Review Article - Interventional Cardiology (2013) Volume 5, Issue 6
Thrombus aspiration in acute myocardial infarction
- Corresponding Author:
- Sorin J Brener
Division of Cardiology, Cardiac Catheterization Laboratory
New York Methodist Hospital, 506 6th Street, KP-2
Brooklyn, NY 11215, USA
Tel: +1 718 780 5143
Fax: +1 718 780 3930
E-mail: sjb9005@nyp.org
Abstract
Cardiovascular disease accounts for the majority of nonaccidental adult deaths in the USA. According to 2011 mortality data, an acute coronary event (acute coronary syndrome [ACS]) occurs every 30 s and is fatal in half of these cases. Most of the deaths are related to acute plaque disruption in the coronary tree, which causes ACS.
Keywords
mortality, myocardial blush grade, pathophysiology, ST-elevation myocardial infarction, ST-segment elevation, thrombectomy, thrombolysis in myocardial infarction flow
Cardiovascular disease accounts for the majority of nonaccidental adult deaths in the USA. According to 2011 mortality data, an acute coronary event (acute coronary syndrome [ACS]) occurs every 30 s and is fatal in half of these cases [1]. Most of the deaths are related to acute plaque disruption in the coronary tree, which causes ACS.
Overall, mortality from ACS has decreased in the last 20 years because of improved detection and treatment, particularly with the introduction of potent antithrombotic therapy and early reperfusion [2]. Since ACS is caused by acute coronary thrombosis, percutaneous coronary intervention (PCI) is superior to medical therapy alone in nearly all patients with high-risk ACS. The benefit of PCI, particularly in patients with ST-elevation myocardial infarction (STEMI), may be enhanced by thrombus removal. In this article, we will discuss the indications, outcomes and recommendations for the use of adjunctive thrombectomy, as well as the relationship between the composition of the aspirated thrombus material and clinical outcome after primary PCI.
Pathophysiology of ACS
Atherosclerosis is a systemic disease of medium and large arteries characterized by multifocal, lipid-rich plaque development [3,4], predominantly at predilection sites characterized by low and oscillatory endothelial shear stress [5]. The disease begins to develop early in life, but progression varies greatly and is difficult to predict. However, it usually takes decades to develop the advanced lesions that are responsible for clinical disease. While most plaques remain asymptomatic (subclinical disease), some become obstructive, causing stable angina, and a few become thrombosisprone (vulnerable) and may lead to ACS.
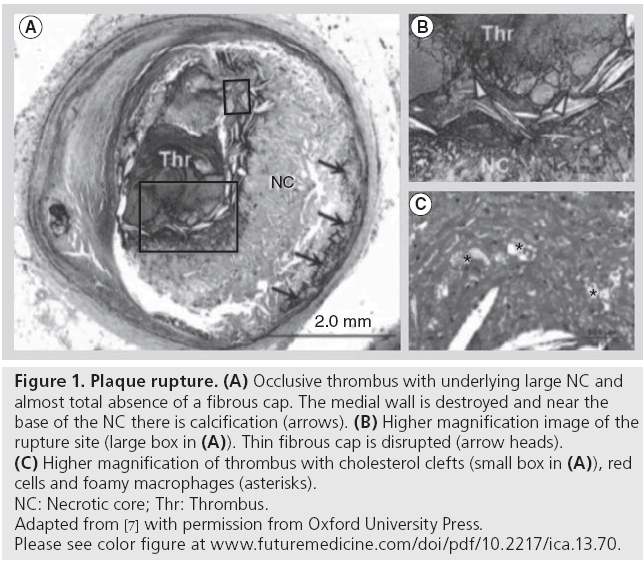
Most data suggest that ACS occurs as a result of some degree of physical disruption to the atherosclerotic plaque with subsequent exposure of the highly thrombogenic subintimal tissue to the blood stream, which leads to the activation of platelets and thrombus formation [6]. There are several forms of plaque disruption that can be identified microscopically. The most frequent type of plaque disruption presents as a gap in the fibrous cap that separates the lipid-rich necrotic core of a plaque from the lumen of the artery (Figure 1). In a worldwide survey [7], plaque rupture was the most frequent cause (~70%) of coronary thrombosis, regardless of clinical presentation, including patients dying from the syndrome and those who survive the acute event [8]. The second form of plaque disruption is plaque fissuring, defined as a lateral tear in an eccentric plaque with underlying small necrotic core. The superficial tear lifts a layer of the intima from the underlying fibrous tissue and the intraplaque hemorrhage extends into the necrotic core, creating a tract usually lined by macrophages. This form of plaque disruption is seen in 10-15% of sudden coronary death cases [9]. Other less common forms of physical disruption of the atherosclerotic plaques include intraplaque hemorrhage and rupture of calcified nodules [10]. In any form of plaque disruption, exposure of the underlying thrombogenic material leads to a cascade of chemical and biological events that result in thrombus formation.
Figure 1: Plaque rupture. (A) Occlusive thrombus with underlying large NC and
almost total absence of a fibrous cap. The medial wall is destroyed and near the
base of the NC there is calcification (arrows). (B) Higher magnification image of the
rupture site (large box in (A)). Thin fibrous cap is disrupted (arrow heads). (C) Higher magnification of thrombus with cholesterol clefts (small box in (A)), red
cells and foamy macrophages (asterisks).
NC: Necrotic core; Thr: Thrombus.
Adapted from [7] with permission from Oxford University Press.
