Review Article - Interventional Cardiology (2015) Volume 7, Issue 1
Transradial peripheral vascular intervention: challenges and opportunities
- Corresponding Author:
- Alexander G Truesdell
PinnacleHealth CardioVascular Institute, 111 South Front Street
Harrisburg, PA, USA
Tel: +1 717 731 0101
Fax: +1 717 731 8359
E-mail: atruesdell@yahoo.com
Abstract
Endovascular therapy for the interventional treatment of peripheral vascular disease is becoming increasingly prevalent. Radial access is replacing femoral access for coronary intervention owing to its superior safety profile. These parallel advances forecast a future paradigm shift to routine transradial peripheral vascular intervention. This review highlights the technical challenges preventing immediate adoption of transradial peripheral vascular intervention, describes the technical aspects of the procedure in different anatomic beds utilizing currently available equipment, details the procedural, morbidity, and possible mortality benefits inherent to transradial access, reviews the available scientific evidence, and recommends a framework for successful transition to a radial strategy for peripheral vascular intervention.
Keywords
Angiography, endovascular intervention, peripheral vascular disease, transradial, vascular access, vascular complications
Peripheral vascular disease is a major source of morbidity and mortality worldwide [1,2]. It is present in up to 20% of persons over age 70, with symptomatic disease affecting nearly 6% of adults over age 60 [3,4]. Alongside efforts to increase public awareness and reduce modifiable risk factors, there have been significant advances in the safety and efficacy of endovascular therapy for peripheral vascular disease. Endovascular intervention is increasingly becoming first-line therapy for the invasive management of vascular disease [4–10]. Due to parallel advances in transradial (TR) techniques for percutaneous coronary intervention (PCI), radial access is replacing femoral access as the dominant approach to PCI in many parts of the world [11,12]. Although high-quality evidence to support transradial peripheral vascular intervention (TRPVI) as a first-line strategy is currently lacking, two decades of TR coronary advances recommend a similar paradigm shift for peripheral vascular intervention (PVI).
The coronary experience
TR access began in earnest following initial reports of radial coronary angiography by Campeau in 1989 and PCI by Kiemeneij in 1992 [13,14]. During the decades since, compared with femoral access, radial artery (RA) access has consistently demonstrated statistically significant reductions in bleeding and access site complications regardless of the clinical condition, patient population or anticoagulation status, despite significant parallel reductions in the incidence of transfemoral (TF) vascular complications [15–28]. These benefits directly translate into decreased morbidity and possibly mortality, particularly in high-risk patient subgroups such as the elderly, obese and those with severe peripheral arterial disease [15,19,20,23,28–42].
The RIVAL, RIFLE-STEACS and STEMI-RADIAL studies, an analysis of 2007–2011 US National Cardiovascular Data Registry data, and several recent metaanalyses all demonstrated both morbidity and mortality benefits to radial access in patients with ST-segment elevation acute coronary syndrome (ACS), most likely as a result of lower rates of bleeding and vascular complications with the TR approach [36,38,40,41,43–45]. A 2014 analysis by Iqbal et al. of 10,095 patients with non-ST-segment elevation myocardial infarction also demonstrated an association between TR access and reduced bleeding, access site complications and all-cause mortality in this population [42]. Most recently, an observational analysis of a nonselected cohort of nearly 350,000 patients between 2006 and 2011 from the British Cardiovascular Intervention Society database demonstrated TR access to be independently associated with reduced 30-day mortality for both ACS and non-ACS populations [46]. Despite the absence of a definitive, adequately powered, multicenter, randomized control trial testing radial versus femoral access with contemporary medications and techniques in ST-elevation ACS, non-STelevation ACS and non-ACS patients to directly assess mortality benefits based on access site alone, the weight of current evidence suggests a statistically significant mortality benefit to TR access for PCI.
Additional practical advantages repeatedly identified in coronary studies are: earlier ambulation, shortened length of stay, reduced resource and staff use, decreased hospital costs and increased patient comfort and satisfaction [19,20,28,30,35,36,38–40,47–49]. As a result, current transatlantic practice guidelines increasingly recommend the RA as the preferred access site for PCI [34,50].
Into the periphery
The performance of peripheral interventions via the RA is designed to apply the numerous benefits of radial access strategies to noncoronary procedures. The proven reduction in bleeding and vascular complications with radial access is particularly pronounced in patients with significant peripheral arterial disease [12,15,17,19,21,51,52]. Absent femoral pulses, bilateral iliac artery disease, severe vessel calcification or tortuosity, coexistent aortic aneurysms or dissections or previous bilateral iliac stenting or aortobifemoral surgical reconstruction all complicate femoral access and intervention strategies, whether for coronary or vascular intervention [34,51,53]. Most of these technical difficulties are obviated by the radial approach.
Procedural advantages
For subclavian, innominate, renal, mesenteric, celiac and some carotid interventions (such as a right internal carotid artery in the presence of a type III or severely diseased aortic arch or a left internal carotid artery arising from the innominate artery), there are anatomic advantages to RA access due to more favorable angles of approach, better sheath and guide support and more coaxial vessel alignment [51,53,54].
Although brachial and axillary access strategies are feasible alternatives to femoral access, they are associated with higher complication rates (as high as 36% in some series) compared with radial access, especially among occasional operators [21,52,55].
Radial access additionally eliminates the need for postprocedure mechanical compression of the femoral arteriotomy, thereby reducing attendant risks of lower extremity ischemia and thrombosis [54]. From a technical standpoint, there is no significant loss of catheter steerability or pushability from the RA compared with the femoral approach. Overall, TRPVI demonstrates at least similar efficacy with improved safety compared with the TF approach [39,56,57].
Limitations
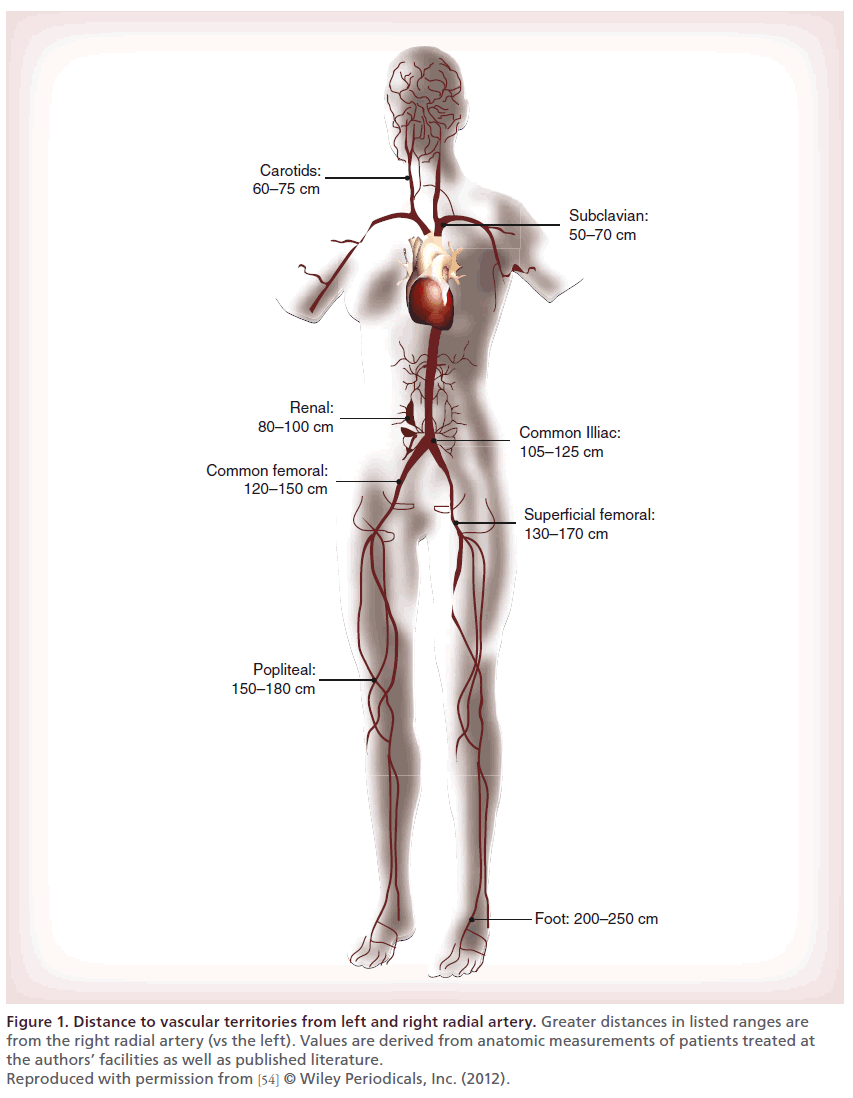
Wholesale adoption of radial access for peripheral intervention has been limited primarily by three major technical issues: the smaller diameter of the RA; radial, brachial, subclavian and aortic tortuosity; and most significantly, the extended distance to the target vessel. Ultrasound, radiographic and anatomic studies demonstrate a range of RA diameters, primarily from 2 to 4 mm, averaging approximately 2.4 mm in women and 2.6 mm in men [12,58–62]. Since the RA can typically expand beyond its resting diameter, most patients can accommodate a 6 French (Fr) radial sheath (outer diameter 2.6–2.9 mm) [61]. Fewer patients’ vessels can routinely accept a 7 Fr sheath, often required for more advanced peripheral equipment, such as atherectomy or thrombectomy devices, cutting balloons and covered stents [63,64]. In highly selected patients, up to 8 Fr sheaths can sometimes be utilized [61,65,66]. In addition to these size constraints, current equipment length limitations also make it impossible to routinely perform comprehensive selective angiography and intervention below the inguinal ligament (Table 1 & Figure 1) [12].
