Efficacy of steroid and nonsteroid caudal epidural injections for low back pain and sciatica: a prospective, randomized, double-blind clinical trial. Spine. 34(14), 1441-1447 (2009).
- Manchikanti L, Cash KA, McManus CD et al. Results of 2-year follow-up of a randomized, double-blind, controlled trial of fluoroscopic caudal epidural injections in central spinal stenosis. Pain. Physician. 15, 371-384 (2012).
- Cervera-Irimia J, Tomé-Bermejo F. Caudal epidural steroid injection in the treatment of chronic discogenic low back pain. Comparative, prospective and randomized study. Rev. Esp. Cir. Ortop. Traumatol. 57, 324-332 (2013).
- Manchikanti L, Cash KA, McManus CD et al. Fluoroscopic caudal epidural injections in managing chronic axial low back pain without disc herniation, radiculitis, or facet joint pain. J. Pain. Res. 5, 381-390 (2012).
- Manchikanti L, Singh V, Cash KA et al. Fluoroscopic caudal epidural injections in managing post lumbar surgery syndrome: two-year results of a randomized, double-blind, active-control trial. Int. J. Med. Sci. 9, 582-591 (2012).
- Kalappa S, Sridhar RB, Nagappa S. Comparing the efficacy of caudal with intravenous dexamethasone in the management of pain following lumbosacral spine surgeries: a randomized double blinded controlled study. Anesth. Essays. Res. 11(2), 416-420 (2017).
- Samagh N, Pai RK, Mathews TK et al. Pre-emptive caudal epidural analgesia with ropivacaine for lumbosacral spine surgery: A randomized case control study. J. Anaesthesiol. Clin. Pharmacol. 34, 237-241 (2018).
- Kamble PC, Sharma A, Singh V et al. Outcome of single level disc prolapse treated with transforaminal steroid versus epidural steroid versus caudal steroids. Eur. Spine. J. 25(1), 217-221 (2016).
- Manchikanti L, Singh V, Pampati V et al. Comparison of the efficacy of caudal, interlaminar, and transforaminal epidural injections in managing lumbar disc herniation: is one method superior to the other? Korean. J. Pain. 28(1), 11-21 (2015).
- Pandey RA. Efficacy of epidural steroid injection in management of lumbar prolapsed intervertebral disc: a comparison of caudal, transforaminal and interlaminar routes. J. Clin. Diagn. Res. 10, RC05-RC11 (2016).
- Karamouzian S, Ebrahimi-Nejad A, Shahsavarani S et al. Comparison of two methods of epidural steroid injection in the treatment of recurrent lumbar disc herniation. Asian. Spine. J. 8(5), 646-652 (2014).
- Singh S, Kumar S, Chahal G et al. Selective nerve root blocks vs. caudal epidural injection for single level prolapsed lumbar intervertebral disc - A prospective randomized study. J. Clin. Orthop. Trauma. 8(2), 142-147 (2017).
- Lee JH, Shin KH, Bahk SJ et al. Comparison of clinical efficacy of transforaminal and caudal epidural steroid injection in lumbar and lumbosacral disc herniation: A systematic review and meta-analysis. Spine. J. 18(12), 2343-2353 (2018).
- Liu J, Zhou H, Lu L et al. The effectiveness of transforaminal versus caudal routes for epidural steroid injections in managing lumbosacral radicular pain: a systematic review and meta-analysis. Medicine (Baltimore). 95, e3373 (2016).
- Kaydu A, Kılıç ET, Gökçek E et al. Unexpected complication after caudal epidural steroid injection: hiccup. Anesth. Essays. Res. 11(3), 776-777 (2017).
- Kaydu A, Kiliç ET, Gökçek E et al. Anosmia after caudal epidural steroid injection. Anesth. Essays. Res. 12(1), 291-293 (2018).
- Lee SH, Park JW, Hwang BM. Anaphylactic shock following nonionic contrast medium during caudal epidural injection. Korean. J. Pain. 28, 280-283 (2015).
- Fujikawa T, Murai S. Portal venous gas after a caudal block. BMJ. Case. Rep. 23 (2014).
- Lee MH, Han CS, Lee SH et al. Motor weakness after caudal epidural injection using the air-acceptance test. Korean. J. Pain. 26, 286-290 (2013).
- Dere K, Akbas M, Bicerer E et al. A complication during caudal steroid injection. J. Back. Musculoskelet. Rehabil. 22(4), 227-229 (2009).
- Kim SG, Yang JY, Kim DW et al. Inadvertent dural puncture during caudal approach by the introducer needle for epidural adhesiolysis caused by anatomical variation. Korean. J. Pain. 26(2), 203-206 (2013).
- Manchikanti L, Malla Y, Wargo BW et al. A prospective evaluation of complications of 10,000 fluoroscopically directed epidural injections. Pain. Physician. 15(2), 131-140 (2012).
- Doo AR, Kim JW, Lee JH et al. A comparison of two techniques for ultrasound-guided caudal injection: the influence of the depth of the inserted needle on caudal block. Korean. J. Pain. 28(2),122-128 (2015).
- Choi JJ, Chang YJ, Jung WS et al. Discordant lumbar epidural hematoma after caudal steroid injection: A case report (CARE-compliant). Medicine (Baltimore). 96(24), e7127 (2007).
- Nanjayan SK, Swamy GN, Yallappa S et al. Arachnoiditis following caudal epidural injections for the lumbo-sacral radicular pain. Asian. Spine. J. 7(4), 355-358 (2013).
- Yue WM, Tan SB. Distant skip level discitis and vertebral osteomyelitis after caudal epidural injection: a case report of a rare complication of epidural injections. Spine. 28(11), E209-E211 (2003).
- Somanchi BV, Mohammad S, Ross R. An unusual complication following caudal epidural steroid injection: a case report. Acta. Orthop. Belg. 74, 720-722 (2008).
- Seo YT, Kong HH, Lee GJ et al. Persistent cauda equina syndrome after caudal epidural injection under severe spinal stenosis: a case report. J. Pain. Res. 10, 1425-1429 (2017).
- Wang G, Liang J, Jia Z et al. Spinal cord infarction caused by sacral canal epidural steroid injection. A case report. Medicine (Baltimore). 97(11), e0111 (2018).
- Berthelot JM, Laredo JD, Darrieutort-Laffite C et al. Stretching of roots contributes to the pathophysiology of radiculopathies. Joint. Bone. Spine. 85(1), 41-45 (2018).