Short Communication - Interventional Cardiology (2019) Volume 11, Issue 4
Transcatheter Heart Valve Replacement: The Successes Achieved Are Not Enough to Disguise the Concern of Thrombosis and Structural Valve Deterioration
- Corresponding Author:
- Francesco Nappi
Department of Cardiac Surgery
North Cardiological Center
of Saint-Denis, Paris, France
E-mail: Email: francesconappi2@gmail.com
Received Date: July 13, 2019 Accepted Date: July 18, 2019 Published Date: July 24, 2019
Abstract
This article summarizes the current research on the benefits of using the transfemoral approach for percutaneous aortic valve implant and the transaortic valve replacement as an operation for severe aortic valve stenosis. Based on the available evidence, the authors provide recommendations for the use of the catheter aortic implantation in intermediate/high risk patients undergoing mechanical operation. Further studies are needed before extensive use of the catheter-based aortic valve procedure in younger patients at lower risk.
Keywords
Percutaneous aortic valve implant; Transaortic valve replacement; Aortic valve stenosis
Introduction
Cribier announcing the first steps of a catheter aortic valve implantation has traced a new way for the treatment of severe aortic valve stenosis in prohibitive/high risk patients [1]. This procedure has proven safety and efficacy over the past 17 years [2]. TAVR has rapidly expanded towards the transmitral valve therapy (TMVT) revealing a common destination of replacing standard surgical valve replacement in favor of transcatheter heart valve therapies. The number of procedures performed has increased exponentially, feeding the Registry of Transcatheter Valve Therapy (TVT) with 54,782 TAVR, with a massive increase from 4,627 in 2012 to 28,808 TAVRs implanted in 2015 (1,898 of which were TViV) [3]. Of the 418 reporting centers that perform TAVR, 176 practice transcatheter mitral leaflet clip (TMC) while 98 perform mitral valve-in-valve implantation for degenerated bioprosthesis (TMViV) and mitral valvein- ring implantation for failed annuloplasty rings (TMViR). Transcatheter valve-in-valve (TViV) techniques are used at 292 centers [3].
This therapy is driven by the seemingly unlimited financial generosity of the industries and supported by astonishing technological advances. As a result, many health providers in highly specialized centers have been attracted by gargantuan investment projects of the leading suppliers of the new technology [4]. The remarkable benefit of percutaneous valve replacement in low/intermediate-risk patients is well documented [2], however, European and American experts have raised concerns regarding early structural valve degeneration and thrombosis of the transcatheter heart valve (THV) (THVT) [5,6]. This should induce caution and serve as food for thought prior to pursuing a paradigm shift in management of severe aortic disease in younger patients in the low risk category [4-6].
The growing influence of industrial sponsors via ‘financial generosity’ is also noted in highimpact scientific journals [4]. Randomized clinical trial studies, several observational studies and multicenter registries provide solid scientific information for determining guidelines. Sponsor-led studies may create a serious deterrent to the impartiality of RCTs from which most international guidelines are based on. Independence from funding and the economic compensation is needed to prevent industry directly influencing the progression of studies [2,4,7].
Finding answers to the current issues in TAVR procedure
The “teething issues” of early structural valve deterioration (SVD) [8] and transcatheter heart valve thrombosis (THVT) [9,10] remain unresolved. Thus, the successes to date in refining the technology of the TAVR procedure are insufficient to firmly declare a winner in the race against the surgical AVR.
Aortic valve and root calcifications: are still a concern?
The bulky calcifications of the native aortic valve, especially those extended deep within the ventricle, increase the risk of paravalvular leakage. To date, there is no device that has shown superiority in dealing with this issue. By performing a biomechanical modeling, we noted that the second generation of balloon expandable catheter-based aortic valves may be ineffective when used on solid and bulky native aortic valve calcifications, with higher values of maximum principal stress obtained in aortic regions close to these solid calcific blocks [11]. Conversely, the self-expanding device may be worse in treating cumbersome calcifications due the increased risk of slippage and malposition due to improper device anchorage. We have demonstrated the crucial role of positioning in determining adequate valve anchorage. Uncrushed calcific blocks may result in failure of the procedure due to intra-valvular or paravalvular regurgitation and post-operative deformation of the device [12].