| Company | Product | Guidewire (in) | Sheath (Fr) | Diameter (mm) | Shaft length (cm) |
|---|---|---|---|---|---|
| PTA balloons | |||||
| Abbott | Armada 14 | 0.014 | 4 | 1.5–4 | 150 |
| Viatrac | 0.014 | 4–5 | 4–7 | 135 | |
| Armada 35 | 0.035 | 5–7 | 3–14 | 135 | |
| Bard | Ultraverse | 0.014–0.018 | 4–6 | 1.5–9 | 150–200 |
| Vascutrak | 0.018 | 5–7 | 4–7 | 140 | |
| Dorado | 0.035 | 5–6 | 3–10 | 135 | |
| Boston Scientific | Coyote | 0.014 | 4 | 1.5–4 | 150 |
| Sterling | 0.018 | 4 | 2–4 | 150 | |
| Mustang | 0.035 | 5–7 | 4–7 | 135 | |
| Cook | Advance Micro | 0.014 | 3 | 1.5–3 | 150 |
| Advance 14 | 0.014 | 4 | 2–4 | 170 | |
| Advance 18–35 | 0.018–0.035 | 4–7 | 3–12 | 135 | |
| Cordis | Sleek | 0.014 | 4 | 1.25–5 | 150 |
| Aviator | 0.014 | 4–5 | 4–7 | 142 | |
| Savvy | 0.018 | 4–5 | 2–6 | 150 | |
| Covidien | NanoCross | 0.014 | 4 | 1.5–4 | 150 |
| PowerCross | 0.018 | 4–6 | 2–6 | 150 | |
| EverCross | 0.035 | 5–7 | 3–12 | 135 | |
| Medtronic | Amphirion | 0.014 | 4 | 1.5–4 | 150 |
| Pacific | 0.018 | 4–5 | 2–7 | 180 | |
| Admiral | 0.035 | 5–7 | 3–12 | 130 | |
| Cutting, scoring or specialty balloons | |||||
| Boston Scientific | Flextome | 0.014 | 137 | ||
| Spectranetics | AngioSculpt | 0.014 –0.018 | 5–6 | 2–6 | 137 |
| TriReme | Chocolate | 0.014–0.018 | 5–6 | 2.5–6 | 120–135 |
| Self-expanding stents | |||||
| Abbott | AccuLink | 0.014 | 6 | 5–10 | 135 |
| Xact | 0.014 | 6 | 5–10 | 135 | |
| Xpert | 0.018 | 4–5 | 3–8 | 135 | |
| Supera | 0.018 | 4.5–6.5 | 6–7 | 120 | |
| Absolute | 0.035 | 6 | 6–10 | 135 | |
| Bard | LifeStent | 0.035 | 6 | 6–10 | 135 |
| E-Luminex | 0.035 | 6 | 4–14 | 135 | |
| Boston Scientific | WallStent | 0.014 | 6 | 6–10 | 135 |
| Epic | 0.035 | 6 | 6–12 | 120 | |
| Cook | Zilver | 0.018–0.035 | 6 | 6–10 | 125 |
| Cordis | Smart | 0.035 | 6 | 6–10 | 120 |
| Covidien | Protege | 0.014 | 6 | 6–10 | 135 |
| EverFlex | 0.035 | 6 | 6–8 | 120 | |
| Medtronic | Complete | 0.035 | 6 | 4–10 | 130 |
| Gore | Viabahn† | 0.014–0.035 | 6–12 | 5–13 | 120 |
| Balloon-expandable stents | |||||
| Abbott | Herculink | 0.014 | 5 | 4–7 | 135 |
| Omnilink | 0.035 | 6–7 | 6–10 | 135 | |
| Atrium | iCast† | 0.035 | 6–7 | 5–12 | 120 |
| Bard | Valeo | 0.035 | 6–7 | 6–10 | 120 |
| Boston Scientific | Express SD | 0.018 | 5–6 | 4–7 | 150 |
| Express LD | 0.035 | 6–7 | 6–10 | 135 | |
| Cook | Formula | 0.014–0.018 | 5–6 | 4–6 | 135 |
| Cordis | Palmaz | 0.018–0.035 | 4–7 | 3–10 | 135 |
| Covidien | VisiPro | 0.018 | 6–7 | 5–10 | 135 |
| Medtronic | Racer | 0.014–0.018 | 5–6 | 4–7 | 130 |
| Assurant | 0.035 | 6 | 6–10 | 130 | |
| Atherectomy devices | |||||
| Bayer Medrad | JetStream | 0.014 | 7 | 1.6–3.4§ | 120–145 |
| Covidien | TurboHawk | 0.014 | 6–8 | 1.5–7# | 104–145 |
| CSI | Stealth360 | 0.014 | 4–6 | 1.25–2‡ | 145 |
| Spectranetics | Turbo Elite Laser | 0.014–0.035 | 4–8 | 1.4–3.8# | 112–150 |
| CTO crossing and re-entry devices | |||||
| Bard | Crosser | 0.014 | 5 | N/A | 146–154 |
| Boston Scientific | TruePath | 0.018 | 4 | N/A | 165 |
| OffRoad | 0.035 | 6 | N/A | 100 | |
| Cordis | FrontRunner | N/A | 5 | N/A | 140 |
| Outback | 0.014 | 6 | N/A | 140 | |
| Covidien | Viance | 0.014 | 5 | N/A | 150 |
| Enteer | 0.014–0.018 | 5 | N/A | 135–150 | |
| Medtronic | Pioneer | 0.014 | 6 | N/A | 120 |
| Filters and embolic protection | |||||
| Abbott | Emboshield Nav6 | 0.014 | 5 | N/A | 135 |
| AccuNet | 0.014 | 6 | N/A | 145 | |
| Boston Scientific | FilterWire | 0.014 | 4 | N/A | 300 |
| Cordis | AngioGuard | 0.014 | 4 | N/A | 135 |
| Covidien | SpiderFX | 0.014 | 4 | N/A | 320 |
| Medtronic | FiberNet | 0.014 | 6–7 | N/A | 150 |
| MoMa | 0.035 | 9 | N/A | 95 | |
#Treatable vessel diameter.
CTO: Chronic total occlusion; Fr: French; N/A: Not applicable or not available; PTA: Percutaneous transluminal angioplasty.
Table 1. Currently available equipment for transradial peripheral vascular intervention
Figure 1. Distance to vascular territories from left and right radial artery. Greater distances in listed ranges are from the right radial artery (vs the left). Values are derived from anatomic measurements of patients treated at the authors’ facilities as well as published literature. Reproduced with permission from [54] © Wiley Periodicals, Inc. (2012).
Concerns
TR access has been associated with increased patient and operator radiation exposure, contrast use and procedural times compared with TF access in the coronary literature, particularly among less experienced operators [12,49,67]. When performing peripheral interventions, in addition to coronary radiation dose reduction strategies (such as positioning the radial access site close to the ipsilateral groin), lower frame rates and road map and mask functions may be used to reduce patient and operator radiation dose. Left radial access may also reduce radiation exposure compared with right radial access [68–70]. However, TR procedure time and radiation exposure is most influenced by operator experience, decreases with training and practice and approximates TF exposure among practiced operators [37,71]. In the end, any differences must be balanced against the parallel reductions in access site complications and bleeding.
Early transradial coronary trials demonstrated a consistently higher rate of access failure with radial versus femoral access (7.3 vs 2.0%) while later studies noted procedural failure and crossover rates of less than 5% [12,15,34,72]. More recent registries reveal crossover rates below 2% (and near 1% for dedicated radialists utilizing modern techniques and equipment), consistent with historical data for femoral access [34,72–74].
Radial compared with femoral access also demonstrates very low and equivalent risks of neurologic complications and silent cerebral microembolization (0.11% for both radial and femoral access in a recent retrospective analysis of 370,328 coronary procedures) [75,76]. Event rates are also equivalent for left versus right radial access (0.11 vs 0.08%) despite greater aortic arch traversal and sometimes increased catheter manipulation from the right radial artery [19,75,76].
Data
Overall there are limited data and no large multicenter randomized studies evaluating TRPVI. Only one randomized trial for carotid intervention has been published to date [77]. Several observational studies, feasibility studies, technical reports, case reports, case series and single-center registries have demonstrated successful TR intervention for carotid, vertebral, subclavian, innominate, renal, iliac, celiac, mesenteric and superficial femoral artery (SFA) disease (Table 2) [12,56–58,78–101]. The existing evidence base for TRPVI is further limited by the potential for literature bias, as very few studies have been published worldwide, most with very small numbers of patients, and all with positive outcomes.
| Study | Design | Population (n) | Vascular territory | Procedural success (n [%]) | TR to TF crossover (n [%]) | Reason for failure or crossover (n) | Major access site complication (n [%]) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Scheinert et al. (2001) | Observational, | 18 TR | Renal | 18(100) | 0 | N/A | 0 | [89] | |
| prospective | |||||||||
| Galli et al. (2002) | Observational, | 25 TR† | Renal | 27(100) | 0 | N/A | 0 | [90] | |
| prospective | |||||||||
| Folmar et al. (2007) | Observational, | 42 TR | Carotid | 35(83) | 7(17) | Inadequate | 0 | [78] | |
| prospective | catheter support | ||||||||
| Pinter et al. (2007) | Observational, | 20 TR | Carotid | 18(90)‡ | N/A | N/A | 0 | [94] | |
| retrospective | |||||||||
| Sanghvi et al. (2008) | Observational, | 15 TR | Iliac and SFA | 14(93) | 1(7) | Inadequate stent | 0 | [83] | |
| retrospective | shaft length | ||||||||
| Patel et al. (2009) | Observational, | 47 TR | Vertebral and | 47(100) | 0 | N/A | 0 | [79] | |
| prospective | basilar | ||||||||
| Trani et al. (2009) | Observational, | 12 TR 12 TF | SFA | 12(100) | 0 | N/A | 0 | [84] | |
| prospective | 12(100) | ||||||||
| Staniloae et al. (2010) | Observational, | 27 TR 41 TF§ | Aortoiliac | 29(88) TR | 3(11) | Subclavian | 0 | TR 0 TF | [57] |
| retrospective, | 46(98) TF | tortuosity (1); | |||||||
| matched | failure to cross (2)## | ||||||||
| Yu et al. (2010) | Observational, | 14 TR | Subclavian | 13(93)¶ | N/A | Failure to cross | 0 | [91] | |
| retrospective | lesion | ||||||||
| Alli et al (2011) | Observational, | 11 TR 44 TF | Renal | 11(100) TR | 1(9) | Insufficient guide | 0 | TR 3(7) TF | [98] |
| retrospective, | 44(100) TF | length | |||||||
| matched | |||||||||
| territory | success (n [%]) | crossover (n [%]) | or crossover (n) | complication (n [%]) | |||||
| Lorenzoni et al. (2011) | Observational, | 25 TR†† | Suprainguinal | 26(81)§§ | N/A | N/A | 0 | [95] | |
| prospective | and | ||||||||
| Infrainguinal‡‡ | |||||||||
| Etxegoien et al. (2012) | Observational, | 382 TR | Carotid | 347(91)♯## | 35(9) | Inadequate | 0 | [96] | |
| retrospective, | catheter support | ||||||||
| multicenter | |||||||||
| Cortese et al. (2014) | Observational, | 149 TR | Iliac | 147(99) | 19(13) | Failure to cross | 0 | [97] | |
| prospective, | lesion | ||||||||
| multicenter | |||||||||
| Coscas et al. (2014) | Observational, | 24 TR | Suprainguinal | 20(91) | 1(4) | Failed radial | 2(10)††† | [86] | |
| retrospective | and | puncture (1); | |||||||
| Infrainguinal¶¶ | failure to cross | ||||||||
| lesion | |||||||||
| Ruzsa et al. (2014) | Randomized, | 130 TR 130 TF | Carotid | 130(100) TR | 13(10)‡‡‡ | Failed puncture | 1(1) TR§§§ 1(1) TF | [77] | |
| prospective, | 130(100) TF | (2), radial | |||||||
| multicenter | spasm (1); radial | ||||||||
| artery loop (1); subclavian stenosis (1); subclavian tortuosity (1); cannulation difficulty (6 | |||||||||
| territory | success (n [%]) | crossover (n [%]) | or crossover (n) | complication (n [%]) | |||||
| Shinozaki et al. (2014) | Observational, | 30 TR | Iliac | 30(100) | 0 | N/A | 0 | [93] | |
| prospective | |||||||||
| Lorenzoni et al. (2014) | Observational, | 110 TR### | Suprainguinal | 154(91) | N/A | N/A | 0 | [58] | |
| prospective | and | ||||||||
| Infrainguinal | |||||||||
| Ruzsa et al. (2014) | Observational, | 27 TR | Renal | 27(100) | 0 | N/A | 0 | [101] | |
| prospective | |||||||||
‡Radial spasm prevented sheath passage in one patient, carotid artery could not be cannulated in one patient.
§80 lesions in 74 patients.
