Research Article - Diabetes Management (2021)
Treatment patterns for chronic venous disease and diabetes mellitus: lessons from an international market-research survey
- Corresponding Author:
- Giacomo Gastaldi
Département de médecine, Service de diabétologie, endocrinologie, nutrition et éducation thérapeutique du patient, HUG, 1211 Geneva, Switzerland
E-mail: giacomo.gastaldi@hcuge.ch
Abstract
Objective: The enormous burden of chronic venous disease among the general population is often overlooked, especially in the context of multi-morbidity. The aim of this study was to assess comorbidity of the pathologically-related conditions chronic venous disease and diabetes mellitus (DM).
Methods: Data were derived from a quantitative market-research survey of physicians in Brazil, Mexico, Turkey, Bulgaria, Switzerland and Egypt (June-July 2019). Generalists and specialist with 3–30 years in practice; ≥ 10 chronic venous insufficiency (CVI) patients per week; and ≥ 1 CVI patient receiving pharmacotherapy were eligible. Interviews assessed practice details, patient population and anonymised data from one patient each treated for CVI, CVI and DM, and CVI and DM with microvascular complications (DmVC).
Results: Over half of patients (53%) with CVI had comorbid DM. DM was likely to be diagnosed first followed by CVI (mean gap 5.3 years) and finally DmVC (mean gap 3.4 years). Patients being treated for CVI alone were diagnosed at an earlier CEAP stage (C2: 34.5%) than those being treated CVI+DM (C2: 24.8%) or CVI+DmVC (C2: 17.8%). A large minority (25.0– 29.3%) of patients self–medicated when presenting with CVI symptoms. Compression with pharmacotherapy was the most common initial treatment in all groups (39.3–42.3%). Most patients in all groups received initial diagnosis and treatment by non–specialists (54.4– 55.4%).
Conclusion: There is a substantial unmet therapeutic need in CVI patients and those with comorbid DM are more likely to be untreated for longer. Earlier specialist intervention may improve care in this population.
Keywords
■ chronic venous disease ■ chronic venous insufficiency ■ diabetes mellitus ■ micro vascular complications ■ diabetic retinopathy ■ diabetic nephropathy ■ diabetic peripheral neuropathy ■ comorbidity complications ■ venoactive drugs
Introduction
Chronic venous disease is a widespread disorder; with a worldwide prevalence of 83.6% in adults over 50 (mean age 50.6 ± 16.9 years). The majority of those classified with the lowest severity of the condition are men. Higher severity is more common amongst women, although sex does not appear to be a risk factor for the most severe disease categories [1]. Chronic venous disease involving persistent morphological or functional abnormalities of the venous system, which shares a number of diagnostic features and risk factors with diabetes mellitus (DM) [2-4]. The recently revised Clinical, Etiological, Anatomical, and Pathophysiological (CEAP) classification system defines the following levels of severity of chronic venous disease: C0: no visible or palpable signs of venous disease; C1: telangiectasiae or reticular veins; C2: varicose veins; C3: edema; C4a: pigmentation and/ or eczema; C4b: lipodermatosclerosis and/or atrophie blanche (white scar tissue); C4c: corona phlebectatica; C5: healed venous ulcer; and C6: active venous ulcer. Classifications highlighting recurrence of both varicose veins (C2r) and venous ulcers (C6r), and simplification of anatomical classifications using common abbreviations are also now present in the updated guidelines [4,5].
Chronic venous disease presents with a variety of symptoms concentrated around the lower limbs including pain, (aching/throbbing/tenderness) and feelings of tightness or heaviness in the legs [2,6]. It is a progressive condition and ambulatory venous pressure will increase over time, leading to deterioration in signs and symptoms in close to half of individuals [3,7]. Chronic venous disease shares a number of metabolic risk factors with cardiovascular disease in particular diabetes mellitus (DM), excess weight, hypertension and age [8-10].
Patients with DM are at increased risk of both the appearance and progression of chronic venous disease. This increased risk is likely driven by a shared pathophysiology driven by haemodynamic abnormalities within the lower limbs, including: vascular wall remodelling and increased vascular permeability; impaired blood flow; increased oxidative stress; vascular inflammation; and endothelial dysfunction [8,9,11-17]. These features are reflected in shared symptoms, including oedema and leg ulcers [11,13,18], and may point towards potential overlap in treatment strategies [8,9]. The prevalence of these symptoms vary with leg ulcers, for example, substantially more likely to be due to venous insufficiency than diabetes [19,20]. In addition, assessment of alternatively aetiologies is key, for example, lower limb oedema, which is present in chronic venous disease of C3 and above (described as chronic venous insufficiency [CVI]) must be carefully assessed in patients with DM in order to rule out the presence of heart failure with preserved ejection fraction (HFpEF) [4, 21-26].
Currently, there is a paucity of literature exploring the shared features of DM and chronic venous disease and the consequent implications for disease management, although this has recently been explored in two review papers [8, 9]. The aim of this study was to address the paucity of data on comorbid chronic venous disease and DM by investigating the diagnostic and therapeutic pathways of patients treated for the two conditions using a dataset from an international market–research study which investigated the clinical features of patients currently being treated for CVI and DM.