Please see color figure at www.futuremedicine.com/doi/pdf/10.2217/ica.13.70.
The initial coronary thrombus consists predominantly of platelets and is subsequently strengthened by fibrin deposition and entrapment of red blood cells [11]. An additional stagnation thrombus may form proximally and distally to the original thrombus, thus greatly increasing thrombus burden. As ACS develops, the patency of the culprit artery may change over time, manifesting intermittent occlusion and recanalization [12,13]. There is frequently microembolization from the rupture plaque into the distal microcirculation, which is partially mitigated by endogenous t-PA secretion from the endothelium. When occlusion persists for a significant time, STEMI presentation is more likely. By contrast, a nonocclusive thrombus or intermittent patency tend to present as ACS without ST-segment elevation (NSTEMI). The severity of ischemia is influenced by the extent of collateral flow to the affected territory and by adjacent vascular tone.
Why use adjunctive thrombectomy in STEMI patients?
Restoration of normal coronary flow and myocardial salvage are the principal objectives in the management of STEMI. The immediate goal of mechanical reperfusion is to promptly restore normal (thrombolysis in myocardial infarction [TIMI] grade 3) epicardial blood flow in the culprit vessel. This can be achieved nearly universally with current technology [14]. By contrast, tissue reperfusion at the level of the myocardium is not normalized in nearly a third of patients, even after successful primary PCI, limiting the benefit of epicardial reperfusion [15].
Potential mechanisms for microvascular dysfunction include distal macro- or microembolization, in situ formation of thrombus, generation of oxygen free radicals, cellular and interstitial edema, endothelial dysfunction, vasoconstriction, inflammation, and microvascular hemorrhage or destruction [15]. It is likely that distal embolization occurs predominantly after stent deployment and may result in no-reflow or slow flow in the infarct-related artery. This has led to growing interest in reducing the thrombus burden as an initial step during primary PCI. The two principal strategies to achieve this goal are thrombus aspiration and intracoronary administration of glycoprotein IIb/IIIa inhibitors (GPIs). A third modality, prevention of distal embolization by proximal or distal occlusion devices, has not been shown to be beneficial [16]. Several large randomized trials evaluated the use of GPIs in STEMI with conflicting results [16,17]. Patients with the highest risk profile and most abnormal baseline characteristics benefit the most from the use of GPIs [18]. Currently, it is a class IIa American College of Cardiology/American Heart Association (ACC/AHA) recommendation to use GPIs for selected patients with high thrombus burden [19]. Routine use of GPIs in STEMI does not appear to be beneficial, although direct intralesion administration of abciximab may reduce infarct size [17,20]. This review will focus on thrombus aspiration and evacuation.
Thrombectomy devices
Several thrombectomy devices have been designed to reduce thrombus burden and prevent distal embolization. These can be divided into mechanical thrombectomy devices (e.g., AngioJet™ [Medrad Interventional/Possis, MN, USA], Rescue™ [BSC, MN, USA], X-Sizer™ [ev3, MN, USA]); and manual thrombectomy devices.
▪ Mechanical thrombectomy
The AngioJet thrombectomy system consists of a hollow catheter through which saline is infused under pressure and forced to exit retrogradely just before the catheter tip. These jets create a localized vacuum, drawing thrombus into the hollow catheter (Venturi effect), where it is mechanically disrupted and evacuated from the body. The US FDA approved the device in 1998 based on its performance among patients with ACS enrolled in the VeGAS-1 and -2 trials [21]. In the subsequent AIMI trial, patients randomized to adjunctive thrombectomy had a larger infarct size compared with conventional PCI, a lower rate of TIMI flow grade 3 and a higher incidence of 30-day major adverse clinical events [22].
The X-Sizer catheter has a helical distal cutter to break the thrombus, which is suctioned out by an external vacuum source. The device is no longer available for coronary use, although it improved microvascular reperfusion in three randomized STEMI trials [23-25].
Manual aspiration thrombectomy
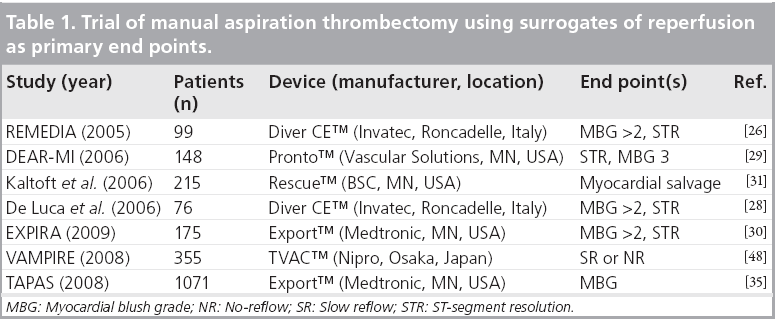
STEMI guidelines predominantly recommend the use of manual, rather than mechanical, aspiration devices. Examples, among more than 15 brands available, include: Diver CE™ (Invatec, Roncadelle, Italy), Export™ (Medtronic, MN, USA), QuickCat™ (Kensey Nash, PA, USA), Xpress-Way™ (Atrium Medical Corporation, NH, USA), and Pronto™ (Vascular Solutions, MN, USA) catheters. The maximal tip diameter for these catheters (fitting 6-F guiding catheters) varies between 1.34 m (Pronto) and 1.73 m (Export). The Diver CE is equipped with side holes (in one version) for fresher thrombus aspiration, and some are available in 7-F (Xpress-Way, Export and Pronto) and even 8-F size (Pronto). Manual aspiration catheters are easy to use and are inexpensive. Based on the pathophysiological considerations described above, they address three distinct objectives in the management of STEMI patients: evacuate as much thrombus as possible to prevent (further) distal embolization of plaque and thrombus material; eliminate circulating pro-inflammatory and -thrombotic substances secreted by activated platelets and injured endothelium; and provide a conduit for intralesion and intracoronary selective administration of vasoactive substances and antithrombotic agents. The early studies with these devices were small and lacked statistical power to detect differences in clinical outcomes. They used surrogate markers of reperfusion as primary end points (Table 1).