#Two TR failures were successful via TF access.
¶One total occlusion could not be crossed by either the radial or femoral approach.
††32 lesions in 25 patients.
‡‡32 target lesions, 16 suprainguinal (6 common iliac, 10 external iliac), 16 infrainguinal (13 superficial femoral, 2 common femoral, 1 popliteal).
§§81% success for suprainguinal lesions, 81% success in infrainguinal lesions.
##93% success right carotid, 88% success bovine left carotid, 88% success standard left carotid.
¶¶38 target lesions, 16 suprainguinal (iliac), 22 infrainguinal (2 common femoral, 17 superficial femoral, 3 popliteal).
†††Radial artery rupture.
‡‡‡TF to TR crossover rate 1.5% (2 iliac artery stenoses).
§§§One symptomatic radial artery occlusion.
###170 lesions in 110 patients.
N/A: Not applicable or not available; SFA: Superficial femoral artery; TF: Transfemoral access; TR: Transradial access.
Table 2. Major studies analyzing the feasibility, safety and efficacy of transradial peripheral vascular intervention.
Preprocedural evaluation
Preprocedural patient evaluation is critical for successful TR intervention [12]. Ideal patients are less than 70 years of age and have an easily palpable radial pulse. The Allen’s, Barbeau (utilizing plethysmography) and ‘reverse’ Allen’s and Barbeau tests are sometimes performed to confirm dual circulation to the hand through the palmar arch [102–104]. These tests are less common outside of the USA and controversy exists regarding the clinical utility of routine testing prior to TR intervention [37,102–106]. Hand ischemia due to periprocedural radial artery occlusion (RAO) or RA harvesting for use as a bypass graft rarely occurs even with an abnormal Allen’s or Barbeau test owing to recruitment of interosseus collaterals [104–106]. Based on the weight of available evidence, the authors do not routinely perform Allen’s or Barbeau tests prior to TR angiography or intervention.
Vascular access
Radial access can be obtained either via a modified Seldinger anterior wall puncture or a standard Seldinger posterior wall puncture. We routinely utilize the former approach in our laboratories, although some experts recommend the latter as a simpler, more reliable technique for less experienced radial operators [107,108]. For poorly palpable radial pulses, a small volume of local anesthetic mixed with nitroglycerine may be injected subcutaneously at the arteriotomy site to promote arterial dilation and improve vessel palpation and access success [12,109,110]. The authors presently use ≤1 ml of a solution of 1% Lidocaine (5–7 ml total volume) admixed with 200–500 μg of nitroglycerine (100 μg/ml concentration). The RA may also be compressed distal to the site of arterial access to improve palpation [108]. Ultrasound-guided access has additionally been shown to improve rates of first-attempt success, reduce arterial trauma and decrease vessel spasm, while also providing helpful real-time anatomic information regarding RA diameter [111].
The ideal arteriotomy site is located approximately 2–3 cm proximal to the radial styloid. More cranial access may be obtained when additional catheter length is required, albeit at the risk of complicating post-procedure hemostasis. More distal, the RA is smaller in diameter, more tortuous and concealed beneath the flexor retinaculum, making access more challenging. Radial-specific sheaths are advised where available, as their progressive tapering and lubricated coating facilitate insertion and reduce rates of spasm compared with standard femoral sheaths [34,112].
Tortuosity/variant anatomy
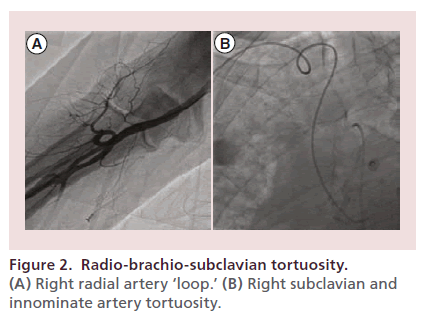
Variant anatomy or tortuosity of the radiobrachial axis, axillary-subclavian axis or the aortic arch significantly impacts the likelihood of procedural success or failure [62,113–116]. Radial ‘loops,’ high-brachial or axillary origin of the RA, hypoplastic or accessory RA or combinations thereof occur in up to 10% of cases and are more common in older, hypertensive patients (Figure 2) [62,115,117]. While atherosclerosis of the radial artery occurs in up to 20% of subjects, multiple ultrasound, angiographic, surgical and anatomic studies demonstrate clinically relevant stenosis that impacts radial artery blood flow or the success of transradial intervention to be rare [73,118–120]. In a recent review of 2211 consecutive radial interventions, a stenotic or hypoplastic radial artery was noted in 1.7 and 7.7% of subjects, respectively, with procedural success rates of 91.9 and 93.9% [120]. Overall, contemporary crossover rates for elective transradial PCI range between 1 and 2%, are primarily due to radio-brachio-subclavian tortuosity or vasospasm, and have only rarely been attributed to atherosclerosis of the radial or brachial artery [34,73,118–120].
Radial artery spasm
RA spasm is another common reason for TR procedural failure and occurs more commonly with bulkier and longer peripheral sheaths. Predictors of vasospasm are: older age, short stature, female sex, diabetes, low body mass index, small wrist circumference and radial sheath to RA ratio of < 1:1 [61,112,121,122]. The RA adventitia is also widely invested with α-adrenoreceptors, making it particularly reactive to local trauma and circulating catecholamines [12,123,124]. So repeated arteriotomy attempts, fear, anxiety and pain routinely contribute to clinically relevant spasm [61,112,121,122].
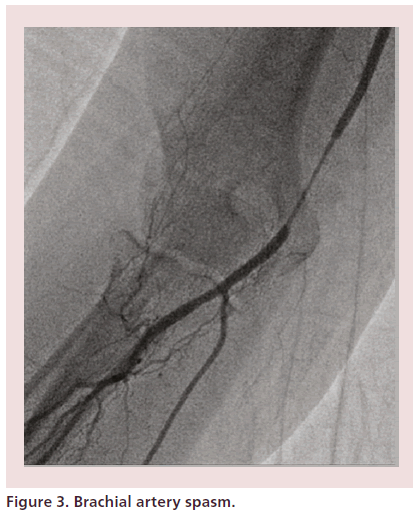
Successful antispasm strategies include generous patient sedation, small diameter hydrophilic sheaths and spasmolytic cocktails [34,112,125–128]. Prophylactic intra-arterial administration of agents known to reduce vascular tone, such as calcium channel blockers (most commonly 2–5 mg of verapamil or 200–500 mcg of nicardipine) and nitrates is critical to limit spasm, increase RA diameter, facilitate larger equipment insertion and improve procedural success [125,129,130]. When spasm does occur, further analgesia, sedation and spasmolytic therapy must be administered immediately. Untreated, severe spasm may prevent catheter advancement and manipulation or result in catheter entrapment (Figure 3) [34]. With contemporary equipment and spasm prevention strategies, the incidence of clinically relevant spasm has decreased to less than 1% [130].
Antithrombotic therapy
Anticoagulation is required for TR angiography in order to reduce the risk of RAO [12,132]. Current expert consensus recommends administration of intra-arterial or intravenous unfractionated heparin at a dose of 50–70 μ/kg for diagnostic angiography [37]. Interventional doses are similar to those recommended for femoral access [50].
Diagnostic angiography
Following successful RA access, a 4 or 5 Fr diagnostic catheter, typically the Judkins right (JR), multipurpose (MP) or vertebral, is advanced over a 0.035 inch wire into the aortic arch. If any tactile resistance is encountered, angiography is performed to delineate the radio-brachio-subclavian anatomy. At the subclavian artery and beyond, fluoroscopy should always be employed to avoid injury to thoracic and abdominal branch vessels. Severe vessel tortuosity at any level between the entry site and the target vessel can usually be overcome using a hydrophilic steerable 0.035, 0.018 or 0.014 inch wire. If difficulty is encountered entering the descending aorta, a JR catheter may be used in the left anterior oblique view to direct a 0.035 inch stiff-angled hydrophilic polymer-coated wire from the subclavian artery.
Aorto-iliac angiography is performed by power injection of the distal abdominal aorta through a straight 125 cm diagnostic pigtail catheter. Selective diagnostic angiography is then performed using a 125 cm JR, MP or vertebral diagnostic catheter or a 150 cm length 0.035 inch support catheter. Until longer length catheters enter production, infrailiac angiography may be more effectively performed from the left RA utilizing a high radial puncture to provide up to 15–20 cm of additional length compared with distal right radial access [12].
Peripheral intervention
Radial, and occasionally brachial, artery angiography should routinely be performed prior to consideration of TRPVI owing to the larger size of some peripheral interventional equipment. During diagnostic angiography, the distance from the RA access site to the iliac bifurcation or target vessel should also be measured to further determine a patient’s eligibility for TR intervention.
Several expert operators advocate performing TRPVI via the left RA to avoid innominate artery tortuosity, catheter manipulation in the aortic arch and to provide additional length [54,108]. However, as with TR coronary procedures, the right-sided approach affords easier access, increased operator and patient comfort, is our preferred approach and in our opinion will likely become the community standard in the future [11,12].
After navigating upper extremity and thoracic tortuosity, the initial hydrophilic wire should be exchanged for a stiff nonhydrophilic 0.035 inch wire for improved support for sheath advancement toward the target vessel. Afterward, the procedure progresses as from the femoral approach.
Adapting a technique utilized for endovascular aortic aneurysm repair, in the absence of radial-specific vascular sheaths for peripheral intervention, the authors apply sterile mineral oil to the external sheath surface as a lubricant to ease the passage of nonhydrophilic sheaths through the radial and brachial artery [133]. For some interventions, newly commercially available hydrophilic sheathless guide catheters with large internal diameters may also be feasible.
In the event of vessel spasm limiting catheter or sheath advancement, liberal doses of intra-arterial vasodilators should be administered. The balloonassisted tracking (BAT) technique, whereby a coronary balloon is partially protruded from the distal end of a guide catheter over a 0.014 inch angioplasty wire and inflated to low pressure, may also be used to facilitate atraumatic catheter passage through tortuous or spastic radial or brachial arteries (Figure 4) [116,117,131]. Until longer peripheral wires and more monorail equipment becomes available, when 300 cm length wires are inadequate for crossing catheter and balloon exchanges in the periphery, the ‘jet exchange’ hydraulic extraction technique is recommended, whereby continuous hydrostatic force is applied to the wire by injecting saline through the lumen of the catheter or balloon to maintain wire position as the catheter or balloon is withdrawn [134,135].
Subclavian & innominate intervention
Upper extremity arterial disease is well suited to ipsilateral TR intervention. Success rates are high and complication rates low compared with the TF approach [82,91]. TR sheaths overall offer improved guide support for ostial lesions and chronic total occlusions (CTO) [54]. Other advantages are reduced guide manipulation in the aortic arch and reduced contrast use. Radial access may sometimes be more challenging due to a poorly palpable radial pulse with severe proximal subclavian stenosis and necessitate ultrasound guidance for successful first-attempt radial access.
A 2010 single-center retrospective review by Yu et al. described 14 cases of subclavian artery stenting using radial access, with procedural success in 13 cases (93%) and no neurologic or access site complications [91]. The single failure in this small series was a CTO that could not be crossed from either the femoral or radial approach [91].