Methodology
Data were derived from a quantitative survey (Supplementary material: S1) designed and developed by a market research company (Axess Research, Lyon, France) and conducted in six countries. Both computer aided web interviews (Brazil, Mexico, Turkey, Bulgaria and Switzerland) and face to face interviews (Egypt) with physicians were conducted. The survey was designed based on available literature and qualitative investigations which highlighted the burden of comorbid conditions in patients treated for chronic venous disease [11, 18, 27, 28].
Structured interviews were conducted with physicians between June and July 2019. Generalists and specialist were targeted at a geographically appropriate ratio. Generalists were internists and general practitioners, and specialists were angiologists/phlebologists, cardiologists, cardiovascular surgeons, general surgeons, and gynaecologists. Physicians were eligible if they had ≥ 3 and ≤ 30 years of practice; a minimum number of CVI patients ≥ 10 per week (reduced from ≥ 15 for generalist and from ≥ 25 for specialists flowing initial recruitment); were responsible for choosing and prescribing CVI treatment; and were treating ≥ 1 CVI patient with a pharmacotherapy. CVI was not defined within the survey, however, CEAP classification at diagnosis/treatment was requested (C1–C6; Supplementary materials S1). Physicians were excluded if they did not treat ≥ 1 patient for both comorbid CVI and DM and ≥ 1 patient for comorbid CVI and DM with microvascular complications (DmVC; defined in the survey as: diabetic retinopathy [DR], diabetic nephropathy [DN], and diabetic peripheral neuropathy [DPN]; Supplementary materials S1).
The structured interviews were split into a screening section determining eligibility and focusing on the physician’s practice details and the overall number of patients of interest being treated (Supplementary materials S1). The eligible physicians then provided details, using their patient records, of one patient treated for CVI, one patient treated for both CVI and DM, and finally one patient under treatment for CVI and DmVC. Data on patient demographics, clinical characteristics, and treatment pathways were collected.
Results
■Overall patient characteristics
Characteristics of recruited physicians are shown in supplementary materials (TABLE S1). In total, physicians estimated that 30% of patients seen per week had CVI, making up 22% of patients seen by generalists and 43% of patients seen by specialists. Amongst specialists, angiologists/ phlebologists saw the greatest proportion of patients with CVI per week (63%), with the lowest proportion seen by cardiologists (24%). Patients with comorbid CVI and DM made up 16.2% of all patients seen per week, comprising 14.1% of patients seen by generalist and 18.7% of those seen by specialists.
Physicians reported that over half of patients (53%) with CVI had comorbid DM, with a higher prevalence of comorbidity in patients seen by generalists (63%) than by specialists (44%). Patients with comorbid CVI and DmVC comprised 7.2% of all patients seen per week, making up 8.2% of patients seen by generalists and 9.7% of those seen by specialists. One third of patients (33%) with CVI had comorbid DmVC, with a higher prevalence of this comorbidity in patients seen by generalists (40%) than by specialists (27%)(TABLE S2).
■ Patients treated for CVI
Patient records from individuals being treated for CVI showed the majority of patients were female (67.3%), mean age was 55.8 years old and body mass index (BMI) was 30.3 kg/m2. Comorbid DM (52%), hypertension (59.7%) and obesity/ overweight (65.7%), were common (TABLE 1).
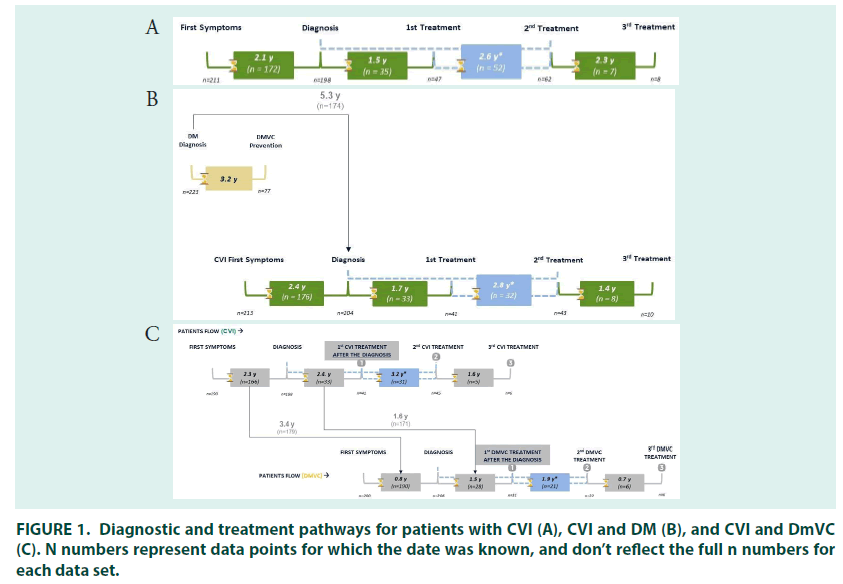
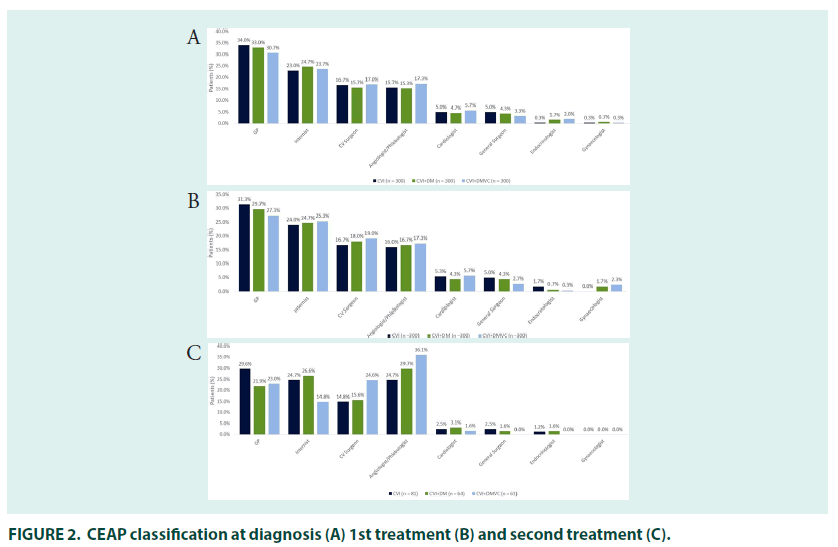
In those people treated for CVI, the mean time between presentation of symptoms and diagnosis was 2.1 years (FIGURE 1). The majority of patients were diagnosed by general practitioners (34.0%), followed by internists (23%). Cardiovascular surgeons were the most common specialists to diagnose CVI (16.7%), followed by angiologists/phlebologists (15.7%), cardiologists (5.0%), general surgeons (5.0%), endocrinologists (0.3%) and gynaecologists (0.3%).