Exciting promises reported in a randomized study using a new device (Lotus Valve System MEV; Boston Scientific Corp) using mechanically expansion have not been backed by convincing results. In a relatively short time MEVs have been withdrawn from trade until 2019 due to a commonly manifesting fault in the locking mechanism [13].
Thrombosis
It is now evident that bioprosthetic valve cusp thrombosis is more common than previously appreciated [9,10]. The guidelines for the administration of anti-platelet and anticoagulant drugs have been modified for new devices to prevent thrombosis, as established in the Partner III RCTs, in which dual antiplatelet treatment is indicated [2]. The ongoing Atlantis trial was conceived to determine the best pharmacological anti-platelet strategy. Thrombosis in surgical bioprosthesis rarely occurs, although high resolution radiological studies have attributed the phenomenon to both procedures [14]. Despite the data reported, a syllogism between the two procedures is not credible [10] because THVT occurs in patients despite dual antiplatelet therapy [2].
Hanson revealed a 7% rate of THVT with 18% of patients experiencing clinically obstructive thrombosis of TAVR despite administration of dual antiplatelet medicament [9]. One of the key indications for SAVR with conventional stented xenograft was the absolute lack of need for these drugs [14].
The current progress of technology and design probably continues to collide with the concept that TAVR was initially designed as a prosthesis for use in the pulmonary artery, which has a higher degree of extensibility and distortion compared to the aortic root [15]. First, due to its function Valsalva’s Sinus of aortic root is not predisposed to receiving any bulky material, moreover with uncrushed solid calcific blocks. It also plays a role in converting the accumulated elastic energy during systole of the left ventricle into kinetic flow energy to ensure distribution of blood in diastole. This delicate function of the Valsalva sinus in TAVR is not preserved by the presence of more deformable or less deformable stent, (nitinol [11] vs chromecobalt [12]), with uncrushed calcifications potentially leading for paravalvular leakage. Secondly, refractory calcific blocks may impact on procedure outcomes such as suboptimal deployment, stent deformation and paravalvular leak which may cause device dislodgment with complications involving the coronary arteries [11,12]. Therefore, the bioprosthetic constituents of the device that normally do not require antiplatelet drugs or anticoagulants when implanted for TAVR may develop thrombosis [2,9,10]. The mechanisms are unclear with a paucity of data available in the literature on the specific relationship existing between persistent noncrushed calcifications and development of thrombosis. Data reported in the literature have rarely focused on specific preoperative measurements of LVOT, annulus, valsalva sinus and STJ in relation to the potential for valve thrombosis. No significant data are available concerning the direction of flow and the geometric axis of the expanded valve in relation to the axis of the LVOT, annulus and Valsalva’s sinus. Ribeiro et al warned that a consistent mismatch with the dimensions of Valsalva’s sinus should raise suspicion for malposition and migration of TAVR that may cause obstruction of the coronary ostia [16-18]. The relationship between different anatomical components of valve placement (LVOT, anatomic ventricular arterial junction, sinotubular junction), as well as the mechanical stresses on leaflet and stent and fluid-dynamic characteristic deserve adequate investigation.