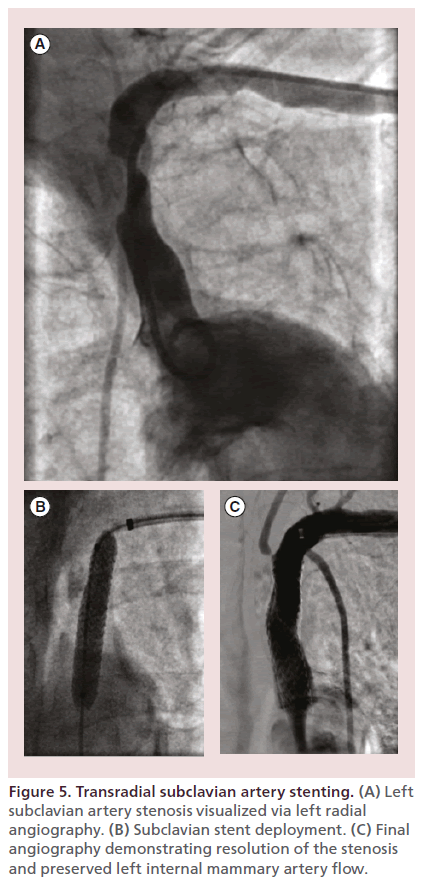
Figure 5 illustrates a case of successful transradial left subclavian artery stenting in a 68-year-old woman with high-grade left subclavian artery stenosis and subclavian steal syndrome scheduled to undergo left internal mammary artery (LIMA) to left anterior descending coronary artery bypass surgery.
Figure 5. Transradial subclavian artery stenting. (A) Left subclavian artery stenosis visualized via left radial angiography. (B) Subclavian stent deployment. (C) Final angiography demonstrating resolution of the stenosis and preserved left internal mammary artery flow.
Carotid artery intervention
Carotid artery stenting (CAS) via the radial approach has been demonstrated to be safe and effective in several small feasibility studies and one multicenter prospective randomized study [77,78,80,136]. The TR approach is most useful in patients with right internal carotid artery lesions, complex arch anatomy or severe aorto-iliac tortuosity or disease [54,78,137].
Etxegoien et al. published the largest series of CAS via RA access in 2012, a retrospective analysis of 382 patients, demonstrating a 91% success rate (93% for right carotid lesions and 88% for both standard and ‘bovine’ left carotid lesions) [96]. Inadequate sheath support was the cause of failure in the unsuccessful cases [96]. There were no bleeding complications [96]. Major and minor strokes occurred in 0.6 and 1.0% of patients, respectively [96]. More recently, in 2014, Ruzsa et al. published the only multicenter prospective randomized study of TRPVI, comparing TR and TF access in 260 consecutive patients undergoing CAS [77]. Procedural success was 100%, with a crossover rate of 10% in the TR group (due to failed puncture, RA spasm, RA and subclavian tortuosity, subclavian stenosis or severe carotid angulation) and 1.5% in the TF group (due to iliac artery stenosis) [77]. Major access site complications occurred in one patient (0.9%) in both the TR and TF arms [77]. Procedure and fluoroscopy times were comparable, but radiation dose was significantly higher in the TR group [77].
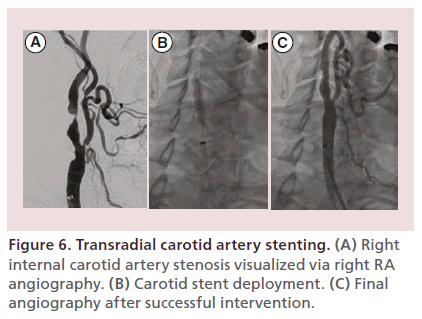
Figure 6 demonstrates a successful case of right internal CAS via the right radial artery in a 64-yearold man with symptomatic high-grade right internal carotid artery stenosis.
Figure 6. Transradial carotid artery stenting. (A) Right internal carotid artery stenosis visualized via right RA angiography. (B) Carotid stent deployment. (C) Final angiography after successful intervention.
Vertebrobasilar intervention
Ipsilateral TR access for vertebral artery intervention is technically easier than the femoral approach and thus preferable to TF intervention [79]. As with subclavian and carotid intervention via the RA, access site complications are also nearly absent.
A feasibility study by Patel et al. in 2009 demonstrated a 100% success rate in 42 vertebral artery and 5 basilar artery interventions [79]. There were no bleeding complications [79]. Transient periprocedural stroke occurred in three patients (6%) and fatal intracranial hemorrhage occurred in one patient (2%), comparable to rates from TF access in historical studies [138].
Renal, celiac & mesenteric intervention
Renal, celiac and mesenteric arteries are ideal for TR intervention due to their downward oriented take-off from the abdominal aorta and typically aorto-ostial disease, maximizing coaxial cannulation and improving guide support from above [56,85,101,139–141]. In concert with the ‘no-touch’ technique (whereby a 0.035 inch J wire is directed caudally from the tip of the guide catheter to prevent contact with the aortic wall, while a 0.014 inch wire inserted through the same guide is used to cannulate the renal artery), TR renal artery intervention also significantly reduces traumatic vessel intubation [142].
Recently, clinical indications for renal artery stenting have become more controversial [142–144]. Although several randomized controlled trials failed to demonstrate significant advantages to renal artery stenting over medical therapy alone, these studies likely excluded groups of patients who may have benefited from intervention [143]. Current expert consensus still recommends consideration of renal artery stenting for severe hypertension with flash pulmonary edema or acute coronary syndrome, resistant hypertension and unexplained ischemic nephropathy with chronic kidney disease, among other indications [144].
Trani et al., in 2009, reported 100% procedural success in 62 consecutive patients undergoing renal artery stenting [85]. A 2011 study by Alli et al. evaluated the feasibility of TR renal intervention in 11 patients and compared safety parameters to a matched group of 44 TF controls [98]. All TR interventions were successful with no complications [98]. There was one access crossover due to insufficient guide length from the right RA [98]. There were no access site complications in the TR group and 3 (7%) in the TF group [98].
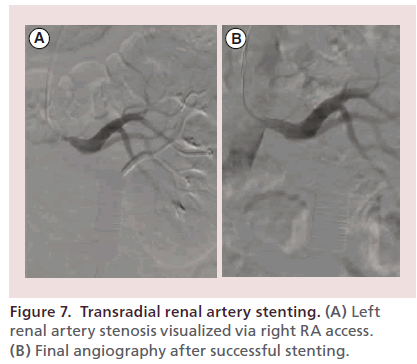
Figure 7 depicts successful left renal artery stenting in a 60-year-old man with severe systemic hypertension refractory to maximal doses of five antihypertensive agents and Duplex arterial ultrasound evidence of bilateral renal artery stenosis. Bilateral stenting was performed and the patient’s hypertension was ultimately managed with lower doses of three antihypertensive agents.
Figure 7. Transradial renal artery stenting. (A) Left renal artery stenosis visualized via right RA access. (B) Final angiography after successful stenting.
Aorto-iliac intervention
TR aorto-iliac angioplasty and stenting is feasible and safe [57,58,83,87,93]. Intervention can typically be performed through a 110 cm 6 Fr introducer sheath positioned in the distal aorta or ostial common iliac artery. For ease of equipment exchange, the authors sometime remove the tuohy-borst valve and replace it with a compatible hemostatic valve. The long straight segment of sheath in the descending aorta provides a high degree of support to iliac intervention from the RA. Antegrade angiography from above facilitates precise stent positioning for ostial common iliac lesions compared with the crossover technique and allows access to the entire length of the external iliac artery compared with the retrograde femoral approach. In addition, bilateral iliac disease can be treated in the same procedure from a single access site [100]. When the distal aorta is involved or kissing balloon or stent technique is required bilateral radial access may be obtained.
Cortese et al. in 2014, published the largest series of TR iliac interventions (149 patients) [97]. Procedural success was achieved in 98.7% of patients [97]. Crossover rates were 12.7% (the TF approach was used in 19 patients after unsuccessful attempts to cross the lesion from above) [97]. There were no reported vascular access or procedure-related complications [97]. Procedure length, fluoroscopy time and contrast volume were comparable to historical TF controls [97].
Infrainguinal intervention
Routine TR femoropopliteal intervention is currently prevented by a lack of sheaths, balloons, stents and atherectomy devices of appropriate length and diameter. Ideally, the common iliac, external iliac or common femoral artery (CFA) should be selectively engaged with a long introducer sheath for sufficient support of infrainguinal intervention. At present, TR femoropopliteal intervention is primarily limited to focal lesions or in-stent restenosis [84]. Made-to-order low-profile long-shaft balloons and self-expanding stents and 400 cm length wires have been used successfully outside of the USA for infrainguinal intervention [92]. Most atherectomy devices are limited by short shaft lengths and larger diameters and at present orbital and laser atherectomy are the only 6 Fr compatible devices [81].
For infrainguinal intervention, radial access may also be expected to reduce the incidence of access site complications compared with the crossover technique or antegrade femoral puncture. Where technically feasible, TR infrainguinal intervention additionally offers the potential benefit of treating bilateral disease during a single procedure.
In 2014, Lorenzoni et al. reported their experience treating 93 infrainguinal lesions in 110 consecutive patients undergoing lower extremity intervention via the TR approach [58]. Success rate was 90% (99% for 74 stenoses and 56% for 19 occlusions) with no bleeding or access site complications [58].
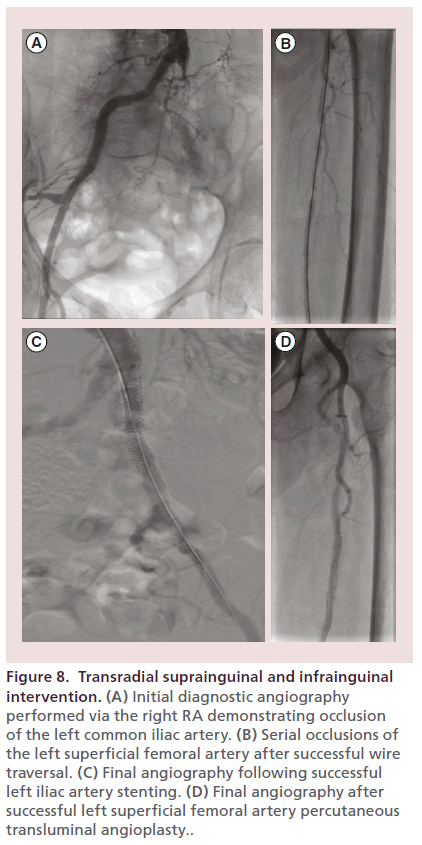
Figure 8 exhibits successful transradial left iliac stenting and left SFA PTA in a 96-year-old woman with chronic kidney disease and limb-threatening left lower extremity critical limb ischemia and nonhealing ulcers. Final angiography demonstrated restoration of inline flow to the left foot. The patient ultimately healed her left lower extremity ulcer.
Figure 8. Transradial suprainguinal and infrainguinal intervention. (A) Initial diagnostic angiography performed via the right RA demonstrating occlusion of the left common iliac artery. (B) Serial occlusions of the left superficial femoral artery after successful wire traversal. (C) Final angiography following successful left iliac artery stenting. (D) Final angiography after successful left superficial femoral artery percutaneous transluminal angioplasty..