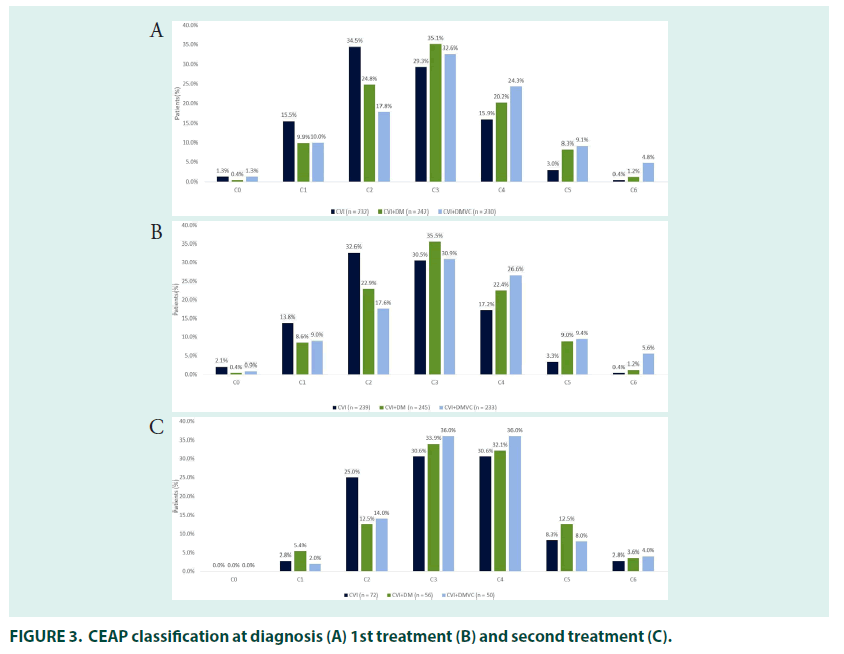
Most patients had either varicose veins (34.5%; CEAP classification C2) or oedema without skin changes (29.3%; C3), at diagnosis. Around one third (29.3%) of patients self–medicated for CVI when their symptoms appeared, and most patients (79.0%) received treatment for their CVI at diagnosis. The mean time to receiving treatment was 1.5 years in patients who were untreated at diagnosis.
First–line treatment prescribers followed the pattern of diagnosis, with the majority of patients treated by general practitioners (31.3%), and followed by internists (24.0%).
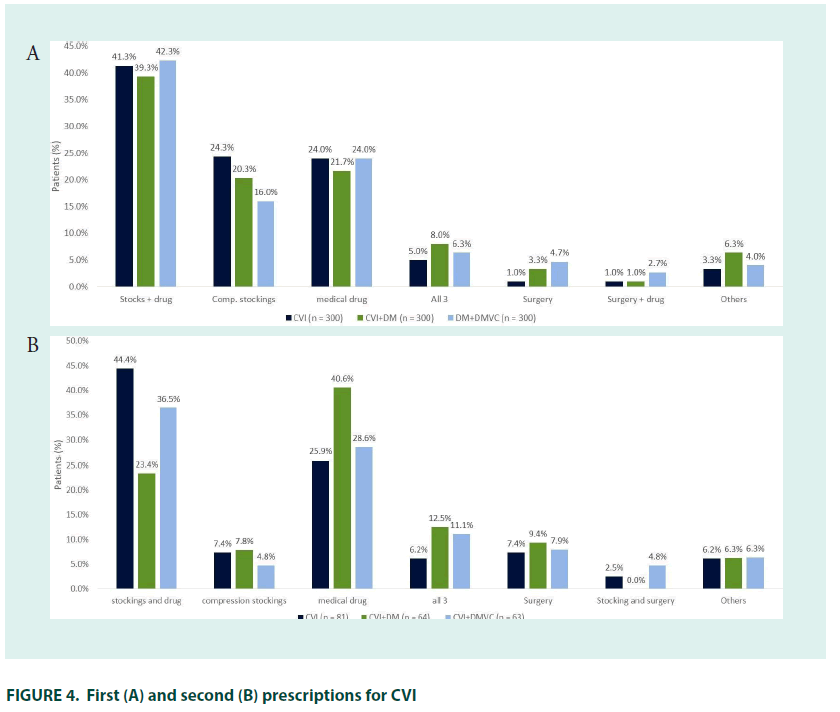
Cardiovascular surgeons were the most common specialist prescribers (16.7%), followed by angiologists/phlebologists (16.0%), cardiologists (5.3%), general surgeons (5.0%), and endocrinologists (1.7%) (FIGURE 2). CEAP classification was similar to that seen at diagnosis. The most common initial treatment was pharmacotherapy with compression stockings (41.3%), followed by compression stockings (24.3%) or pharmacotherapy alone (24.0%); 7.0% of patients were treated with surgery either alone or in combination with other therapies.