Structural valve degeneration
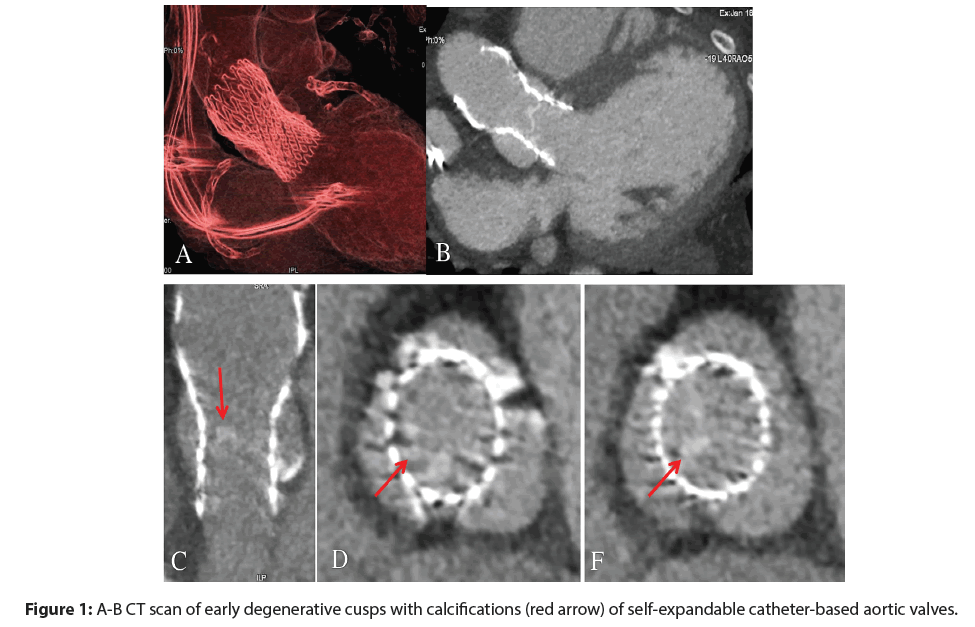
There have been no replicable results concerning the structural valve degeneration from the PARTNER I trial (Placement of Aortic Transcatheter Valve Trial). One study described no SVD requiring redo SAVR at 5 years after SAPIEN implantation [19] while a later analysis reported few cases of reintervention in patients with TAVR from the same RCT with continued access to the registry [20]. Another report revealed the absence of SVD 4 years after SAPIEN implantation but a rate of 9.7% of living patients with moderate prosthetic valve failure lacking the need for reoperation or reintervention at 5-year follow-up [21]. The Medtronic CoreValve device has a prosthesis failure rate of 1.4%. of at 5-years compared to the Sapien (Edwards Life Sciences) [22]. Concerns described by Del Trigo on the early manifestation of SVD have not been fully explained yet. The author described a significantly increased transvalvular gradient in a large cohort of patients within just 2 years (minimum follow-up 6 months) post-implantation and showed an occurrence of patient prosthetic mismatch ranging between 40% and 55% regardless of SVD [8]. The occurrence of SVD was associated to specific subcategories of patients (<23 mm sized valve, high BMI, absence of anticoagulation, valve-in-valve procedures). This risk of SVD could be accentuated during crimping movements in the 14F dispensing systems that are provided with the latest generations of TVT which have thinner leaflets [23] (Figure 1). THVT and SVD could be interconnected as documented in a study supported by refined 4-dimensional computed tomography [10]. The highspeed scanners revealed early and late leaflet immobility, thickness, and thrombosis in both SAVR and TAVR procedures [10]. Concerns have been raised about the immobility of bioprosthetic cusps in determining SVD. However, this condition does not always cause thrombosis because other factors such as valve deformation and unusual tensile loads on the prosthetic leaflets may also play a role [11,12]. The effectiveness of valve durability especially when implanted in younger intermediate risk patients (class IIA LOE B-R), empowered by an RCT which showed non-inferiority of TAVR vs surgical AVR in symptomatic patients at stage D of AS36, deserves further examination. This new potential complication in combination with the fragility of level B-R evidence, dictated by moderate quality of evidence from one or more RCTs require a lot of attention in choice of patients [24].
What clearly emerges from this analysis? Data on the reliability of the duration of device-bound bioprosthesis are not yet sufficient to extend its use in younger-low risk patients. We noted a rather low incidence ranging from <1% before 5 years and 10% at 10 years for patients>65 years old when analyzing the midterm failure rates of porcine and bovine bioprosthesis [14]. Secondgeneration porcine Hancock II valves (Medtronic) include actuarial survival rates with documented longterm outcomes without SVD at 10, 15, and 20 years of 95%, 75%, and 49%, respectively [25]. Evidence on SVD from the Carpentier-Edwards Perimount (Edwards Lifesciences) pericardial valve in the aortic position revealed an actuarial freedom from SVD at 15 and 20 years of 79% and 54%, respectively, with an expected valve durability (median survival time without SVD) of 19 years [14]. Currently, our knowledge on the stress loads of TAV leaflets are limited and and cannot be directly measured. This gap in understanding can only be filled by studying the areas of increased stresses and correlating them to regions of calcific degeneration or leaflet tearing by application of finite element analysis. FEA involves calculating the stress and strain coefficients of the complex structures within a small geometric area whereby its behaviour can be mathematically anticipated. Therefore, its application to medical design of TAVR may allow quantification of stresses and investigate potential failure modes and locations [26].