In the 100 STEMI patients of the REMEDIA trial, aspiration thrombectomy with the Diver CE device improved myocardial reperfusion (ST-segment resolution [STR] ≥70%: 44.9 vs 36.7%; p = 0.02 and myocardial blush grade [MBG] ≥2: 68.0 vs 58.0%; p = 0.034). Despite the favorable effect on surrogate markers, the REMEDIA trial failed to show a benefit in 30-day major adverse cardiac events (MACE - death, stroke, reinfarction and target vessel revascularization) for the aspiration arm, but the study lacked power for MACE [26]. In a 50-patient substudy using myocardial contrast echocardiography, thrombus aspiration reduced early microvascular obstruction and marginally improved 6-month adverse left ventricular remodeling [27].
De Luca and colleagues analyzed data from 76 patients with anterior STEMI who had aspiration thrombectomy and found that it improved microvascular function (MBG and STR) and reduced frequency of adverse left ventricular remodeling at 6 months; again, there was no difference in MACE rate [28].
The DEAR-MI trial evaluated the Pronto aspiration catheter. The thrombectomy group had improved microvascular perfusion, less frequent no-reflow (3 vs 15%; p < 0.05), and reduced angiographic embolization (5 vs 19%; p < 0.05). There was also less myonecrosis (lower peak CK-MB), but no improvement was seen in in-hospital outcomes [29].
The EXPIRA trial randomized 175 patients with STEMI to primary PCI alone versus primary PCI with manual thrombectomy and showed a significant improvement in the rates of MBG 3 and complete STR. This study was unique in that it evaluated infarct size by MRI and also found that microvascular obstruction extent was lower in the acute phase with aspiration (1.7 vs 3.7 g; p = 0.0003). An improvement in infarct remodeling (size reduction) at 3 months was also seen with aspiration (from 17 to 11% of left ventricular mass; p = 0.004), but not in the control group (from 14 to 13%; p = not significant) [30].
The INFUSE-AMI trial was a multicenter, randomized, single-blinded trial with a 2 × 2 factorial design (intralesion abciximab vs no abciximab [Clearway™ catheter; Atrium Medical Corporation] and aspiration thrombectomy [Export catheter] vs no aspiration) and included 452 patients with anterior STEMI at 37 sites in six countries. The study used infarct size as a percentage of left ventricular mass measured by cardiac MRI after 30 days as the primary end point. There was no significant difference between patients randomized to aspiration thrombectomy versus no aspiration in infarct size (median: 17.0 [interquartile range (IQR): 9.0-22.8%] vs 17.3% [IQR: 7.1-25.5%], respectively; p = 0.51), absolute infarct mass (median: 20.3 [IQR: 9.7-31.7 g] vs 21.0 g [IQR: 9.1-34.1 g], respectively; p = 0.36), or abnormal wall motion score (median score 7.5 in both groups; p = 0.89) [17].
Finally, in a randomized study using the Rescue catheter, Kaltoft and colleagues reported that thrombectomy had no impact on myocardial salvage, measured by sestamibi single-photon emission computed tomography (SPECT) nuclear imaging. In fact, infarct size was increased in the thrombectomy group (15 vs 8%; p = 0.004) [31]. It is possible that bulky catheters cause distal embolization of thrombotic debris before aspiration occurs. Two recent meta-analyses refute this speculation, showing that aspiration thrombectomy is associated with a lower rate of angiographically visible distal embolization (AVDE) [32,33].