The 27% (n=81) of patients who received second line treatment did so 2.6 years after their initial treatment prescription, most had oedema with skin changes (30.6%; C3) or with skin/ subcutaneous changes (30.6%; C4) (FIGURE 3). General practitioners (29.6%) remained the most common prescribers, followed by interns and angiologists/phlebologists (both 24.7%), prescriptions by cardiologist (2.5%), general surgeons (2.5%) and gynaecologists (1.2%) were relatively uncommon.
Combined pharmacological therapy and compression stockings remained the most common therapy (44.4%), while use of stockings alone was less common and use of surgical interventions increased.
■ Patients treated for CVI and DM
Patient records from individuals being treated for CVI and DM showed the majority of patients were female (62.0%), mean age was 60.7 years old and BMI was 30.5 kg/m2. Comorbid hypertension (80.0%) and obesity/overweight (72.3%), depression (20.7%), and PAD (15.6%) were common (TABLE 1).
| Patient characteristic | CVD treated (n=300) | CVD and DM treated | CVD and DmVC treated | Total (n=900) |
|---|---|---|---|---|
| Age, mean (range) years | 55.8 (25–99) | 60.7 (30–100) | 64.8 (35–90) | 60.4 (25–100) |
| Female, n (%) | 202 (67.3) | 186 (62.0) | 141 (47.0) | 529 (58.8) |
| BMI, mean (range) kg/m2 | 30.3 (18–50) | 30.5 (19–50) | 30.5 (17–50) | 30.4 (17–50) |
| Overweight/obesity, n (%) | 197 (65.7) | 216 (72.3) | 215 (71.7) | 629 (69.9) |
| HbA1C, mean % (range) | 7.0 (4.9–20) | 7.6 (4.0–14) | 8.2 (4.9–15.4) | 7.6 (4.0–20.0) |
| Total cholesterol, mean g/L (range) | 2.6 (0.9–7.5) | 2.4 (0.6–5.5) | 2.6 (0.7–8.0) | 2.5 (0.6–8.0) |
| Blood pressure, mean systolic/diastolic mm/Hg | ||||
| 137/83 | 141/85 | 146/87 | 141/85 | |
| Creatinine (µmol/L), mean (range) | 103.3 (44.2–265.2) | 105.2 (53.0–247.5) | 148.7 (44.0–1326.0) | 119.6 (44.0–1326.0) |
| CVI ( ≥ C3) at diagnosis | 133 (48.7) | 157 (64.9) | 163 (70.9) | 453 (64.3) |
| Hypertension | 179 (59.7) | 240 (80.0) | 254 (84.7) | 675 (75) |
| Diabetes | 157 (52.3) | 300 (100) | 300 (100) | 757 (84.1) |
| Depression | 42 (14.0) | 62 (20.7) | 77 (25.6) | 181 (20.1) |
| PAD | 28 (9.3) | 47 (15.6) | 98 (32.6) | 173 (19.2) |
| Cardiomyopathy | 25 (8.3) | 34 (11.3) | 63 (21.0) | 122 (13.6) |
| Other comorbidity | 9 (3.0) | 16 (5.3) | 19 (6.3) | 44 (4.8) |
| No comorbidity | 39 (13.0) | 22 (7.3) | 17 (5.6) | 79 (8.8) |
TABLE 1. Characteristics of patients being treated for CVI; CVI and DM; and CVI and DmVC.
In people being treated for both DM and CVI, there was a mean gap of 2.4–years between the first symptoms of CVI and diagnosis. DM diagnosis occurred before (78.7%) or in the same year (10.3%) as diagnosis of CVI in most patients, with a mean gap between the two diagnoses of 5.3 years. The majority of patients had their CVI diagnosed by general practitioners (33.0%), followed by internists (24.7%), cardiovascular surgeons (15.7%), angiologists/ phlebologists (15.3%), general surgeons (4.7%) and cardiologists (4.3%). Most patients had either varicose veins (24.8%; CEAP classification C2) or oedema without skin changes at diagnosis (35.1%; C3).
