Research Paper - Imaging in Medicine (2017) Volume 9, Issue 5
The value of fetal trans cerebellar diameter in detecting GA in different fetal growth patterns in Egyptian fetuses
Mahmoud Alalfy*, Omaima Idris, Hassan Gaafar, Hany Saad, Omar Nagy, Yossra Lasheen, Hadeer Meshaal Sherif Elsirgany & Ahmed HassanNational Research Centre, Algeza, Egypt
- Corresponding Author:
- Mahmoud Alalfy
National Research Centre
Algeza, Egypt
E-mail: mahmoudalalfy@ymail.com
Abstract
Ultrasonographic assessment of fetal gestational age is very critical for clinical decision making in obstetrics, many fetal biometric parameters are extensively used but in this research study we mainly targeted transcerebellar diameter in second and third trimester among Egyptian population and its accuracy and clinical reliability to calculate fetal gestational age.
Aim of the research: The main goal and corner stone purpose of this research was to measure the accuracy of the transcerebellar diameter Transcerebellar diameter in calculating the gestational age of fetuses among Egyptian population and if it is more accurate than other fetal biometric measurements (Biparietal diameter, Head circumference, Abdominal circumference, Femur Length) in gestations with no medical disorders or not and the additional different methodology implemented in this research in comparison to other studies was using the Transcerebellar diameter in diabetic mothers with macrosomic fetuses, in pregnant Egyptian ladies with hypertension during pregnancy that have growth restricted fetuses with evaluation of uteroplacental blood flow by Doppler flow indices confirming uteroplacental insufficiency, also in fetuses with congenital structural abnormalities and in cerebellar anomalies.
Results: The IQR interquartile range of the discrepancy between menstrual and sonographic gestational ages was the least when Transcerebellar diameter, in comparison to Biparieteal Diameter, Head Circumference, Abdominal Circumference and Femur Length (0.43 mm, 1.27 mm, 1.0 mm, 1.56 mm and 1.28 mm, respectively). The difference, however, was statistically significant only when compared to that using Abdominal Circumference.
Conclusion: We concluded that Transcerebellar diameter is the most accurate biometric measurement in both uncomplicated pregnancies and in complicated pregnancies with medical disorders such as Diabetes mellitus and hypertension especially when associated with fetal macrosomia or fetal intrauterine growth restriction and in structural abnormalities affecting organs other than cerebellum as the cerebellar growth is not affected by these circumstances.
Keywords
transcerebellar diameter (TCD) ▪ biparietal diameter (BPD) ▪ head circumference (HC) ▪ abdominal circumference (AC) ▪ femur length (FL)
Introduction
Confirmation of gestational age obtained by LMP is made by ultrasound especially when there is discrepancy between fetal measurements. This avoids fallacies caused by different times of ovulation and aids in proper decision making in obstetrics. Second trimester fetal biometry is not accurate as LMP or first trimester CRL for proper estimation of gestational age even more some authors consider CRL measurement is more reliable than LMP which raises the clinical value [1].
First trimester screening is also essential to detect fetal anomalies and is more accurate significantly in estimation of fetal gestational age than second trimester fetal biometry [2]. In pregnancies that are not complicated by any medical disorders with physiologically normal uniform fetal pattern of growth, evaluation and clinically essential estimation of fetal growth in relation to estimated fetal gestational age can be calculated using BPD, HC, AC, FL and TCD [3].
One of the most popularly used and clinically applied fetal sonographic parameters is the BPD using the transthalamic plane and/or transventricular plane [4]. In transventricular plane ultrasound image will reveal a midline echogenic falx cerebri with an echolucent box shaped cavum septum pellucidum and right and left lateral ventricles [5].
Femur length is measured using an angle of insonation 45 to 90 degrees ends of ossified diaphysis are used as land marks for caliper placement during measurement triangular spur artifacts could falsely increase femur length [6].
Brain development and growth is a complex continuing process especially in second half of gestation including 3 main critical physiological and morphological aspects including proliferation, migration and mylenation.
Cerebellar normal development and growth in some studies have shown a reflection of fetal growth pattern. Therefore transcerebellar diameter is implemented to determine fetal age although multifactorial causes of Small for Gestational age may result in confounding and deceiving measurements as uncovered and displayed from various previously performed studies [7-9].
Pathological alteration in fetal growth pathway due to macrosomia or IUGR does not seem to affect TCD even changes in vault development due to external pressure didn’t alter TCD [10-12].
Different tools have been used to study reliably and clarify cerebellar growth and normal morphological development through various imaging tools such as 2D ultrasound, MRI and even postmortem examination including histological studies to reflect accurate development at microscopic and macroscopic level. All these tools confirmed reliability of TCD to estimate gestational age [13-15].