TAPAS trial
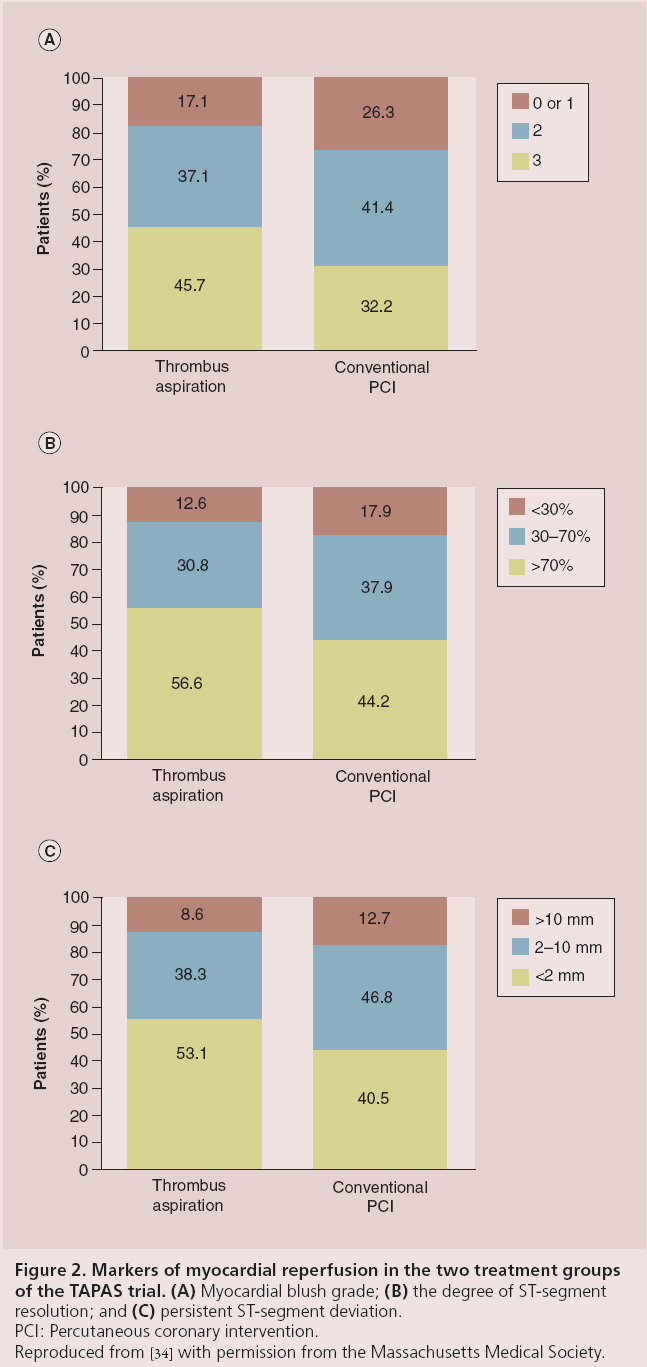
The TAPAS trial is the largest randomized study to date evaluating the effects of manual thrombectomy [34]. It was performed at a single center in The Netherlands and included 1071 patients with STEMI. Patients were randomized to either aspiration thrombectomy with the 6-F Export catheter before conventional PCI (n = 535) or to conventional PCI alone (n = 536). PCI could be performed by direct stenting or by balloon angioplasty followed by stent deployment, and bare-metal stents were used in all patients. The primary end point was final MBG. Secondary end points included the degree of STR, and clinical outcomes (death or death/MI) at 30 days and 1 year. Inclusion criteria were very broad. Treatment allocation was performed before angiography, independent of thrombus burden. Less than 10% of patients crossed over from one arm to the other. There were no significant differences in baseline characteristics between the two groups. Angiographically visible thrombus was noted in 48.6% of the thrombectomy group and in 44.0% of the conventional PCI group (p = 0.14). Successful aspiration of debris was confirmed by histopathological examination in 72.9% of cases. The primary end point of postprocedural MBG 0 or 1 occurred in 17.1% of patients in the thrombectomy group compared with 26.3% in the conventional PCI group (risk ratio [RR]: 0.65; 95% CI: 0.51-0.83; p < 0.001; Figure 2). Frequency of postprocedural TIMI grade 3 flow was similar between groups (86 vs 82.5%; p = 0.12). Other secondary end points such as complete STR (56.6 vs 44.2%; p < 0.001) and absence of persistent ST-segment deviation (53.1 vs 40.5%; p < 0.001) were more common in the thrombectomy group (Figure 2).
Figure 2: Markers of myocardial reperfusion in the two treatment groups
of the TAPAS trial.(A) Myocardial blush grade; (B) the degree of ST-segment
resolution; and (C) persistent ST-segment deviation.
PCI: Percutaneous coronary intervention.
Reproduced from [34] with permission from the Massachusetts Medical Society.
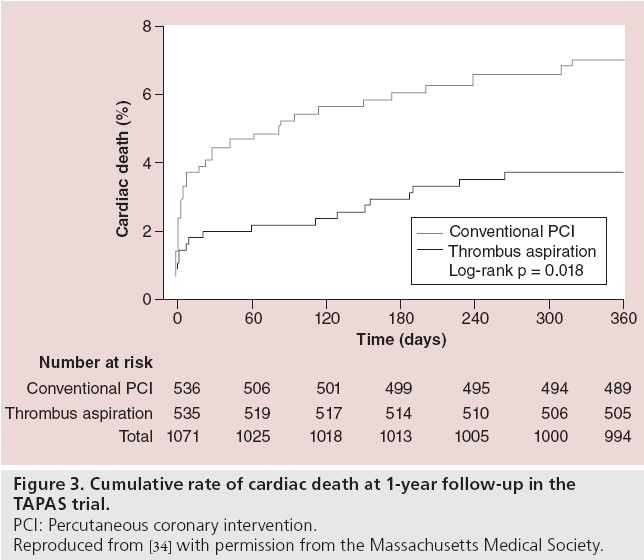
Importantly, improved myocardial perfusion was associated with a significantly lower 30-day mortality (1.1 vs 2.9 vs 5.7% for MBG 3, 2 and 0/1, respectively; p = 0.001). The early mortality benefit associated with MBG 3 persisted at 1 year (3.7 vs 4.7 vs 11%, respectively; p = 0.001). There were no significant differences in reinfarction (0.8 vs 1.9%; p = 0.11), target vessel revascularization (4.5 vs 5.8%; p = 0.34), major bleeding (3.8 vs 3.4%; p = 0.11) and MACE (6.8 vs 9.4%; p = 0.12) at 30 days between the thrombectomy and conventional PCI groups, respectively. At 1 year, cardiac mortality was 3.6% in the thrombectomy group and 6.7% in the conventional PCI group (p = 0.020) (Figure 3) [101]. The 1-year composite end point of cardiac mortality or nonfatal reinfarction was higher in the conventional PCI group (hazard ratio [HR]: 1.81; 95% CI: 1.16-2.84; p = 0.009) [35].