A quarter of patients (25.0%) being treated for DM and CVI self–medicated when symptoms of CVI first appeared, and 77.7% of patients received treatment when they were first diagnosed. The mean time to receiving treatment was 1.7 years in patients who were untreated at diagnosis. The majority of patients were prescribed their first CVI treatment by general practitioners (29.7%), followed by internists (24.7%), cardiovascular surgeons (18.0%), angiologists/phlebologists (16.7%), general surgeons (4.3%), cardiologists (4.3%), gynaecologists (1.7%), and endocrinologists (0.7%). CEAP classification was in line with that seen at diagnosis (FIGURE 3).
The most common initial treatment was combined pharmacotherapy with compression stockings (39.3%), followed by pharmacotherapy (20.3%) or compression stockings (20.3%) alone. Surgery alongside pharmacotherapy and stockings was received by 8.0% of patients (FIGURE 4). The 21.3% (n=64) of patients who received second–line treatment did so 2.8 years after their initial treatment prescription, most had oedema with skin changes (33.9%; C3) or with skin/subcutaneous changes (32.1%; C4). Angiologists/phlebologists (29.7%), and internists (26.6%) were the most common prescribers, followed by general practitioners (21.9%) cardiovascular surgeons (15.6%), cardiologists (3.1%), endocrinologists and gynaecologists (both 1.6%).
Pharmacological therapy alone was the most common therapy (40.6%), followed by combined pharmacotherapy and stockings (23.4%); 12.5% of patients received surgery alongside stockings and pharmacotherapy, use of stockings alone was less common than at first–line. In total, 41.1% of patients received preventative therapy for DmVC. The mean time between preventative DM diagnosis and DmVC preventative therapy was 3.2 years.
■ Patients treated for CVI and DmVC
Patient records from individuals being treated for CVI and DmVC showed the majority of patients were male (53.0%), mean age was 64.8 years old and BMI was 30.5 kg/m2. Comorbid hypertension (84.7%), obesity/ overweight (71.7%), and depression (25.6%), were common. As were PAD (32.6%) and cardiomyopathy (21.0%) (TABLE 1). DPN (75.7%) was the most common form of DmVC, followed by DN (50%), and DR. Close to half of the patients had co-morbid DPN and DN (46.4%), 37.3% of patients had DR and DPN, 35.3% of patients had DN and DR, and 28.7% of patients had all three issues.
In patients treated for CVI and DmVC, the mean time between presentation with symptoms of CVI and diagnosis was 2.3 years and the mean time between presentation with symptoms of DmVC and diagnosis was 0.8 years. Presentation with symptoms of CVI occurred before (65.5%) or in the same year (19.5%) as symptoms of DmVC in most patients, with a mean time between CVI and DmVC symptom presentation of 3.4 years. CVI diagnosis occurred before (53.3%) or in the same year (29.7%) as diagnosis of DmVC in most patients, with a mean gap between the two diagnoses of 1.7 year. The majority of patients with CVI were diagnosed by general practitioners (30.7%), followed by internists (23.7%). Angiologist/phlebologists (17.3%) and cardiovascular surgeons (17.0%) were the most common specialists to diagnose CVI, followed by cardiologists (5.7%), general surgeons (3.3%), endocrinologists (2.0%) and gynaecologist (0.3%). Most patients had either oedema without skin changes (32.6%; CEAP classification C3) or with skin/subcutaneous changes (24.3%; C4) at diagnosis.
Just over a quarter of patients (27.7%) with CVI self–medicated when symptoms first appeared, and 73.0% of patients received treatment when they were first diagnosed. The mean time to receiving CVI treatment was 2.4 years in patients who were untreated at diagnosis. Prescriber patterns and CEAP classification at time of first treatment were roughly in line with that at diagnosis. The most common initial treatment was pharmacotherapy with compression stockings (42.3%), followed by pharmacotherapy (24.0%) and compression stockings alone (16.0%). The 21.0% (n=63) of patients who received second–line treatment did so 3.2 years after their initial treatment, most had oedema with skin changes (36.0%; C3) or with skin/subcutaneous changes (36.0%; C4). Angiologists/phlebologists (36.1%) and cardiovascular surgeons (24.6%) were the most common prescribers, followed by general practitioners (23.0%), internists (14.8%) and cardiologists (1.6%). Combined compression stocking and pharmacological therapy was the most common therapy (36.5%), followed by pharmacological therapy alone (28.6%) and combined pharmaco/compression therapy and surgery (11.1%). As with other groups, use of compression stockings alone was rare at second– line.
Discussion
In this quantitative cross–sectional market– research derived data set which investigated patients currently under treatment for CVI, patients being treated for CVI were majority female, with a high burden of comorbid obesity/ overweight, hypertension, and diabetes. Patients being treated for DM and CVI were marginally more likely to be male than those treated for CVI alone (43% vs 48%, respectively), with slightly higher rates of hypertension, obesity/overweight, as well as higher rates of depression and PAD. There was a higher proportion of men being treated for CVI and DmVC (54%), as well as increased rates of hypertension (85%), PAD (33%), cardiomyopathy (21%), and depression (26%).