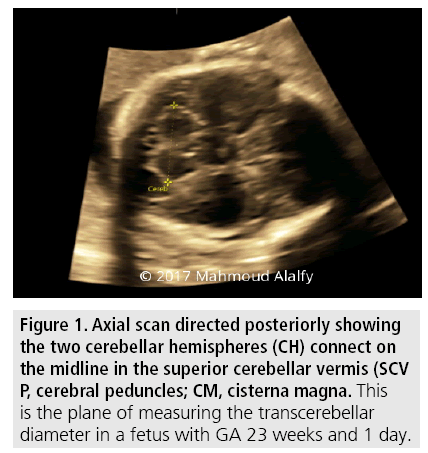
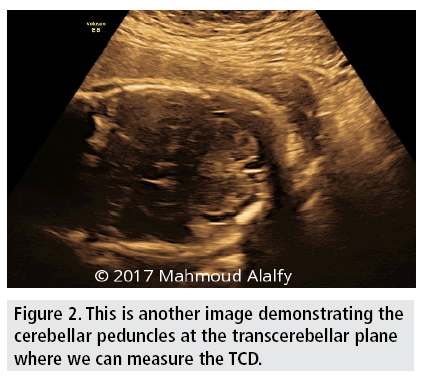
The cerebellum is normally dumb-bell-shaped in fetal sonographic views and composed of 2 cerebellar hemispheres connected centrally by a triangular-shaped vermis. Suboccipitobregmatic view is used for TCD measurement [16,17] Second trimester measurement of TCD is in millimeters and is numerically equivalent to gestational age weeks [18,19].
So According to our prospective case control research data obtained we found that (TCD) measurement is a very crucial parameter in estimating and calculating the gestational age of fetuses better and even more precise than other fetal biometric measurements (BPD, HC, AC, FL) which are routinely used in pregnant women with no medical disorders and also our research revealed the high value of the TCD in clinical scenarios and obstetric conditions where other biometric parameters are significantly affected causing false estimation and improper calculation of the GA as in hypertensive gestations that have IUGR fetuses, diabetic mothers with macrosomic fetuses, in fetuses with congenital abnormalities but the only pitfall and significant sonographic drawback of TCD measurement is in existence of cerebellar morphologic and developmental abnormalities as it becomes small and not accurate with fetal gestational age giving a false estimation in the form of lower value than the actual gestational age.
Therefore after performing the present study among Egyptian population decreasing the confounding variables caused by ethnic and racial differences in growth patterns we realized and clearly concluded that TCD is a reliable biometric measurement in uncomplicated pregnancies and in complicated pregnancies with medical disorders such as D.M and hypertension especially when associated with macrosomic or IUGR fetuses and even in morphological and structural abnormalities viewed sonographically affecting organs other than cerebellum as the cerebellar pattern of sonographic growth and normal development is not affected or manipulated by these circumstances (FIGURES 1 and 2).
Patients and Methods
This study had been carried out in accordance with The Code of Ethics of the World Medical Association.
Consent: All pregnant women participated in this study were signed an informed consent. The Privacy Rights of human’s subjects was observed.
■ Study design
A prospective case control study at Cairo fetal medicine unit, Cairo university, Egypt.
■ Time of the study
Study was conducted from February 2016 till February 2017 for the pregnant women coming for our unit for routine ultrasound scan.
■ Aim of the study
To measure the accuracy of the transcerebellar diameter (TCD) in accurately determining the gestational age (GA) of fetuses in comparison to other fetal biometric measurements (BPD, HC, AC, FL) in pregnant women with no medical disorders and in hypertensive gestations that have IUGR fetuses, in diabetic women with macrosomic fetuses, in fetuses with congenital structural abnormalities not involving cerebellum and in congenital anomalies of cerebellum.
Patients were divided into 2 groups:
• Control group
Inclusion criteria:
- Pregnant women with sure date of her last menstrual period
- Pregnant women from 18 weeks till 38 weeks
- Pregnant women with no medical disorders
• Case group
Divided into 4 groups:
1. Hypertensive gestations that have IUGR fetuses with affected Doppler indices (umbilical artery and MCA).
2. Diabetic mothers with macrosomic fetuses.
3. Pregnant ladies that had congenital structural abnormalities that involve any organ other than cerebellum.
4. Pregnant ladies that had a fetus with structural abnormalities in the cerebellum.
Inclusion criteria:
- Pregnant women with sure date of her last menstrual period
- Pregnant women from 18 weeks till 38 weeks
Exclusion criteria:
• Pregnant women those are unsure of their dates
• Multiple pregnancy
• Pregnant women before 18 weeks or after 38 weeks
Results
The current study was conducted at Cairo fetal medicine unit during the period between (February 2016) and (February 2017) with approval of ethical committee of Cairo university school of medicine.