Patent hemostasis
At the conclusion of TR angiography or intervention, the RA sheath is immediately removed and hemostasis achieved with external vessel compression. Utilizing any number of commercially available radial compression bands, compression pressure should be titrated to establish nonocclusive hemostasis (using confirmatory plethysmography) and progressively adjusted over 1–2 h to maintain adequate antegrade arterial flow throughout the hemostasis process [12,27,145]. Attention to patent hemostasis reduces the risk of RAO and increases the likelihood of successful repeat radial access in the future [145–147].
Radial-specific complications
Vascular complications are trivial in number and severity with TR intervention compared with TF intervention owing primarily to the RA’s superficial course, isolation from other vascular structures, and easy compressibility. Radial-specific complications include RA spasm, vessel perforation, bleeding, pseudoaneurysm formation and RAO [124]. Although uncommon compared with femoral access, these complications are more frequently observed in the presence of variant anatomy and vessel tortuosity [62,115,148].
Catheter entrapment
Prolonged RA exposure to large, long peripheral sheaths increases the likelihood of clinically significant RA spasm. It is best prevented with liberal intraarterial vasodilator administration, pain control, sedation and patience [125,129,130]. Severe spasm preventing catheter removal post-procedure may warrant axillary nerve block, deeper conscious sedation with propofol or general anesthesia [130,149]. Forceful removal of an entrapped catheter may cause partial or complete RA transection or eversion endarterectomy [25,149].
Vascular injury
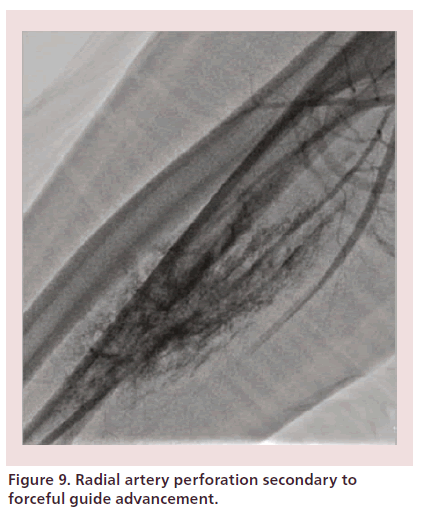
Radial or brachial artery dissections are retrograde events and often seal spontaneously. Perforations are rare, occur in 0.1% of cases, and are easily managed compared with similar vascular injuries in the femoral and iliac region [150,151]. The preferred course of action is to obtain or maintain access across the site of injury and internally tamponade the site with the catheter, permitting the procedure to continue [106,150,151]. A recent small case series demonstrated 100% success with this technique [151]. Removing the catheter will leave an unsealed dissection or perforation that may require external control with brachial sphygmomanometer cuff inflation and placement of a loose elastic bandage around the forearm (Figure 9) [34,53]. Associated hematomas are often easily controlled with manual pressure and rarely (0.004% in a recent large case series) progress to limb-threatening forearm compartment syndrome [49]. Immediate therapy includes cessation of anticoagulation, blood pressure control and external compression [12,34,152,153]. Vascular surgery consultation is recommended in the rare case of threatened limb ischemia.
Radial artery occlusion
Nonocclusive RA injury and asymptomatic RAO occur in up to 10% of patients after transradial catheterization, most commonly with large artery-catheter mismatch, female sex, diabetes, occlusive hemostasis and lack of heparin and antiplatelet pretreatment [12,61,132,145,154]. RAO may prevent the use of the RA for future catheterization or for use as a bypass conduit or hemodialysis fistula. It is best prevented by immediate sheath removal and patent hemostasis. With proper procedural heparin dosing and patent hemostasis strategies, RAO rates have dropped to 2–5% at 24 h post-procedure in recent studies [145]. Spontaneous recanalization also occurs commonly, and rates of RAO are now less than 1–2% at 30 days [133,154,155]. With proper procedural technique, repeated TR arterial access has been performed for up to ten procedures at some centers [146,147].
Training
Existing North American and European guidelines for recommended learning steps and competency recommendations for TR coronary interventional training should be used as a model for any operator aiming to explore TRPVI [12,34,37]. Similarly established TR coronary best practices should be translated to TRPVI [37].
The novice TRPVI operator should already have demonstrated competency in both TR coronary intervention and peripheral endovascular intervention [37,156]. While the learning curve has been suggested to be at least 50 cases for coronary TR competence among experienced operators, it is unknown for TRPVI, but may be similar for physicians already skilled in both peripheral vascular and TR intervention [157]. Building on the successful coronary model, there is also a need for structured TR peripheral vascular training in fellowship programs as well as organized professional courses, simulators and mentorships [37].
Future perspective
The movement toward increased performance of TR coronary procedures should ultimately translate into greater adoption of the TR approach for peripheral vascular angiography and intervention. As technological advances in sheath and catheter design and miniaturization of interventional equipment proceeds, routine TRPVI may be expected to become more feasible and popular.
At present, there are little clinical data comparing the TR and TF approaches for PVI. Future randomized controlled trials are needed for head-to-head comparison of TR and TF access.
In the near-term, there is also a need for a larger variety and longer length of radial-specific hydrophilic introducer sheaths in 125 and 150 cm lengths. Thinner wall peripheral sheaths with smaller outer diameters and larger inner diameters should be developed to permit interventions via 5 or 6 Fr sheaths. At the same time, future self-expanding stents need to be downsized without loss of radial force [158].
The 0.014 inch, 0.018 inch and 0.035 inch guidewires should extend to 400 cm without sacrificing torque control and crossing capability [63]. Continued development of longer length support catheters for over-the-wire exchanges and new rapid exchange systems is advised. Balloons should be produced in shaft lengths up to 200 cm. Finally, CTO devices, re-entry devices, atherectomy tools and intravascular ultrasound catheters should be developed in longer lengths and smaller diameters. The numerous potential benefits of TRPVI clearly justify continued development of such radial-specific devices and equipment.
Drug-coated balloon (DCB) technology may be the best immediate answer to TR femoropopliteal interventions, and ultimately below the knee (BTK) interventions. DCBs mechanically disrupt plaque and infuse an antiproliferative agent throughout the treated lesion, and may be particularly useful in anatomic situations where stents perform poorly such as bifurcations, distal pedal arteries, complex lesions, long segments and common femoral and popliteal artery lesions [159,160]. This may also increase use of atheroablative devices for improved vessel preparation once longer shaft length and smaller diameter equipment comes to market.
Beyond DCBs, bioresorbable vascular scaffolds may soon provide optimal transient scaffolding of the healing vessel and continued antiproliferative drug elution to counteract excessive neointimal hyperplasia and then be reabsorbed, with restoration of normal vessel endothelial structure and function [161,162]. These and other technical advances should increase the type and severity of lesions amenable to TR endovascular intervention.
BTK interventions may also someday benefit from dual radial and pedal access techniques to improve success rates [163–166]. Many of the procedural skills learned perfecting TR intervention translate well into pedal access for BTK intervention [167,168]. This additional expertise will provide the endovascular interventionalist with a wider spectrum of therapeutic options. The day is not far off when ‘radial first’ may apply to interventions throughout the vascular tree.
Conclusion
The benefits of TR compared with TF access are by now well established. Peripheral vascular intervention continues to transition to an endovascular first approach. Building upon these advances, TRPVI holds the promise of superior safety and superior efficacy for the treatment of peripheral vascular disease. A radial-first strategy is currently hampered by the absence of: randomized clinical trials and expert consensus, full-spectrum radial-specific equipment and a critical mass of suitably trained operators. The immense potential benefits of TRPVI justify the development of validated training pathways and competency standards and the manufacture and marketing of smaller, longer and radial-specific peripheral vascular equipment. Combined with parallel ongoing advances in DCB and BVS technology as well as increased adoption of pedal access strategies, another major revolution in the interventional treatment of peripheral vascular disease is on the horizon.
Acknowledgement
The authors would like to thank EA Morgan, MLS for library services support.
Executive summary
Advantages of radial access
• Compared to femoral access, the radial approach for coronary angiography and intervention has demonstrated consistent reductions in bleeding, access site complications, morbidity, and possibly mortality across most patient populations studied.
• The clinical benefits of radial access are even more pronounced in patients with significant peripheral vascular disease.
• Clear anatomic advantages to radial access exist for peripheral intervention in innominate, subclavian, renal, mesenteric and celiac arteries, as well as carotid arteries in the presence of complex arch anatomy or severe aorto-iliac tortuosity or disease.
Current limitations of transradial peripheral vascular intervention
• Wholesale immediate adoption of transradial peripheral vascular intervention (TRPVI) is limited in part by the smaller diameter of the RA and the larger Fr size of many peripheral vascular devices.
• Existing equipment length limitations constrain default infrainguinal angiography and intervention.
• The coronary literature evidences increased contrast use, radiation exposure and procedure times for transradial (TR) versus transfemoral intervention during the learning period.
Lack of prospective data, randomized clinical trials, expert consensus & clinical guidelines
• There is limited high-quality scientific data and no large multicenter randomized controlled trials to support TRPVI.
• Only one randomized trial for TRPVI (for TR carotid intervention) has been published to date.
• Several observational studies, feasibility studies, technical reports, case reports, case series and single-center registries have demonstrated successful TR intervention throughout the vascular tree.
Technical aspects of TR angiography & intervention
• Detailed preprocedural evaluation is critical to identify suitable candidates for TRPVI.
• Comprehensive spasm prevention strategies are key to successful TR intervention.
• Validated TR coronary procedural techniques may be utilized to successfully negotiate tortuosity and variant anatomy of the radio-brachial and aorto-subclavian axes during TRPVI.
• During diagnostic angiography, measuring the distance from the RA access site to the target vessel is mandatory to determine a patient’s eligibility for TR intervention.
• Proven guideline-supported femoral endovascular and coronary TR techniques can be combined for the effective performance of TRPVI by experienced operators.
Training & competency
• Existing TR coronary guidelines and best practices should be used as a model for physicians expanding to TRPVI.
• Operators should demonstrate TR coronary and peripheral vascular interventional proficiency as prerequisites to the performance of TRPVI.
• Structured training programs are needed to develop more widespread TRPVI expertise among endovascular interventionalists.
Financial & competing interests disclosure
WB Bachinsky has received honoraria and research grants from Abbott Vascular and Cordis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- Abola MT, Bhatt DL, Duval, et al. Fate of individuals with ischemic amputations in the REACH Registry: three-year cardiovascular and limb-related outcomes. Atherosclerosis 221, 527–535 (2012).
- Mahoney EM, Wang K, Keo HH et al. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ. Cardiovasc. Qual. Outcomes 3, 642–651 (2010).
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 110, 738–743 (2004).
- Norgen L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg. 45, S5-S67 (2007).
- Anderson JL, Halperin JL, Albert NM et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations). Circulation 127, 1425–1443 (2013).
- Jones WS, Dolor DJ, Hasselblad V et al. Comparative effectiveness of endovascular and surgical revascularization for patients with peripheral artery disease and critical limb ischemia: systematic review of revascularization in critical limb ischemia. Am. Heart J. 167, 489–498 (2014).
- Adam DJ, Beard JD, Cleveland T et al. Bypass versus angioplasty in severe ischemia of the leg (BASIL): multicenter, randomized controlled trial. Lancet 366, 1925–1934 (2005).
- Klein AJ, Feldman DN, Aronow HD et al. SCAI expert consensus statement for aorto-iliac arterial intervention appropriate use. Catheter Cardiovasc. Interv.84, 520–528 (2014).