More than half of the patients being treated for CVI, had comorbid DM. These conditions share a number of risk factors such as increasing age, lifestyle factors including poor nutrition and lack of exercise, as well as excess weight or obesity [11]. Previous clinical studies have demonstrated a higher prevalence of diabetes in patients with chronic venous disease/CVI (11–30%,) than that seen in the general population (approximately 9%) [11,18,27,28]. The substantially higher results seen in our population, are likely in part due to inclusion criteria, however, they are in line with those seen in a Chinese study which investigated clinical characteristics of patients with early chronic venous disease (C0–C1) and at least one cardiometabolic risk factor [29]. There was a high prevalence of diabetes in patients with early chronic venous disease and this was significantly higher in female patients with early-stage chronic venous disease compared with controls (72.2% versus 50.3%, P=0.0001; 63.1% versus 66.4%, P=0.470) [29].
In our data set, patients being treated for CVI or CVI and DM were more likely to be female, however, there was a higher prevalence of male sex in those being treated for CVI and DmVC. This may be explained by higher incidence of microvascular complications in men with diabetes [30]. In a large (N=2,636) retrospective study of patients with CVI presence of comorbid PAD or metabolic disease significantly correlated with the development of advanced venous insufficiency (P<0.0001 and P=0.02, respectively) (28). Similarly, in our data set PAD was substantially higher in the CVI and DmVC treated population who also had the most advanced stages of CVI.
In patients being treated for both CVI and DM, our data suggests that DM is likely to be diagnosed first followed by CVI and finally DmVC. These data are supported by the high prevalence of diabetes found in patients with early stage chronic venous disease by Zong et al. [29]. Patients being treated for CVI waited approximately 2 years for a diagnosis following presentation of symptoms, with similar waits in those receiving additional treatment for comorbid DM and DmVC. Those patients being treated for CVI alone were more likely to be diagnosed at an early CEAP stage than those being treated for comorbid DM or DmVC, with more than half (51%) of patients being diagnosed at CEAP ≤ 2, compared with 35% and 29% for the DM and DmVC groups, respectively. These results are in line with previous data showing that patients with DM exhibit more severe forms of CVI than patients with CVI alone [11,29,31]. It is worth noting that over 50% of the CVI– treated group in our study also had DM so there may be an even more stark difference in CVI stage between those with CVI only and comorbid CVI/DM patients.
The presence of chronic venous disease characteristics, including microcirculation disorder in the lower limbs, high BMI, arterial hypertension and endothelial dysfunction, in patients with type 2 DM makes these patient particularly susceptible to more sever forms of chronic venous disease [32,33]. Patients being treated for DM and DmVC had more advanced chronic venous disease, even though there was no substantial difference in time between CVI- symptom presentation and diagnosis between the three groups. This suggests that the diagnosis of chronic venous disease at a later stage in the two DM groups may be driven by patients presenting with symptoms at a later disease stage. There is an overlapping symptomology between diabetes and CVI with oedema and trophic changes in the lower limbs particularly common in patients with type 2 DM [13]. In addition, patients with multiple comorbidities and polypharmacy may well put their symptoms of CVI down to the presence of other conditions or side effects from therapy. Despite symptoms of venous disease being a common feature in patients with diabetes, they are often overlooked and are rarely discussed in clinical appointments [8,12]. Targeted efforts towards patient education on signs of chronic venous disease may help patients recognize symptoms earlier and encourage them to seek medical advice.
Between 25 and 30% of patients self– medicated for CVI at the point of presenting with symptoms, suggesting a substantial unmet therapeutic need in this population. Although there was a substantial gap between presentation of symptoms and diagnosis, the majority of patients in all groups received treatment at diagnosis. Combined compression and pharmacotherapy was the most commonly prescribed initial treatment in all groups, in line with the current guidance which suggests both these therapeutic approaches have a place at all stages of chronic venous disease [34]. The slightly lower rates of compression-therapy use in the groups being treated for DM and DmVC may have been driven by higher rates of cardiomyopathy and PAD as caution is advised when using compression therapy in patients with cardiac failure/PAD [34,35].
Some pharmacotherapies such as calcium dobesilate have efficacy in multiple micro- vascular damage-related diseases including chronic venous disease, DR, and DN, with significant improvements demonstrated following treatment of patients and in animal models [36].
However, we did not see substantial differences in the use of pharmacotherapy in patients being treated for both CVI and DmVC in our study population, suggesting such dual efficacy is not be utilised [37-42]. Surgical interventions were relatively rare initially, despite the high prevalence of patients with chronic venous disease of C2 and above, where surgery is recommended as a potential therapeutic approach [8,34].
The majority of patients in all groups received initial diagnosis and treatment by non– specialists (general practitioners/internists). We believe this may be indicative of a potential unmet need for early intervention by a specialist in chronic venous disease. In a large systematic review comparing specialist and generalist care in various conditions, outcomes were better in patients receiving care by specialists [43]. While a German study of chronic venous disease, showed that patients referred to specialists by general practitioners were older and had a more advanced stage of disease than those who self- referred. Additionally, there was little difference in the number of patients who did not go on to be diagnosed with CVI in the two groups, suggesting a limited benefit from generalists acting as gate keepers [44]. In patients who received a second therapeutic intervention treatment by specialists was more common, particularly in those patients receiving therapy for CVI and DmVC. As would be expected, disease was more advanced in this subgroup, and surgical interventions increased. Prescriptions involving compression therapy dropped, particularly in those patients receiving treatment for CVI and DM, however, this may reflect the fact that many patients had already been prescribed compression therapy.