Figure 3: Cumulative rate of cardiac death at 1-year follow-up in the
TAPAS trial.
PCI: Percutaneous coronary intervention.
Reproduced from [34] with permission from the Massachusetts Medical Society.
▪ Clinical significance of the TAPAS trial
The TAPAS trial is the largest clinical trial of adjunctive manual thrombectomy to date and may be more representative of contemporary practice than other studies because of its broad inclusion criteria. Most patients were treated with stents and GPIs. TAPAS confirmed the observations from other small randomized trials regarding the improvement in myocardial perfusion after adjunctive aspiration thrombectomy. The association with a reduction in mortality supports the concept that microvascular protection is technically feasible and clinically relevant in STEMI; however, TAPAS was a single, highvolume center with experienced operators and very short door-to-aspiration time (28 min; IQR: 14-42 min), and thus may overestimate the true advantages of this intervention. Recently, the TASTE trial reported that manual thrombus aspiration did not reduce mortality at 30 days among 7012 Scandinavian patients with STEMI (2.8 vs 3.0% in conventional PCI arm; HR: 0.94; 95% CI: 0.72-1.23; p = 0.63) [36]. Currently, the TOTAL trial is randomizing approximately 4000 patients with STEMI to PCI alone versus PCI with adjunctive aspiration thrombectomy. The primary end point of the study is the first occurrence of cardiovascular death, recurrent myocardial infarction, cardiogenic shock, or new or worsening New York Heart Association Class IV heart failure within 180 days [102]. The study should provide clear data as to whether aspiration thrombectomy should be routinely performed during primary PCI. Another trial, ‘Effect of Thrombus Aspiration in Patients With Myocardial Infarction Presenting Late After Symptom Onset’, is being conducted in Germany and uses microvascular obstruction by cardiac MRI as its primary end point [103].
Meta-analysis of randomized controlled trials
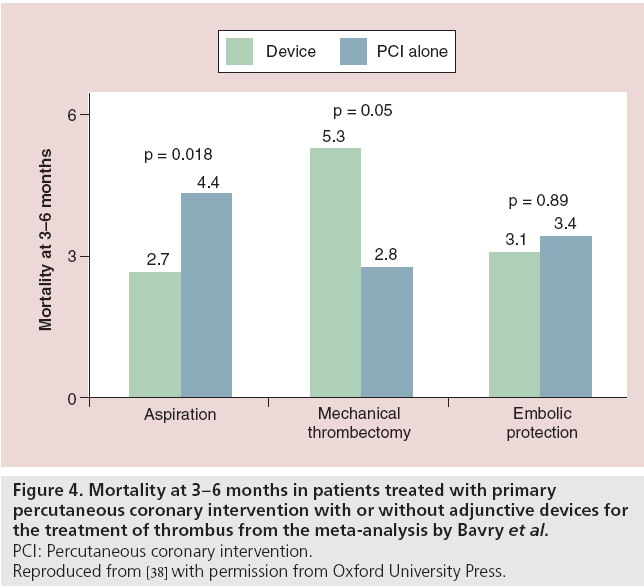
As most of the trials testing adjunctive thrombectomy were not powered to detect differences in clinical outcome, several meta-analyses have been conducted to evaluate its role in primary PCI. Bavry et al. analyzed 30 trials (6415 patients) that tested adjunctive thrombectomy or emboli protection devices during primary PCI, among which 13 trials used manual thrombus aspiration (47% of the total populations) [37]. In this metaanalysis, manual thrombectomy resulted in better MBGs (the incidence of MBG 3 after revascularization was 53% for adjunctive devices vs 40% for PCI alone [RR: 1.38; 95% CI: 1.20-1.58; p < 0.0001]) and improved STR (the incidence of complete STR was 63% for adjunctive devices vs 53% for PCI alone [RR: 1.27; 95% CI: 1.15-1.41; p < 0.0001]). Importantly, the 3-6 month mortality was significantly lower in patients receiving manual aspiration compared with the control group (2.7 vs 4.4%; p = 0.018) [37]. In the same meta-analysis, manual aspiration was superior to emboli protection devices and mechanical thrombectomy, neither of which showed clinical benefit (Figure 4).
Figure 4: Mortality at 3-6 months in patients treated with primary
percutaneous coronary intervention with or without adjunctive devices for
the treatment of thrombus from the meta-analysis
by Bavry et al.
PCI: Percutaneous coronary intervention.
Reproduced from [38] with permission from Oxford University Press.