Biomechanics
Recent reports on the innovative use of Finite Element Analysis (FEA) for research in cardiovascular science may shed light on structural changes in biological systems, such as the degeneration of leaflets and vesselwall stress. Measurements of biomechanical stress have revealed interesting findings regarding leaflet stresses related to the geometry of stented porcine and bovine pericardium xenografts [11,12-26]. Recently, Xuan et al. fully evaluated TAVR, including the leaflets, stent, and sutures. One study performed in quasi-static simulations at 120 mm Hg, evaluated leaflet stresses in 22 mm-diameter self-expandable bovine and porcine valves. The authors considered the measured geometry of leaflets and changes in thickness to determine the stress exerted. The results revealed that the maximum principal stress for bovine and porcine pericardial leaflets was 915.62 kPa and 1565.80 kPa, respectively, in the fully loaded position [27-34]. The measurement of leaflet stresses showed higher deformation and peak stresses along the leaflet-stent attachment along the commissures. Other reports have provided various explanations for the biomechanical performance of a SAPIEN™ TAVR in patient-specific simulations using 3D computed tomography reconstruction, and evaluated the relationship between the geometry of the aortic root and the location of the self-expandable valve [11]. The results of these studies support the notion that a calcified aortic valve and root are associated with a higher risk of coronary obstruction and paravalvular leakage. In addition, aortic root rupture may be visible. Nevertheless, no valid information on the distribution of stresses in leaflets are currently available. Morganti and co-workers studied the correlations among stresses in the aortic root and leaflet asymmetry to estimate the rate of rupture [11,12].
Conclusion
However, their study was limited by the small number of patients and lack of data regarding the influences of asymmetry on TAV leaflet stresses. The study by Xuan et al. provided more emphatic evidence because it showed that peak stresses for both the stent and leaflets of a 26- mm SAPIEN™ Transcatheter Heart Valve were present at commissural tips where the leaflets were attached, which is consistent with, albeit not conclusive of, a risk of leaflet degeneration [27].
An aggressive and timely surgical strategy is likely the best means of managing large complex coronary pseudoaneurysms for lasting benefit.
References
- Yerramareddy VC. A rare case of iatrogenic coronary pseudoaneurysm following pericardiocentesis. IHJ Cardiovascular Case Reports. 1(2): 83-85 (2017).
- Pu L. A giant pseudoaneurysm of coronary artery in a young patient with Behçet's disease. Echocardiography. 34(11): 1736-1737 (2017).
- Harrison A. Cardiovascular complications in behçet syndrome acute myocardial infarction with late stent thrombosis and coronary, ventricular, and femoral pseudoaneurysms. Tex Heart Inst J. 36(5): 498-500 (2019).
- Liu L. Coronary artery pseudoaneurysm following blunt trauma. J Cardiac Surg. 27(5): 563-565 (2012).
- Flum DR, McGinn JT, Tyras DH, et al. Coronary pseudoaneurysm after angioplasty. Am Surg. 61: 1035-1038 (1995).
- Kawazoe H. Spontaneous disappearance of coronary pseudoaneurysm due to coronary artery perforation following percutaneous coronary intervention. Cardiovas intervand therapeut. 28: 408-414 (2013).
- Schöbel WA. Occurrence of a saccular pseudoaneurysm formation two weeks after perforation of the left anterior descending coronary artery during balloon angioplasty in acute myocardial infarction. Catheter Cardiovasc Interv. 47(3): 341-346 (1999).
- Ichikawa M, Kijima Y. Spontaneous resolution of pseudoaneurysm after zotarolimus-eluting stent implantation: imaging evidence at 13 months of follow-up. Catheter Cardiovasc Interv. 30(2): 168-170.
- Chen D. Spontaneous resolution of coronary artery pseudoaneurysm consequent to percutaneous intervention with paclitaxel-eluting stent. Tex Heart Inst J. 35(2): 189-192 (2008).
- Alfonso F. Coronary aneurysms after drug-eluting stent implantation: clinical, angiographic, and intravascular ultrasound findings. JACC 53(22): 2053-2060 (2009).
- Kawai Y. A case of coronary rupture and pseudoaneurysm formation after fracture of implanted paclitaxel-eluting stents. Cardiovas intervand therapeut. 31(3): 231-237 (2016).