As previously noted, our statements regarding prevalence of comorbidities in this population should be interpreted with significant caution given the nature of the dataset. Nevertheless, we believe these data are robust enough to act as a call to action regarding a substantial unmet treatment burden driven by late diagnosis and late presentation of symptoms in the above patient groups. Other limitations included, differences in the data provided in each patient group which retarded our ability to compare data sets on aspects such as diagnosis of diabetes, or preventative therapy for DmVC. Selection of patient records was left to the discretion of the physician allowing potential for bias. In addition, the research was conducted on physicians with a high number of patients with chronic venous disease, which likely skewed the patient population. Exclusion of physicians who were not treating at least one patient with pharmacotherapy may have similarly affected the patient population. There were substantial reporting gaps for some patient and treatment characteristics and physicians were asked to report on patients treated for CVI rather than chronic venous disease which may have skewed the data towards more serious disease.
There is an enormous burden of chronic venous disease among the general population. Patients tend to minimize the importance of chronic venous disease, particularly during initial stages, and the signs and symptoms are often overlooked by physicians during appointments especially in the context of multi-morbidity. Efforts should be made towards screening for chronic venous disease in patients with DM and vice versa, in particular signs of CVI like oedema must be addressed as they may mask other serious conditions like HFpEF. While the coexistence of DM and CVI was frequent in our cohort, the gap between diagnosis of DM and CVI was short, hinting at a common pathophysiological pathway rather than one being the consequence of the other. Indeed, vascular complications of DM are typically seen after a period of at least 5 to 10 years from DM onset. Venous damage or progression of CVI is usually worse in patients living with DM. Hence the treatment of patients with both diseases should be more aggressive, and dual action treatments considered, with a more intensive follow-up advised.
Conclusion
There is a substantial unmet therapeutic need in CVI patients and those with comorbid DM are more likely to be untreated for longer. Earlier specialist intervention may improve care in this population. There is a substantial unmet therapeutic need in CVI patients with comorbid DM and earlier intervention from a multidisciplinary team of specialists may improve care in this population.
Acknowledgement
The quantitative market research survey was designed and funded by Vifor Pharma. Editorial and writing support, provided by Ewen Legg, PhD, of Halcyon Medical Writing Ltd., was funded by Vifor Pharma.
Conflicts of interest
JRS, DL, FG, JLS, AKB, JRG, SS: Have no conflicts of interest to disclose; MS: Honoraria/ payment from Servier and Laboratoire Innotech; GG: Educational grants from Lilly, NovoNordisk, Sanofi, Dexcom, Roche, Medtronic, Insulet, Abbott; Honoraria/payment from OM Pharma and NovoNordisk; Board participation for Ascensia.
Financial support
Study funded by Vifor Pharma.
References
- Rabe E, Guex JJ, Puskas A, et al. Epidemiology of chronic venous disorders in geographically diverse populations: results from the vein consult program. Int Angiol. 31(2), 105-115 (2012).
- Barros SB, Kakkos SK, Maeseneer DM, et al. Chronic venous disease: from symptoms to microcirculation. Int Angiol. 38(3), 211-218 (2019).
- Lee BB, Nicolaides AN, Myers K, et al. Venous hemodynamic changes in lower limb venous disease: the UIP consensus according to scientific evidence. Int Angiol. 35(3), 236-352 (2016).
- Eklof B, Perrin M, Delis KT, et al. Updated terminology of chronic venous disorders: the VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg. 49(2), 498-501 (2009).
- Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 8(3), 342-352 (2020).
- Santler B, Goerge T. Chronic venous insufficiency-a review of pathophysiology, diagnosis, and treatment. J Dtsch Dermatol Ges. 15(5), 538-556 (2017).
- Raffetto JD. Pathophysiology of chronic venous disease and venous ulcers. Surg Clin North Am. 98(2), 337-347 (2018).
- Pannier F. Chronic venous disease and diabetic microangiopathy. Part 2: A review of commonalities. (2018).
- Gastaldi G. Chronic venous disease and diabetic microangiopathy. Part 1: Background and pathophysiology. (2018).
- Auzky O, Lanska V, Pitha J, et al. Association between symptoms of chronic venous disease in the lower extremities and cardiovascular risk factors in middle-aged women. Int Angiol. 30(4), 335-341 (2011).
- Fejfarová V, Roztočil K, Svědínková A, et al. The relationship between chronic venous insufficiency and diabetes mellitus. Int Angiol. 36(1), 90-91 (2017).
- Mani R, Yarde S, Edmonds M. Prevalence of deep venous incompetence and microvascular abnormalities in patients with diabetes mellitus. Int J Low Extrem Wounds. 10(2), 75-79 (2011).
- Shlyakova AA, Strongin LG, Kudykin MN, et al. Clinical and pathogenetic features of lesions of the lower extremities in patients with type 2 diabetes mellitus and chronic venous insufficiency. Diabetes Mellitus. 19, 212-220 (2016).
- Rajendran P, Rengarajan T, Thangavel J, et al. The vascular endothelium and human diseases. Int J Biol Sci. 9(10), 1057-1069 (2013).
- Barrett EJ, Liu Z, Khamaisi M, et al. Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab. 102(12), 4343-4410 (2017).
- Domingueti CP, Dusse LM, Carvalho M, et al. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 30(4), 738-745 (2016).
- Bergan JJ, Schönbein SGW, Smith PD, et al. Chronic venous disease. N Engl J Med. 355(5), 488-498 (2006).