A total of 60 pregnant women were included in the study. TABLE 1 shows the initial characteristics of included women.
| Age (years) Range Mean ± SD |
20–32 25.77 ± 3.95 |
| BMI (years) Range Mean ± SD |
19.15–38.22 27.03 ± 4.45 |
| Gestational Age (weeks) Range Mean ± SD |
18.57–37 26.72 ± 5.43 |
BMI: Body Mass Index; SD: Standard Deviation
Table 1: Initial characteristics of included women.
This table demonstrates the demographic data of the patients showing the range of age and BMI and GA of the fetuses.
TABLE 2 shows the descriptive of sonographic fetal biometry in included women.
| BPD (mm) Range Median (IQR) |
41-99.2 66 (54.01-77.45) |
| HC (mm) Range Median (IQR) |
150-344.3 235.5 (198.88-282) |
| AC (mm) Range Median (IQR) |
141-358 208.75 (177.5-260.43) |
| FL (mm) Range Median (IQR) |
29.5-76.3 45.8 (37.6-57.75) |
| TCD (mm) Range Median (IQR) |
17-50.5 26.75 (23.08-36.65) |
IQR: Interquartile Range; BPD: Biparietal Diameter; HC: Head Circumference; AC: Abdominal Circumference; FL: Femur Length
Table 2: Sonographic fetal biometry in included women.
TABLE 3 shows the Doppler ultrasound velocimetry indices of umbilical artery and middle cerebral artery in included women.
| UA PI Range Median (IQR) |
0.64-1.8 0.97 (0.83-1.2) |
| UA RI Range Median (IQR) |
0.48-1.0 0.63 (0.58-0.70) |
| MCA PI Range Median (IQR) |
0.7-3.4 1.72 (1.51-2.0) |
| MCA RI Range Median (IQR) |
0.63-1.0 0.82 (0.76-0.89) |
IQR: Interquartile Range; UA: Umbilical Artery; MCA: Middle Cerebral Artery; PI: Pulsatility Index; RI: Resistance Index
Table 3: Doppler ultrasound velocimetry indices of UA and MCA in included women.
TABLE 4 demonstrated that from 60 women, 4 (6.6%) had fetal Dandy-Walker Malformation, 1 (3.3%) had echogenic foci in right and left lateral ventricles and 1 (3.3%) had mild bilateral hydronephrosis (TABLE 5 and FIGURE 1).The case with Dandy-Walker malformation was excluded from subsequent analyses.
| Sonographic EFW Average IUGR Fetal Macrosomia |
53 (88.33%) 4 (6.6%) 3 (5%) |
EFW: Estimated Fetal Weight; IUGR: Intrauterine
Growth Restriction
Data presented as number (percentage)
Table 4: Rates of IUGR and macrosomia in included women.
| Congenital Malformations Dandy-Walker Malformation Echogenic Foci in Lateral Ventricles Mild Bilateral Hydronephrosis |
4 (6.6%) 1 (3.3%) 1 (3.3%) |
Data presented as number (percentage)
Table 5: Congenital malformation in included women.
TABLE 6 shows differences between discrepancies between menstrual gestational age and sonographic estimation of gestational age using different fetal biometry in included women.
| Discrepancy between menstrual gestational age and sonographic gestational age | IQR (mm) |
P* | |
|---|---|---|---|
| BPD Range Median (IQR) |
-9.57–4 -0.71 (-1.28–0) |
1.27 | <0.001 1 (HS) <0.001 2 (HS) <0.001 3 (HS) 0.134 4 (NS) 0.0085 (S) 0.7456 (NS) 0.453 7 (NS) 0.0658 (NS) 0.004 9 (S) 0.131 10 (NS) |
| HC Range Median (IQR) |
-9.0–4.14 0 (-0.57–0.43) |
1.0 | |
| AC Range Median (IQR) |
-11.14–6.85 0.43 (-0.28–1.28) |
1.56 | |
| FL Range Median (IQR) |
-10.14–3.71 0.15 (-0.57–0.71) |
1.28 | |
| TCD Range Median (IQR) |
-13.86–2.43 0 (-0.28–0.15) |
0.43 | |
IQR: Interquartile Range; BPD: Biparietal Diameter; HC: Head Circumference; AC: Abdominal
Circumference; FL: Femur Length; NS: Non-Significant
* Analysis using Wilcoxon Signed Rank Test
1 Difference between Discrepancy between Menstrual GA and Sonographic Estimation using Fetal
BPD and Discrepancy using Fetal HC
2 Difference between Discrepancy between Menstrual GA and Sonographic Estimation using Fetal
BPD and Discrepancy using Fetal AC
3 Difference between Discrepancy between Menstrual GA and Sonographic Estimation using Fetal
BPD and Discrepancy using Fetal FL
4 Difference between Discrepancy between Menstrual GA and Sonographic Estimation using Fetal
BPD and Discrepancy using Fetal TCD
5 Difference between Discrepancy between Menstrual GA and Sonographic Estimation using Fetal
HC and Discrepancy using Fetal AC
6 Difference between Discrepancy between Menstrual GA and Sonographic Estimation using Fetal
HC and Discrepancy using Fetal FL
7 Difference between Discrepancy between Menstrual GA and Sonographic Estimation using Fetal
HC and Discrepancy using Fetal TCD
8 Difference between Discrepancy between Menstrual GA and Sonographic Estimation using Fetal
AC and Discrepancy using Fetal FL
9 Difference between Discrepancy between Menstrual GA and Sonographic Estimation using Fetal
AC and Discrepancy using Fetal TCD
10 Difference between Discrepancy between Menstrual GA and Sonographic Estimation using Fetal
FL and Discrepancy using Fetal TCD
Table 6: Sonographic fetal biometry in included women.