In another meta-analysis of 18 prospective randomized controlled trials, Burzotta and colleagues used patient-level data that allowed evaluation of outcomes in subgroups [32]. No statistically significant difference was found between adjunctive device therapy and PCI versus standard PCI for early death or reinfarction (odds ratio [OR]: 0.85; 95% CI: 0.54-1.33; 13 RCTs; n = 2818). Subgroup analyses found a statistically significant effect in favor of treatment with thrombectomy in reducing AVDE (OR: 0.51; 95% CI: 0.28-0.92; seven RCTs; n = 1281), absence of STR (OR: 0.46; 95% CI: 0.32-0.66; 12 RCTs; n = 1934) and absence of MGB <3 (OR: 0.42; 95% CI: 0.23-0.75; nine RCTs; n = 1562). All the benefit was confined to aspiration thrombectomy, while none was observed with distal emboli protection devices. This metaanalysis has the advantage of a patient-level analysis, but also has the potential for bias, as only 11 of 18 trial investigators agreed to participate and provide data for the study. There was also significant heterogeneity among studies and statistical evidence for small trial bias. In a subsequent meta-analysis from the same group, using patient-level data from 2686 patients enrolled in 11 trials, adjunctive thrombectomy was associated with reduced 1-year mortality (p = 0.049). Subgroups analysis showed that thrombectomy is associated with improved survival in patients treated with GPIs (p = 0.045) and that the survival benefit is confined to patients treated with manual thrombectomy (p = 0.011) [38].
In the meta-analysis performed by De Luca and colleagues in 2008, which included nine randomized trials with 2417 patients (1209 patients in the manual thrombectomy device group and 1208 in the control group), PCI preceded by manual thrombectomy was compared with PCI alone. Adjunctive manual thrombectomy was associated with significantly improved postprocedural TIMI grade 3 flow (87.1 vs 81.2%; p < 0.0001) and postprocedural MBG 3 (52.1 vs 31.7%; p < 0.0001), less distal embolization (7.9 vs 19.5%; p < 0.0001), and a significant reduction in 30-day mortality (1.7 vs 3.1%; p = 0.04) [39].
Finally, in a Bayesian meta-analysis of 21 randomized trials, thrombectomy (performed in 16 studies) resulted in significantly less no-reflow (OR: 0.39; 95% credible interval [CrI]: 0.18-0.69), more complete STR (OR: 2.22; 95% CrI: 1.60-3.23) and higher rates of TIMI grade 3 flow (OR: 2.50; 95% CrI: 1.48-4.41). There was no reduction in death (OR: 0.94; 95% CrI: 0.47-1.80), or in the composite of death, recurrent MI or stroke (OR: 1.07; 95% CrI: 0.63-1.92) with thrombectomy. Restriction of the analysis to trials that used manual aspiration thrombectomy devices yielded similar results [40].
A special clinical scenario that may be particularly well suited for thrombus aspiration is the treatment of acute stent thrombosis, because of the large thrombus burden present in these patients. In a propensity-adjusted analysis of 205 patients from multiple centers with angiographically proven stent thrombosis, manual aspiration was used in 56% of the subjects. After adjustment for clinical and angiographic characteristics, aspiration thrombectomy resulted in higher rates of final TIMI grade 3 flow and improved procedural success (96 vs 83% for conventional PCI; p < 0.001), There was no significant difference in long-term mortality (adjusted HR: 0.99; 95% CI: 0.44-2.24) [41].
Current guidelines for adjunctive thrombectomy
Based on these data, the ACC/AHA guidelines have given aspiration thrombectomy a class IIa (level of evidence B) indication in primary PCI for STEMI. The European Society of Cardiology guidelines also conferred manual thrombus aspiration a class IIa indication (level of evidence A) [42,43]. Some authors advocate aspiration thrombectomy only in patients with high thrombus burden [44,45]. Such a strategy may be difficult to implement as at least 75% of patients with STEMI have no-flow (TIMI 0) on initial angiogram, rendering evaluation of thrombus burden difficult or impossible.
Aspirated thrombus composition and its relation to clinical outcome
Investigators at a high-volume STEMI center in The Netherlands correlated thrombus age with outcome in 1315 consecutive STEMI cases undergoing primary or rescue PCI [46]. Aspirated material was assessed by histopathology for thrombus presence. When found (75.3% of the cohort), thrombus was classified as either fresh (<24 h, containing mostly erythrocytes, granulocytes, platelets and fibrin) or older thrombus (characterized by the presence of necrotic fragments from red and white blood cells, as well as smooth muscle growth, neovascularization and connective tissue deposition). Fresh thrombus was present in 552 patients (42.0%), while older thrombus was seen in 372 patients (28.2%). Patients with older thrombus had a significantly higher 4-year mortality (16.0 vs 7.4%; HR: 1.82; 95% CI: 1.17-2.85; p = 0.008), potentially related to less myocardial salvage. A more recent study assessed the association between composition of aspirated thrombi and the incidence of AVDE during PCI in patients with STEMI treated with thrombus aspiration. The study included 164 patients (126 men; mean age 65 ± 12 years). In aspirated samples, histopathological analysis revealed thrombus components alone in 118 patients (72%), and both thrombus and plaque fragments (containing macrophage-derived foam cells and occasional smooth muscle cells) in 46 patients (28%). The authors concluded that an erythrocyte- rich component in aspirated coronary thrombi and plasma glucose levels on admission were closely associated with the incidence of AVDE [47].
Conclusion
Manual thrombus aspiration, but not distal embolic protection devices or mechanical thrombectomy, improves markers of reperfusion during primary PCI. One single-center study and a meta-analysis of smaller trials indicated the possibility that manual thrombectomy reduced mortality. Large-scale multicenter randomized trials are needed to confirm its utility in all STEMI patients.
Future perspective
The data presented in this review suggest that we have a fairly mature understanding of the pathophysiological processes leading to STEMI. The disparity between the effect of thrombus aspiration on mortality in older versus newer trials suggests that we need to better identify the patient and vessel characteristics that are most likely to benefit from this intervention. We predict that in the next few years we will be able to better image the thrombus, identify its components and select the patients best suited for thrombectomy. Better and more selective delivery of antithrombotic medications may further assist in improving outcome in STEMI.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Atherosclerotic plaque rupture results in thrombus formation and acute myocardial infarction.