- Klein AJ, Pinto DS, Gray BH, Jaff MR, White CJ, Drachman DE. SCAI expert consensus statement for femoro-popliteal arterial intervention appropriate use. Catheter Cardiovasc. Interv. 84, 529–536 (2014).
- Gray BH, Diaz-Sandoval LJ, Dieter RS, Jaff MR, White CJ. SCAI expert consensus statement for infrapopliteal arterial intervention appropriate use. 84, 539–545 (2014).
- Bertrand OF, Rao SV, Pancholy S et al. Transradial approach for coronary angiography and interventions: results of the first international transradial practice survey. J. Am. Coll. Cardiol. Interv. 3, 1022–1031 (2010).
- Caputo RP, Tremmel JA, Rao S et al. Transradial arterial access for coronary and peripheral procedures: executive summary by the transradial committee of the SCAI. Cathet. Cardiovasc. Interv. 78, 823–839 (2011).
- Campeau L. Percutaneous radial approach for coronary angiography. Cathet. Cardiovasc. Diagn. 16, 3–7 (1989).
- Kiemeneij F, Laarman GJ. Percutaneous transradial artery approach for coronary stent implantation. Cathet. Cardiovasc. Diagn. 30, 173–178 (1993).
- Agostoni P, Biondi-Zoccai GG, de Benedictis ML et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: Systematic overview and meta-analysis of randomized trials: J. Am. Coll. Cardiol. 44, 349–356 (2004).
- Applegate RJ, Sacrinty MT, Kutcher MA et al. Trends in vascular complications after diagnostic cardiac catheterization and percutaneous coronary intervention via the femoral artery. J. Am. Coll. Cardiol. Interv. 1, 317–326 (2008).
- De Belder AJ, Smith RE, Wainwright RJ, Thomas MR. Transradial artery coronary angiography and intervention in patients with severe peripheral vascular disease. Clin. Rad. 52, 115–118 (1997).
- Helft G, Dambrin G, Zaman A et al. Percutaneous coronary intervention in anticoagulated patients via radial artery access. Catheter Cardiovasc. Interv. 73, 44–47 (2009).
- Jolly SS, Yusuf S, Cairns J et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomized, parallel group, multicentre trial. Lancet 377, 1409–1420 (2011).
- Jolly SS, Alana S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am. Heart J. 157, 132–140 (2009).
- Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, Van der Wieken R. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the Access study. J. Am. Coll. Cardiol. 29, 1269–1275 (1997).
- Lee MS, Applegate B, Rao S, Kirtane AJ, Seto A, Stone GW. Minimizing femoral artery access complications during percutaneous coronary intervention: a comprehensive review. Catheter Cardiovasc. Interv. 84, 62–69 (2014).
- Chase AJ, Fretz, EB, Warburton WP et al. Association of the arterial access site at angioplasty with transfusion and mortality: the M.O.R.T.A.L. study (Mortality benefit of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg). Heart 94, 1019–1025 (2008).
- Cox N, Resnic FS, Popma JJ, Simon DI, Eisenhauer AC, Rogers C. Comparison of the risk of vascular complications associated with femoral and radial access coronary catheterization procedures in obese versus nonobese patients. Am. J. Cardiol. 94, 1174–1177 (2004).
- Nathan S, Rao SV. Radial versus femoral access for percutaneous coronary intervention: implications for vascular complications and bleeding. Curr. Cardiol. Rep. 14, 502–209 (2012).
- Vorobcsuk A, Konyi A, Aradi D et al. Transradial versus transfemoral percutaneous coronary intervention in acute myocardial infarction: systematic overview and meta-analysis. Am. Heart J. 158, 814–821 (2009).
- Ziakas AG, Koskinas KC, Gavrilidis S et al. Radial versus femoral access for orally anticoagulated patients. Catheter Cardiovasc. Interv. 76, 493–499 (2010).
- Hibbert B, Simard T, Wilson KR et al. Transradial versus transfemoral artery approach for coronary angiography and percutaneous coronary intervention in the extremely obese. Am. Coll. Cardiol. Interv. 5, 819–826 (2012).
- Caputo RP. Transradial access: economic considerations. Invasive Cardiol. 21, 18A–20A (2009).
- Cooper CJ, El-Shiekh RA, Cohen DJ et al. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am. Heart J. 138, 430–436 (1999).
- Dauerman HL, Rao SV, Resnic FS, Applegate RJ. Bleeding avoidance strategies: consensus and controversy. J. Am. Coll. Cardiol. 58, 1–10 (2011).
- Doyle BJ, Rihal CS, Gastineau DA, Holmes DR JR. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J. Am. Coll. Cardiol. 53, 2019–2027 (2009).
- Feldman DN, Swaminathan RV, Kaltenbach LA et al. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention: an updated report from the National Cardiovascular Data Registry (2007–2012). Circulation 127, 2295–2306 (2013).
- Hamon M, Pristipino C, Di Mario C et al. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care and Thrombosis of the European Society of Cardiology. EuroIntervention 8, 1242–1251 (2013).
- Louvard Y, Benamer H, Garot P et al. Comparison of transradial and transfemoral approaches for coronary angiography and angioplasty in octogenarians (the OCTOPLUS study). Am J. Cardiol. 94, 1177–1180 (2004).
- Mamas MA, Ratib K, Routledge H et al. Influence of access site selection on PCI-related adverse events in patients with STEMI: meta-analysis of randomized clinical trials. Heart 98, 303–311 (2012).
- Rao SV, Tremmel JA, Gilchrist IC et al. Best practices for transradial angiography and intervention: a consensus statement from the Society for Cardiovascular Angiography and Intervention’s transradial working group. Catheter Cardiovasc. Interv. 83, 228–236 (2014).
- Rao SV, Cohen MG, Kandzari DE, Bertrand OF, Gilchrist IC. The transradial approach to percutaneous coronary intervention: historical perspective, current concepts, and future directions. J. Am. Coll. Cardiol. 55, 2187–2195 (2010).
- Rao SV, Ou FS, Wang TY et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary interventions: a report from the national cardiovascular data registry. J. Am. Coll. Cardiol. Interv. 1, 379–386 (2008).
- Romagnoli E, Biondi-Zoccai G, Sciahbasi A et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS study. J. Am. Coll. Cardiol. 60, 2481–2489 (2012).
- Baklanov DV, Kaltenbach LA, Marso SP et al. The prevalence and outcomes of transradial percutaneous coronary intervention for ST-segment elevation myocardial infarction: analysis from the National Cardiovascular Data Registry (2007 to 2011). J. Am. Coll. Cardiol. 61, 420–426 (2013).
- Iqbal MB, Arujuna A, Ilsley C et al. Radial versus femoral access is associated with reduced complications and mortality in patients with non-ST-segment-elevation myocardial infarction: an observational cohort study of 10095 patients. Circ. Cardiovasc. Interv. 7, 456–464 (2014).
- Mehta SR, Jolly SS, Cairns J et al. Effects of radial versus femoral artery access in patients with acute coronary syndromes with or without ST-segment elevation. J. Am. Coll. Cardiol. 60, 2490–2499 (2012).
- Bernat I, Horak D, Stasek J et al. ST-segment elevation myocardial infarction treated by radial or femoral approach in a multicenter randomized clinical trial: the STEMI-RADIAL trial. J. Am. Coll. Cardiol. 63, 964–972 (2013).
- Bertrand OF, Belisle P, Joyal D et al. Comparison of transradial and femoral approaches for percuaneous coronary interventions: a systematic review and hierarchial Bayesian meta-analysis. Am. Heart J. 163, 632–648 (2012).
- Mamas MA, Anderson SG, Carr M et al. Baseline bleeding risk and arterial access site practice in relation to procedural outcomes after percutaneous coronary intervention. J. Am. Coll. Cardiol. 64, 1554–1564 (2014).
- Amorosa G, Sarti M, Bellucci R et al. Clinical and procedural predictors of nurse workload during and after invasive procedures: the potential benefit of a systematic radial access. Eur. J. Cardiovasc. Nurs. 4, 234–241 (2005).
- Mitchell MD, Hong JA, Lee BY, Umscheid CA, Bartsch SM, Don CW. Systematic review and cost-benefit analysis of radial artery access for coronary angiography and intervention. Circ. Cardiovasc. Qual. Outcomes 5, 454–462 (2012).
- Rao SV, Bernat I, Bertrand OF. Remaining challenges and opportunities for improvement in percutaneous transradial coronary procedures. Eur. Heart J. 33, 2521–2526 (2012).
- Levine GN, Bates ER, Blakenship JC et al. 2011 ACCF/ AHA/SCAI guideline for Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 58, e44–e122 (2011).
- Coppola JT, Staniloae C. Radial access for peripheral vascular procedures. Endovascular Today 1, 38–44 (2012).
- Hildick-Smith DJ, Walsh JT, Lowe MD et al. Coronary angiography in the presence of peripheral vascular disease: femoral or brachial/radial approach? Catheter Cardiovasc. Interv. 49, 32–37 (2000).
- Romagnoli E, Mann T, Sciahbasi A, Pendenza G, Biondi-Zoccai GG, Sangiorgi GM. Transradial approach in the catheterization laboratory: pros/cons and suggestions for successful implementation. Int J. Cardiol. 163, 116–124 (2013).
- Staniloae CS, Korabathina R, Coppola JT. Transradial access for peripheral vascular interventions. Catheter Cardiovasc. Interv. 81, 1194–1203 (2013).
- Hildick-Smith DJ, Khan ZI, Shapiro LM, Patch MC. Occasional-operator percutaneous brachial artery coronary angiography: first, do no arm. Catheter Cardiovasc. Interv. 57, 161–165 (2002).
- Yamashita T, Imai S, Tamada T. Transradial approach for noncoronary angiography and interventions. Catheter Cardiovasc. Interv. 70, 303–308 (2007).
- Staniloae CS, Korabathina R, Yu J, Kurian D, Coppola J. Safety and efficacy of transradial aortoiliac interventions. Catheter Cardiovasc. Interv. 75, 659–662 (2010).
- Lorenzoni R, Lisi C, Corciu A, Lazzari M, Bovenzi F. Tailored use of transradial access for above the knee angioplasty. J. Endovasc. Ther. 21, 635–640 (2014).
- Okuyan H, Hzal F, Tacay G, Timurkaynak T. Angiographic evaluation of the radial artery diameter in patients who underwent coronary angiography or intervention. J. Invasive Cardiol. 25, 353–357 (2013).
- Oshima A, Takeshita S, Kozuma K et al. Intravascular ultrasound analysis of the radial artery for coronary artery bypass grafting. Ann. Thorac. Surg. 79, 99–103 (2005).
- Saito S, Ikei H, Hosokawa G, Tanaka S. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc. Interv. 46, 173–178 (1999).
- Yoo BS, Yoon J, Ko JY et al. Anatomical consideration of the radial artery for transradial coronary procedures: arterial diameter, branching anomaly and vessel tortuosity. Int J. Cardiol. 101, 421–427(2005).
- Lorenzoni R, Roffi M. Transradial access for peripheral and cerebrovascular interventions. J. Invasive Cardiol. 25, 529–536 (2013).
- Rogers JH, Laird JR. Overview of new technologies for lower extremity revascularization. Circulation 116, 2072–2085 (2007).