- Florea I, Stoica LE, Tolea I. Chronic venous insufficiency-clinical-evolutional aspects. Curr Health Sci J. 37 (1):21-25 (2011).
- Körber A, Klode J, Al-Benna S, et al. Etiology of chronic leg ulcers in 31,619 patients in Germany analyzed by an expert survey. J Dtsch Dermatol Ges. 9(2), 116-121 (2011).
- Moffatt CJ, Franks PJ, Doherty DC, et al. Prevalence of leg ulceration in a London population. QJM. 97(7), 431-437 (2004).
- Yeboah J, Bertoni A, Qureshi W, et al. Pedal edema as an indicator of early heart failure in the community. Circ Heart Fail. 9(12), e003415 (2016).
- Kenny H, Abel E. Heart failure in type 2 diabetes mellitus: impact of glucose- lowering agents, heart failure therapies, and novel therapeutic strategies. Circ Res. 124, 121-141 (2019).
- Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. Circulation. 119(14), e391-e479 (2009).
- Redfield MM, Jacobsen SJ, Burnett JC, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 289(2), 194-202 (2003).
- Loehr LR, Rosamond WD, Chang PP, et al. Heart failure incidence and survival (from the atherosclerosis risk in communities study). Am J Cardiol. 101(7), 1016-1022 (2008).
- McHugh K, DeVore AD, Wu J, et al. heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art Review. J Am Coll Cardiol. 73(5), 602-611 (2019).
- Çevik ZG. Patients with chronic venous insufficiency: treatment modalites of venous impairment and its complications during two years period in primary care center. OALib. 2(8), 1-9 (2015).
- Reka IE, Imre M. Socio-demographic Characteristics of patients diagnosed with advanced chronic venous insufficiency (c4-c6) correlated with clinical and para-clinical findings. Acta Med Marisiensis. 61(2), 94 (2015).
- Zhong J, Chen J, Zhao ZG, et al. Diabetes mellitus is associated with early chronic venous disorder of the lower extremities in Chinese patients with cardiometabolic risk factors. Diabetes Metab Res Rev. 30(6), 505-512 (2014).
- An J, Nichols GA, Qian L, et al. Prevalence and incidence of microvascular and macrovascular complications over 15 years among patients with incident type 2 diabetes. BMJ Open Diabetes Res Care. 9(1), e001847 (2021).
- Agus GB, Jawien A, Carelli F. Nautilus survey on chronic venous diseases. Panminerva Med. 52(2 Suppl 1), 5-9 (2010).
- Matic M, Matic A, Djuran V, et al. Frequency of peripheral arterial disease in patients with chronic venous insufficiency. Iran Red Crescent Med J. 18(1), e20781-e (2016).
- Ferrara F, Ferrara G. Sclerotherapy in the patient with diabetes: indications and results. Phlebolymphology. 19, 193-198 (2012).
- Nicolaides A, Kakkos S, Baekgaard N, et al. Management of chronic venous disorders of the lower limbs. guidelines according to scientific evidence. Part I. Int Angiol. 37(3), 181-254 (2018).
- Wittens C, Davies AH, Bækgaard N, et al. Editor's choice - management of chronic venous disease: clinical practice guidelines of the european society for vascular surgery (ESVS). Eur J Vasc Endovasc Surg. 49(6), 678-737 (2015).
- Liu J, Li S, Sun D. Calcium dobesilate and micro-vascular diseases. Life sciences. 221, 348-353 (2019).
- Zhang X, Liu W, Wu S, et al. Calcium dobesilate for diabetic retinopathy: a systematic review and meta-analysis. Sci China Life Sci. 58(1), 101-107 (2015).
- Rabe E, Jaeger KA, Bulitta M, et al. Calcium dobesilate in patients suffering from chronic venous insufficiency: a double-blind, placebo-controlled, clinical trial. Phlebology. 26(4), 162-168 (2011).
- Zhou Y, Qi C, Li S, et al. Diabetic nephropathy can be treated with calcium dobesilate by alleviating the chronic inflammatory state and improving endothelial cell function. Cell Physiol Biochem. 51(3), 1119-1133 (2018).
- Qin L, Qin W, Wang J, et al. Combined treatment of diabetic nephropathy with alprostadil and calcium dobesilate. Exp Ther Med. 14(5), 5012-5016 (2017).
- Haller H, Ji L, Stahl K, et al. Molecular mechanisms and treatment strategies in diabetic nephropathy: new avenues for calcium dobesilate-free radical scavenger and growth factor inhibition. Biomed Res Int. 1909258 (2017).
- Han K, Liu C, Shi X, et al. Effects of alprostadil combined with calcium dobesilate in patients with diabetic peripheral neuropathy. Neuro Endocrinol Lett. 39(2), 143-147 (2018).
- Smetana GW, Landon BE, Bindman AB, et al. A comparison of outcomes resulting from generalist vs specialist care for a single discrete medical condition: a systematic review and methodologic critique. Arch Intern Med. 167(1), 10-20 (2007).
- Fischer T, Hähnel A, Jordan M, et al. [Referral by a general practitioner versus self-referral to a specialist practice. A comparison using chronic venous insufficiency as an example]. Dtsch Med Wochenschr (1946). 128(43), 2242-2247 (2003).