▪ Manual thrombus aspiration improves infarct vessel reperfusion and prevents distal embolization of plaque and thrombus material.
▪ There is conflicting evidence regarding the effect of thrombectomy on short- and long-term survival.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Roger VL, Go AS, Lloyd-Jones DM et al. Executive summary: heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation 125, 188–197 (2012).
- Lloyd-Jones D, Adams RJ, Brown TM et al. Executive summary: heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation 121, 948–954 (2010).
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325 (2011).
- Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res. 95, 156–164 (2012).
- Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 49, 2379–2393 (2007).
- Crea F, Liuzzo G. Pathogenesis of acute coronary syndromes. J. Am. Coll. Cardiol. 61, 1–11 (2013).
- Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur. Heart J. 34, 719–728 (2013).
- Kubo T, Imanishi T, Takarada S et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J. Am. Coll. Cardiol. 50, 933–939 (2007).
- Schaar JA, Muller JE, Falk E et al. Terminology for high-risk and vulnerable coronary artery plaques. Report of a meeting on the vulnerable plaque, June 17 and 18, 2003, Santorini, Greece. Eur. Heart J. 25, 1077–1082 (2004).
- Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation 111, 3481–3488 (2005).
- Falk E. Dynamics in thrombus formation. Ann. NY Acad. Sci. 667, 204–223 (1992).
- Falk E. Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death. Autopsy evidence of recurrent mural thrombosis with peripheral embolization culminating in total vascular occlusion. Circulation 71, 699–708 (1985).
- Ambrose JA, Winters SL, Arora RR et al. Coronary angiographic morphology in myocardial infarction: a link between the pathogenesis of unstable angina and myocardial infarction. J. Am. Coll. Cardiol. 6, 1233–1238 (1985).
- Prasad A, Stone GW, Aymong E et al. Impact of ST-segment resolution after primary angioplasty on outcomes after myocardial infarction in elderly patients: an analysis from the CADILLAC trial. Am. Heart J. 147, 669–675 (2004).
- Prasad A, Gersh BJ. Management of microvascular dysfunction and reperfusion injury. Heart 91, 1530–1532 (2005).
- Stone GW, Webb J, Cox DA et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA 293, 1063–1072 (2005).
- Stone GW, Maehara A, Witzenbichler B et al. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA 307, 1817–1826 (2012).
- De Luca G, Navarese E, Marino P. Risk profile and benefits from Gp IIb-IIIa inhibitors among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: a meta-regression analysis of randomized trials. Eur. Heart J. 30, 2705–2713 (2009).
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; O’Gara PT, Kushner FG, Ascheim DD et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 61(4), e78–e140 (2013).
- Eitel I, Wohrle J, Suenkel H et al. Intracoronary compared with intravenous bolus abciximab application during primary percutaneous coronary intervention in STsegment elevation myocardial infarction: cardiac magnetic resonance substudy of the AIDA STEMI trial. J. Am. Coll. Cardiol. 61, 1447–1454 (2013).
- Kuntz RE, Baim DS, Cohen DJ et al. A trial comparing rheolytic thrombectomy with intracoronary urokinase for coronary and vein graft thrombus (the Vein Graft AngioJet Study [VeGAS 2]). Am. J. Cardiol. 89, 326–330 (2002).
- Ali A, Cox D, Dib N et al. Rheolytic thrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarction: 30-day results from a multicenter randomized study. J. Am. Coll. Cardiol. 48, 244–252 (2006).
- Lefevre T, Garcia E, Reimers B et al. X-sizer for thrombectomy in acute myocardial infarction improves ST-segment resolution: results of the X-sizer in AMI for negligible embolization and optimal ST resolution (X AMINE ST) trial. J. Am. Coll. Cardiol. 46, 246–252 (2005).
- Napodano M, Pasquetto G, Sacca S et al. Intracoronary thrombectomy improves myocardial reperfusion in patients undergoing direct angioplasty for acute myocardial infarction. J. Am. Coll. Cardiol. 42, 1395–1402 (2003).
- Beran G, Lang I, Schreiber W et al. Intracoronary thrombectomy with the X-sizer catheter system improves epicardial flow and accelerates ST-segment resolution in patients with acute coronary syndrome: a prospective, randomized, controlled study. Circulation 105, 2355–2360 (2002).
- Burzotta F, Trani C, Romagnoli E et al. Manual thrombus-aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus-aspiration in primary and rescue angioplasty (REMEDIA) trial. J. Am. Coll. Cardiol. 46, 371–376 (2005).
- Galiuto L, Garramone B, Burzotta F et al. Thrombus aspiration reduces microvascular obstruction after primary coronary intervention: a myocardial contrast echocardiography substudy of the REMEDIA trial. J. Am. Coll. Cardiol. 48, 1355–1360 (2006).
- De Luca L, Sardella G, Davidson CJ et al. Impact of intracoronary aspiration thrombectomy during primary angioplasty on left ventricular remodelling in patients with anterior ST elevation myocardial infarction. Heart 92, 951–957 (2006).