- Coroleu SF, Burzotta F, Fernandez-Gomez C et al. Feasibility of complex coronary and peripheral interventions by trans-radial approach using large sheaths. Catheter Cardiovasc. Interv. 79, 597–600 (2012).
- Wu SS, Galani RJ, Bahro A, Moore JA, Burket MW, Cooper CJ. 8 French transradial coronary interventions: clinical outcome and late effects on the radial artery and hand function. J. Invasive Cardiol. 12, 605–609 (2009).
- Bertrand OF, Arsenault J, Mongrain R. Operator vs. patient radiation exposure in transradial and transfemoral coronary interventions. Eur. Heart J. 29, 2577–2578 (2008)
- Dominici M, Diletti R, Milici C et al. Left radial versus right radial approach for coronary artery catheterization: a prospective comparison. J. Invasive Cardiol. 25, 203–209 (2012).
- Sciahbasi A, Romagnoli E, Trani C et al. Operator radiation exposure during percutaneous coronary procedures through the left or right radial approach: the TALENT dosimetric substudy. Catheter Cardiovasc. Interv. 4, 226–231 (2011).
- Pelliccia F, Trani C, Biondi-Zoccai GG et al. Comparison of the feasibility and effectiveness of transradial coronary angiography via right versus left radial artery approaches (from the PREVAIL) study. Am J. Cardiol. 110, 771–775 (2012).
- Pancholy SB, Bertrand OF. Radiation exposure with transradial and transfemoral procedures. Am. Heart J, 165, 254–255 (2013).
- Burzotta F, Trani C, Mazzari MA et al. Vascular complications and access crossover in 10,676 transradial percutaneous coronary procedures. Am. Heart J. 163, 230–238 (2012).
- Abdelaal E, Brousseau-Provencher C, Montminy S et al. Risk score, causes, and clinical impact of failure of transradial approach for percutaneous coronary interventions. J. Am. Coll. Cardiol. Intv. 6, 1129–1137 (2013).
- Vink MA, Amoroso G, Dirksen MT et al. Routine use of the transradial approach in primary percutaneous coronary intervention: procedural aspects and outcomes in 2209 patients treated in a single high-volume centre. Heart 97, 1938–1942 (2011).
- Hamon M, Lipiecki J, Carrie D et al. Silent cerebral infarcts after cardiac catheterization: a randomized comparison of radial and femoral approaches. Am. Heart J. 164, 449–454 (2012).
- Ratib K, Mamas MA, Routledge HC, Ludman PF, Fraser D, Nolan J. Influence of site choice on incidence of neurologic complications after percutaneous coronary intervention. Am. Heart J. 165, 317–324 (2013).
- Ruzsa Z, Nemes B, Pinter L et al. A randomized comparison of transradial and transfemoral approach for carotid artery stenting: RADCAR (RADial access for CARotid artery stenting) study. EuroIntervention 10, 381–391 (2014).
- Folmar J, Sachar R, Mann T. Transradial approach for carotid artery stenting: a feasibility study. Catheter Cardiovasc. Interv. 69, 355–361 (2007).
- Patel T, Shah S, Malhorta H, Radadia R, Shah L, Shah S. Transradial approach for stenting of vertebrobasilar stenosis: a feasibility study. Catheter Cardiovasc. Interv. 74, 925–931 (2009).
- Patel T, Shah S, Ranjan A, Malhorta H, Pancholy S, Coppola J. Contralateral transradial approach for carotid artery stenting: a feasibility study. Catheter Cardiovasc. Interv. 75, 268–275 (2010).
- Sanghvi K, Nachtigall J, Luft U. Transradial endovascular treatment of severe common femoral artery stenosis. J. Invasive Cardiol. 25, 616–619 (2013).
- Brountzos EN, Malagari K, Kelekis DA. Endovascular treatment of occlusive lesions of the subclavian and innominate artery. Cardiovasc. Intervent Radiol. 29, 503–510 (2006).
- Sanghvi K, Kurian D, Coppola J. Transradial intervention of iliac and superficial femoral artery disease is feasible. J. Invasive Cardiol. 21, 385–387 (2008).
- Trani C, Burzotta F, Tommasino A, Giammarinaro M. Transradial approach to treat superficial femoral artery in-stent restenosis. Catheter Cardiovasc. Interv. 74, 494–498 (2009).
- Trani C, Tommasino A, Burzotta F. Transradial renal stenting: why and how. Catheter Cardiovasc. Interv. 74, 951–956 (2009).
- Coscas R, de Blic R, Capdevila C, Javerliat I, Goëau-Brissonniere O, Coggia M. Percutaneous radial access for peripheral transluminal angioplasty. J. Vasc. Surg. 20 doi:10.1016/j.jvs.2014.07.009(2014) (Epub ahead of print).
- Cortese B, Peretti E, Troisi N, Siquilberti E, Setti M, Piti A. Transradial percutaneous iliac intervention, a feasible alternative to the transfemoral route. Cardiovasc. Revasc Med. 13, 331–334 (2012).
- Flachskampf FA, Wolf T, Daniel WG, Ludwig J. Transradial stenting of the iliac artery: a case report. Catheter Cardiovasc. Interv. 65, 193–195 (2005).
- Scheinert D, Bräunlich S, Nonnast-Daniel B et al. Transradial approach for renal artery stenting. Catheter Cardiovasc. Interv. 54, 442–227 (2001).
- Galli M, Tarantino F, Mameli S et al. Transradial approach for renal percutaneous transluminal angioplasty and stenting: a feasibility pilot study. J. Invasive Cardiol. 14, 386–390 (2002).
- Yu J, Korabathina R, Coppola J, Staniloae C. Transradial approach to subclavian artery stenting. J. Invasive Cardiol. 22, 204–206 (2010).
- Trani C, Tommasino A, Burzotta F. Pushing the limits forward: transradial superficial femoral artery stenting. Catheter Cardiovasc. Interv. 76, 1065–1071 (2010).
- Shinozaki N, Ogata N, Ikari Y. Initial results of transradial iliac artery stenting. Vasc Endovasc Surg. 48, 51–54 (2014).
- Pinter L, Cagiannos C, Ruzsa Z, Bakoyiannis C, Kolvenbach R. Report on initial experience with transradial access for carotid artery stenting. J. Vasc Surg, 45, 1136–1141 (2007).
- Lorenzoni R, Mazzoni A, Lazzari M, Boni A, Gemignani C, Bovenzi F. Radial artery access for above the knee angioplasty: a feasibility study. Eurointervention 7, 924–929 (2011).
- Etxegoien N, Rhyne D, Kedev S, Sachar R, Mann T. The transradial approach for carotid artery stenting. Catheter Cardiovasc. Interv. 80, 1081–1087 (2012).
- Cortese B, Trani C, Lorenzoni R et al. Safety and feasibility of iliac endovascular interventions with a radial approach: results from a multicenter study coordinated by the Italian Radial Force. Int J. Cardiol. 175, 280–284 (2014).
- Alli O, Mathew V, From AM, Barsness G, Misra S, Gulati R. Transradial access for renal artery intervention is feasible and safe. Vasc. Endovasc. Surg. 45, 738–742 (2011).
- Castriota F, Cremonesi A, Manetti R, Lamarra M, Noera G. Carotid artery stenting using radial artery access. J. Endovasc. Surg. 6, 385–386 (1999).
- Staniloae CS, Kurian DC, Coppola JT. Transradial bilateral iliac stenting. J. Invasive Cardiol. 18, E256–E257 (2006).
- Ruzsa Z, Toth K, Jambrik Z et al. Transradial access for renal artery intervention. . Interv. Med. Appl. Sci.. 6, 97–103 (2014).
- Allen EV. Thromboangiitis obliterans: methods of diagnosis of chronic occlusive arterial lesions distal to the wrist with illustrative cases. Am J. Med Sci. 178, 237–244 (1929).
- Barbeau GR, Arsenalt F, Dugas L, Simard S, Lariviere MM. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with Allen’s test in 1010 patients. Am. Heart J. 147, 489–493 (2004).
- Greenwood MJ, Della-Siega AJ, Fretz EB et al. Vascular communications of the hand in patients being considered for transradial coronary angiography: is the Allen’s test accurate. J. Am. Coll. Cardiol. 46, 2013–2017 (2005).
- Hata M, Sezai A, Niino T et al. Radial artery harvest using the sharp scissors method for patients with pathological findings on Allen’s test. Surgery Today 36, 790–792 (2006).
- Valgimigli M, Campo G, Penzo C et al. Transradial coronary catheterization and intervention across the whole spectrum of Allen test results. J. Am. Coll. Cardiol. 63, 1833–1841 (2014).
- Pancholy SB, Sanghvi KA, Patel TM. Radial artery access technique evaluation trial: randomized comparison of Seldinger versus modified Seldinger technique for arterial access for transradial catheterization. Catheter Cardiovasc. Interv. 80, 288–291 (2012).
- Sanghvi KA. Ten critical lessons for performing transradial catheterization. Endovascular Today. 3, 62–67 (2014).
- Pancholy SB, Coppola J, Patel T. Subcutaneous administration of nitroglycerine to facilitate radial artery cannulation. Catheter Cardiovasc. Interv. 68, 389–391 (2006).
- Beyer AT, Ng R, Zimmet J et al. Topical nitroglycerin and lidocaine to dilate the radial artery prior to transradial cardiac catheterization: a randomized, placebo-controlled, double-blind clinical trial: the PRE-DILATE Study. Int. J. Cardiol. 168, 2575–2578 (2013).
- Roberts J, Manur R. Ultrasound-guided radial artery access by a non-ultrasound trained interventional cardiologist improved first-attempt success rates and shortened time for successful radial artery cannulation. J. Invasive Cardiol. 25, 676–679 (2013).
- Rathore S, Stables RH, Pauriah M et al. Impact of length and hydrophilic coating of the introducer cheetah on radial artery spasm during transradial coronary intervention. J. Am. Coll. Cardiol. Interv. 3, 475–483 (2010).
- Burzotta F, Brancati MF, Trani C et al. Impact of radial-to-aorta vascular anatomical variants on risk of failure in trans-radial coronary procedures. Catheter Cardiovasc. Interv. 80, 298–303 (2012).
- Masuda N, Matsukage T, Ogata N, Morino Y, Tanabe, , Ikari Y. Analysis of peripheral arterial bends that interfere with coronary catheterization. J. Invasive Cardiol. 22, 197–203 (2010).
- Lo TS, Nolan J, Fountzopoulos E et al. Radial artery anomaly and its influence on transradial coronary procedural outcome. Heart 95, 410–415(2009).
- Patel T, Shah S, Pancholy S et al. Working through complexities of radial and brachial vasculature during transradial approach. Catheter Cardiovasc. Interv. 83, 1074–1088 (2014).
- Patel T, Shah S, Pancholy S et al. Working through challenges of subclavian, innominate, and aortic arch regions during transradial approach. Catheter Cardiovasc. Interv. 84, 224–235 (2014).
- Gaudino M, Tondi, P, Serricchio M et al. Atherosclerotic involvement of the radial artery in patients with coronary artery disease and its relation with midterm radial artery graft patency and endothelial function. J. Thorac Cardiovasc. Surg. 126, 196–1971 (2003).
- Ruengsakulrach P, Sinclair R, Komeda M, Raman J, Gordon I, Buxton B. Comparative histopathology of radial artery versus internal thoracic artery and risk factors for development of intimal hyperplasia and atherosclerosis. Circulation 100, I139-I144 (1999).