- Sandhu PS, Kaul U. Coronary stent fracture resulting in pseudoaneurysm. Indian Heart J. 64: 622-623.
- Choi JH, Song BG, Song YB, et al. Catastrophic coronary stent fracture and coronary perforation presenting as cardiogenic shock: a rare but fatal complication of stenting. Circ Cardiovasc Imaging. 1: 7-8 (2008).
- Aqel RA. Spontaneous coronary artery dissection, aneurysms, and pseudoaneurysms: review. Echocardiography. 21(2): 175-82 (2004).
- Aoki J. Coronary artery aneurysms after drug-eluting stent implantation. JACC: Cardiovasc Interven. 1(1): 14-21 (2008).
- Bell MR. Relation of deep arterial resection and coronary artery aneurysms after directional coronary atherectomy. J Am Coll Cardiol 20: 1474-1481 (1992).
- Dralle JG. Coronary artery aneurysms after angioplasty and atherectomy. Ann Thorac Surg. 59: 1030-1035 (1995).
- Le MQ, Narins CR. Mycotic pseudoaneurysm of the left circumflex coronary artery: a fatal complication following drug‐eluting stent implantation. Catheter Cardiovasc Interv. 69: 508-512 (2007).
- Furtado AD, Bhat SPS, Peer SM, et al. Infected pseudoaneurysm involving a drug-eluting stent. Interact CardioVasc Thorac Surg. 12: 636-638 (2011).
- Svensson A. Variable size of aortic subvalvular pseudoaneurysm. Acta Radiol Open. 7(6): 205-284 (2018).
- Yan J. Stress signaling JNK2 crosstalk with CaMKII underlies enhanced atrial arrhythmogenesis. Circ Res. p: 16 (2019).
- Yan J. Role of stress kinase JNK in binge alcohol-evoked atrial arrhythmia. J Am Coll Cardiol. 71(13): 1459-1470 (2018).
- Qingyan Liu. IL-34 promotes foam cell formation by enhancing CD36 expression through p38 MAPK pathway. Sci Rep. 8: 17347 (2018).
- Nunes RA. Spontaneous closure of post-intervention left anterior descending coronary pseudoaneurysm. Rev Port Cardiol. 33(6): 381 (2014).
- Mikhail B, Brewer RJ, Clark VL, et al. Spontaneous closure of a perforation-induced coronary artery pseudoaneurysm. J Invas Cardiol. 14: 282-284 (2002).
- Lell E. Delayed development of a coronary artery pseudoaneurysm after angioplasty. Cathet ardiovasc Intervent. 47: 186-190 (1999).
- Amano T, Nakamura S. A Case of percutaneous coronary intervention using a polytetrafluorethylene-covered stent for an iatrogenic pseudoaneurysm of the left main coronary artery. J of Cardiol and Vasc Med. 3: 1-5 (2018).
- Johnson NP. Post-intervention coronary pseudoaneurysm treated with a covered stent. Tex Heart Inst J. 39(3): 448-449 (2012).
- Bogaard K. Coronary artery pseudoaneurysm: closure with pericardium-covered stents, guided by cardiac computed tomography angiography. Canadian J of Cardiol. 29: 1014-1014 (2013).
- Sallam T. Coil embolization of left coronary artery pseudoaneurysms arising as a complication of percutaneous coronary intervention. Catheter Cardiovasc Interv. 80(7): 1228-1231 (2012).
- Sasaguri S. A surgical case report of off-pump onlay patch grafting for pseudoaneurysm with diffusely calcified coronary artery. Ann Thorac Cardiovasc Surg. 17: 94-96 (2011).
- Hagau AMT. Large coronary pseudoaneurysm with pulmonary artery fistula, six months after left main trunk stenting with paclitaxel-eluting stent. Med Ultrason. 15(1): 59-62 (2013).
- Cabarrus MBS. Iatrogenic giant coronary artery pseudoaneurysm with "daughter aneurysm" formation: serial imaging findings and natural history. J Thorac Imaging. 27(6): 185-7 (2012).
- Aggarwal P. Successful management of a giant unruptured mycotic coronary artery aneurysm after coronary angioplasty. Indian Heart J. 68(2): 44-46 (2016).