- Silva-Orrego P, Colombo P, Bigi R et al. Thrombus aspiration before primary angioplasty improves myocardial reperfusion in acute myocardial infarction: the DEAR-MI (Dethrombosis to Enhance Acute Reperfusion in Myocardial Infarction) study. J. Am. Coll. Cardiol. 48, 1552–1559 (2006).
- Sardella G, Mancone M, Bucciarelli-Ducci C et al. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J. Am. Coll. Cardiol. 53, 309–315 (2009).
- Kaltoft A, Bottcher M, Nielsen SS et al. Routine thrombectomy in percutaneous coronary intervention for acute ST-segmentelevation myocardial infarction: a randomized, controlled trial. Circulation 114, 40–47 (2006).
- Burzotta F, Testa L, Giannico F et al. Adjunctive devices in primary or rescue PCI: a meta-analysis of randomized trials. Int. J. Cardiol. 123, 313–321 (2008).
- De Luca G, Suryapranata H, Stone GW, Antoniucci D, Neumann FJ, Chiariello M. Adjunctive mechanical devices to prevent distal embolization in patients undergoing mechanical revascularization for acute myocardial infarction: a meta-analysis of randomized trials. Am. Heart J. 153, 343–353 (2007).
- Svilaas T, Vlaar PJ, van der Horst IC et al. Thrombus aspiration during primary percutaneous coronary intervention. N. Engl. J. Med. 358, 557–567 (2008).
- Vlaar PJ, Svilaas T, van der Horst IC et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet 371, 1915–1920 (2008).
- Fröbert O, Lagerqvist B, Olivecrona GK et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N. Engl. J. Med. doi:10.1056/NEJMoa1308789 (2013) (Epub ahead of print).
- Bavry AA, Kumbhani DJ, Bhatt DL. Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction: a comprehensive meta-analysis of randomized trials. Eur. Heart J. 29, 2989–3001 (2008).
- Burzotta F, De Vita M, Gu YL et al. Clinical impact of thrombectomy in acute STelevation myocardial infarction: an individual patient-data pooled analysis of 11 trials. Eur. Heart J. 30, 2193–2203 (2009).
- De Luca G, Dudek D, Sardella G, Marino P, Chevalier B, Zijlstra F. Adjunctive manual thrombectomy improves myocardial perfusion and mortality in patients undergoing primary percutaneous coronary intervention for STelevation myocardial infarction: a meta-analysis of randomized trials. Eur. Heart J. 29, 3002–3010 (2008).
- Mongeon FP, Belisle P, Joseph L, Eisenberg MJ, Rinfret S. Adjunctive thrombectomy for acute myocardial infarction: a Bayesian meta-analysis. Circ. Cardiovasc. Interv. 3, 6–16 (2010).
- Waldo SW, Armstrong EJ, Yeo KK et al. Procedural success and long-term outcomes of aspiration thrombectomy for the treatment of stent thrombosis. Catheter. Cardiovasc. Interv. (2013).
- Kushner FG, Hand M, Smith SC Jr et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 120, 2271–2306 (2009).
- Van de Werf F, Bax J, Betriu A et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the task force on the management of ST-segment elevation acute myocardial infarction of the European society of cardiology. Eur. Heart J. 29, 2909–2945 (2008).
- Javaid A, Siddiqi NH, Steinberg DH et al. Adjunct thrombus aspiration reduces mortality in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction with high-risk angiographic characteristics. Am. J. Cardiol. 101, 452–456 (2008).
- Margheri M, Vittori G, Chechi T et al. Thrombus aspiration with export catheter in ST elevation myocardial infarction. J. Interv. Cardiol. 20, 38–43 (2007).
- Kramer MC, van der Wal AC, Koch KT et al. Presence of older thrombus is an independent predictor of long-term mortality in patients with ST-elevation myocardial infarction treated with thrombus aspiration during primary percutaneous coronary intervention. Circulation 118, 1810–1816 (2008).
- Yunoki K, Naruko T, Inoue T et al. Relationship of thrombus characteristics to the incidence of angiographically visible distal embolization in patients with ST-segment elevation myocardial infarction treated with thrombus aspiration. JACC Cardiovasc. Interv. 6, 377–385 (2013).
- Ikari Y, Sakurada M, Kozuma K et al. Upfront thrombus aspiration in primary coronary intervention for patients with ST-segment elevation acute myocardial infarction: report of the VAMPIRE (Vacuum Aspiration Thrombus Removal) trial. JACC Cardiovasc. Interv. 1, 424–431 (2008).
▪▪ Excellent review of the biology of atherosclerosis.
▪ Updated review of all the novel aspects of acute coronary syndrome pathosphyisology.
▪ Meta-analysis of the effect of glycoprotein inhibitors on outcomes in ST-segment elevation myocardial infarction.
▪▪ Only study to demonstrate a reduction in mortality with annual thrombus aspiration.
▪ Meta-analysis of the effect of manual aspiration thrombectomy on outcomes in ST-segment elevation myocardial infarction.
▪ Websites
- American Heart Association. New drugs and technologies. http://circ.ahajournals.org/ content/119/9/1311.full – F2
- A Trial Of Routine Aspiration Thrombectomy With Percutaneous Coronary Intervention (PCI) Versus PCI Alone In Patients With ST-Segment Elevation Myocardial Infarction (STEMI) Undergoing Primary PCI (TOTAL). www.clinicaltrials.gov/show/NCT01149044
- Effect Of Thrombus Aspiration In Patients With Myocardial Infarction Presenting Late After Symptom Onset. www.clinicaltrials.gov/show/NCT01379248