- Valsecchi O, Vassileva A, Musumeci G et al. Failure of transradial approach during coronary interventions: anatomic considerations. Catheter Cardiovasc. Interv. 67, 870–878 (2006).
- Ruiz-Salmeron RJ, Mora R, Velez-Gimon M et al. Radial artery spasm in transradial cardiac catheterization: assessment of factors related to its occurrence, and of its consequences during follow-up. Rev. Esp. Cardiol. 58, 504–511 (2005).
- Uhlemann M, Mobius-Winkler S, Mende M et al. The Leipzig prospective vascular ultrasound registry in radial artery catheterization: impact of sheath size on vascular complications. J. Am. Coll. Cardiol. Interv. 5, 36–43 (2012).
- He GW, Yang CQ. Characteristics of adrenoreceptos in the human radial artery: clinical implications. J. Thorac. Cardiovasc. Surg. 115, 1136–1141 (1998).
- Kanei Y, Kwan T, Nakra NC et al. Transradial cardiac catheterization: a review of access site complications. Catheter Cardiovasc. Interv. 78, 840–846 (2011).
- Coppola J, Patel T, Kwan T et al. Nitroglycerine, nitroprusside, or both, in preventing radial artery spasm during transradial artery catheterization. J. Invasive Cardiol. 18, 155–158 (2006).
- Kiemeneij F. Prevention and management of radial artery spasm. J. Invasive Cardiol. 18, 159–160 (2006).
- Varenne O, Jegou A, Cohen R et al. Prevention of arterial spam during percutaneous coronary interventions through radial artery: the SPASM study. Catheter Cardiovasc. Interv. 68, 231–235 (2006).
- Caussin C, Gharbi M, Durier C et al. Reduction in spasm with a long hydrophilic transradial sheath. Catheter Cardiovasc. Interv. 76, 668–672(2010).
- Boyer N, Beyer A, Gupta V et al. The effects of intra-arterial vasodilators on radial artery size and spasm: implications for contemporary use of trans-radial access for coronary angiography and percutaneous coronary intervention. Cardiovasc. Revasc. Med. 14, 321–324 (2013).
- Ho HH, Jafary FH, Ong PJ. Radial artery spasm during transradial cardiac catheterization and percutaneous coronary intervention: incidence, predisposing factors, prevention, and management. Cardiovasc. Revasc. Med. 13, 193–195 (2012).
- Patel T, Shah S, Pancholy S, Rao S, Bertrand OF, Kwan Balloon-assisted tracking: a must-know technique to overcome difficult anatomy during transradial approach. Catheter Cardiovasc. Interv. 83, 211–220 (2014).
- Pancholy SB. Comparison of the effect of intra-arterial versus intravenous heparin on radial artery occlusion after transradial catheterization. Am. J. Cardiol. 104, 1083–1085 (2009).
- Kpodonu J. Equipment required for aortic endografting. In: Endovascular and Hybrid Therapies for Structural Heart and Aortic Disease. Kpodonu J and Bonan R (Eds). John Wiley &Sons, Oxford, UK, 15–21 (2013).
- Nanto S, Ohara T, Shimonagata T, Hori M, Kubori S. technique for changing a PTCA balloon catheter over regular-length guidewire. Cathet Cardiovasc. Diagn. 32, 274–277 (1994).
- Feiring AJ, Olson LE. Coronary stent and over-the-wire catheter exchange using standard length guidewires: jet exchange (JEX) practice and theory. Cathet. Cardiovasc. Diagn. 42, 457–466 (1997).
- Dahm JB, van Buuren F, Hansen C, Becker J, Wolpers HG. The concept of an anatomy related individual arterial access: lowering technical and clinical complications with transradial access in bovine- and type-III aortic arch carotid artery stenting. Vasa 40, 468–473 (2011).
- Shaw JA, Gravereaux EC, Eisenhauer AC. Carotid stenting in the bovine arch. Catheter Cardiovasc. Interv. 60, 566–569 (2003).
- Eberhardt O, Naegele T, Raygrotzki S, Weller M, Ernemann U. Stenting of vertebrobasilar arteries in symptomatic atherosclerotic disease and acute occlusion: case series and review of the literature. J. Vasc. Surg. 43, 1145–1154 (2006).
- Zeller T. Renal artery stenosis: epidemiology, clinical manifestations, and percutaneous endovascular therapy. J. Invasive Cardiol. 18, 497–506 (2005).
- Braunlich S, Ludwig J, Scheinert D. Transradial renal artery angioplasty and stenting. J. Invasive Cardiol. 14, 147–149 (2002).
- Raghu C, Louvard Y. Transradial approach for percutaneous transluminal angioplasty and stenting in the treatment of chronic mesenteric ischemia. Catheter Cardiovasc. Interv. 61, 450–454 (2004).
- Feldman RL, Wargovivh TH, Bittl JA. No-touch technique for reducing aortic wall trauma during renal artery stenting. Catheter Cardiovasc. Interv. 46, 245–248 (1999).
- Cooper CJ, Murphy TP, Cutlip DE et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N. Engl. J. Med. 370, 13–22 (2014).
- Parikh SA, Shishehbor MH, Gray BH, White CJ, Jaff MR. SCAI expert consensus statement for renal artery stenting appropriate use. Catheter Cardovasc Interv. 84, 1163–1171 (2014).
- Pancholy S, Coppola J, Patel T, Roke-Thomas M. Prevention of radial artery occlusion patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc. Interv. 72, 335–340 (2008).
- Abdelaal E, Molin P, Plourde G et al. Successive transradial access for coronary procedures: experience of Quebec Heart-Lung Institute. Am. Heart J. 165, 325–331 (2013).
- Yoo BS, Lee SH, Ko JY et al. Procedural outcomes of repeated transradial coronary procedure. Catheter Cardiovasc. Interv. 58, 301–314 (2003).
- Dehghani P, Mohammad A, Bajaj R et al. Mechanism and predictors of failed transradial approach for percutaneous coronary interventions. J. Am. Coll. Cardiol. Interv. 2(11), 1057–1064 (2009).
- Shroff A, Siddiqui, , Burg A, Singla I. Identification and management of complications of transradial procedures. Curr. Cardiol. Rep. 15, 350 (2013).
- Calvino-Santos RA, Vazquez-Rodriguez JM, Salgado-Fernandez, et al. Management of radial artery perforation. Catheter Cardiovasc. Interv. 61, 74–78 (2004).
- Patel T, Shah S, Sanghvi K, Pancholy S. Management of radial and brachial artery perforations during transradial procedures a practical approach. J. Invasive Cardiol. 21, 544–547 (2009).
- Tizon-Marcos H, Barbeau GR. Incidence of compartment syndrome of the arm in a large series of transradial approach for coronary procedures. J. Invasive Cardiol. 21, 380–384 (2008).
- Bertrand OF. Acute forearm muscle swelling post transradial catheterization and compartment syndrome: prevention is better than treatment. Catheter Cardiovasc. Interv. 75, 366–368 (2010).
- Stella PR, Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der Wieken R. Incidence and outcome of radial artery occlusion following transradial artery coronary angioplasty. Cathet. Cardiovasc. Diagn. 40, 156–158 (1997).
- Cubero JM, Lobardo J, Pedrosa C et al. Radial compression guided by mean artery pressure versus standard compression with a pneumatic device (RACOMAP). Catheter Cardiovasc. Interv. 73, 467–472 (2009).
- Creager MA, Goldstone J, Hirshfeld JW Jr et al. ACC/ ACP/SCAI/SVMB/SVS clinical competence statement on vascular medicine and catheter-based peripheral vascular interventions. J. Am. Coll. Cardiol. 44, 941–957 (2004).
- Hess CN, Peterson ED, Neely ML et al. The learning curve for transradial percutaneous coronary intervention among operators in the United States: a study from the national cardiovascular data registry (NCDR). Circulation 129, 2277–2286 (2014).
- Schmidt W, Wissgott C, Anderson R, Behrens P, Schitz KP. Performance characteristics of modern self-expanding nitinol stents indicated for SFA. Rofo 183, 818–825 (2011).
- Krokidis M, Spilhiopoulos S, Katsanos K, Sabharwal T. Peripheral applications of drug-coated balloons: past, present and future. Cardiovasc. Intervent. Radiol. 36, 281–291 (2013).
- Biondo-Zoccai G, Sangiorgi G, D’Ascenzo, et al. Drug-eluting balloons for peripheral artery disease: a meta-analysis of 7 randomized clinical trials and 643 patients. Int. J. Cardiol. 168, 570–571(2013).
- Iqbal J, Onuma Y, Ormiston J, Abizaid A, Waksman R, Serruys P. Bioresorbable scaffolds: rationale, current status, challenges, and future. Eur. Heart J. 35, 765–776 (2014).
- Onuma Y, Ormiston J, Serruys PW. Bioresorbable scaffold technologies. Circ. J. 75, 509–520 (2011).
- Mustapha JA, Saab F, McGoff T et al. Tibio-pedal arterial minimally invasive retrograde revascularization in patients with advanced peripheral vascular disease: the TAMI technique, original case series. Catheter Cardiovasc. Interv. 83, 987–994 (2014).
- Spinosa DJ Harthun NL, Bissonette EA et al. Subintimal arterial flossing with antegrade-retrograde intervention (SAFARI) for subintimal recanalization to treat critical limb ischemia. J. Vasc. Interv. Radiol. 16, 37–44 (2005).
- Rogers RK, Dattilo PB, Garcia JA, Tsai T, Casserly IP. Retrograde approach to recanalization of complex tibial disease. Catheter Cardiovasc. Interv. 77, 915–925 (2011).
- Yeh KH, Tsai YJ, Huag HL, Chou HH, Chang HJ, Ko YL. Dual vascular access for critical limb ischemia: immediate and follow-up results. Catheter Cardiovasc. Interv. 77, 296–302 (2001).
- Londono JC, Singh V, Martinez CA. Posterior tibial artery access using transradial techniques: retrograde approach to inaccessible lower extremity lesions. Catheter Cardiovasc.Interv. 79, 1194–1198 (2012).
- Patel PM, Kern MJ. Mastering transradial techniques: essential skills for complex endovascular peripheral artery intervention. Catheter Cardiovasc. Interv. 79, 1199–1200 (2012).
• US cardiology societies consensus guidelines on the management of patients with peripheral vascular disease.
•• Expert consensus recommendations for effective performance of transradial (TR) percutaneous revascularization by the Transradial Committee of the US Society for Cardiovascular Angiography and Interventions.
• Retrospective review of contemporary prevalence and outcomes of transradial versus transfemoral access for percutaneous coronary intervention (PCI) from the US National Cardiovascular Data Registry.
•• Review of best practices for TR angiography and intervention by the Transradial Working Group of the US Society for Cardiovascular Angiography and Interventions.
•• Comprehensive technical review of contemporary transradial peripheral vascular intervention techniques.
• Ten practical procedural tips for successful performance of transradial peripheral vascular intervention.
• Technical review of strategies for successful negotiation of radio-brachial anatomic challenges during TR intervention.
• Technical review of strategies for successful negotiation of aorto-subclavian anatomic challenges during TR intervention.
• US multispecialty society clinical competency recommendations for the performance of catheter-based peripheral vascular interventions.