The IQR of the discrepancy between menstrual and sonographic gestational ages was the least when TCD, in comparison to BPD, HC, AC and FL (0.43 mm, 1.27 mm, 1.0 mm, 1.56 mm and 1.28 mm, respectively). The difference, however, was statistically significant only when compared to that using AC.
TABLE 7 shows the correlation between fetal biometry, gestational age and Doppler ultrasound velocimetry indices of UA and MCA.
| Menstrual Gestational Age | UA PI | UA RI | MCA PI | MCA RI | ||
|---|---|---|---|---|---|---|
| BPD | rs P |
0.940 <0.001 HS |
-0.253 0.051 NS |
-0.266 0.040 S |
0.085 0.516 NS |
0.065 0.620 NS |
| HC | rs P |
0.946 <0.001 HS |
-0.283 0.029 S |
-0.292 0.023 S |
0.076 0.563 NS |
0.048 0.715 NS |
| AC | rs P |
0.940 <0.001 HS |
-0.222 0.089 NS |
-0.224 0.085 NS |
0.021 0.874 NS |
0.035 0.790 NS |
| FL | rs P |
0.955 <0.001 HS |
-0.205 0.116 NS |
-0.218 0.094 NS |
0.004 0.978 NS |
-0.004 0.978 NS |
| TCD | rs P |
0.858 <0.001 HS |
-0.027 0.837 NS |
-0.010 0.938 NS |
0.011 0.931 NS |
-0.053 0.687 NS |
Table 7: Correlation between fetal biometry, gestational age and Doppler ultrasound velocimetry indices in included women.
TCD was significantly positively correlated to menstrual gestational age, but not to any of the measured Doppler velocimetry indices of UA and MCA.
Discussion
Previous research studies that are performed on TCD measurements evaluation, clearly displayed and showed that TCD was a reliable trusted tool of measurement to calculate the fetal gestational age, it is widely believed to be superior in reliability than HC, FL, BPD and AC when comes to precision of calculating fetal gestational age. It could be even to considered an accurate, dependable and reliable tool of measurement assisting formulas used to settle on fetal gestational age in both singleton and twin gestation [20,21].
The IQR of the discrepancy between menstrual and sonographic gestational ages was the least when TCD, in comparison to BPD, HC, AC and FL [(0.43 mm, 1.27 mm, 1.0 mm, 1.56 mm and 1.28 mm, respectively). The diversity of measurements, however, was statistically significant only when correlated to that using AC [10].
So, from our research results and data obtained, we settled and concluded that the most, reliable, precise showing superiority in accurate analysis and assessment of fetal gestational age was the TCD followed by the HC then the BPD followed by the FL and the least precise tool of calculating fetal gestational age was AC this finding is similar to other research studies which strengthens and augments our research study findings and results reliability [11,14].
Previous research uncovers the linkage between GA and TCD to evaluate predictability of GA by TCD causing increased clinical reliability and trust to use TCD measurements.
TCD was obtained from a total of two hundred and twenty one fetuses with a defined GA. The research showed that TCD matched with GA and expected the GA to ± 2.33 weeks [15,16].
Another research applied on 50 patients made from the start of the 2nd trimester till term of pregnancy. TCD measurement was done to determine the gestational age. The regression analysis indicated a significant relationship between TCD and GA, concluding that TCD is a useful and a precise tool for the estimation of GA [3].
Another study stated that there was a slight fluctuations and variability in the normal growth curves of the fetal cerebellum, denoting multiple conditions that would result in difficulties in measuring the TCD in late gestations, so we also analyzed the data at the 31 and 36 weeks of gestation. This explores and uncovers the outstanding value of our study in which cerebellar fetal growth is restricted in the study in a limited gestational age period of 5 weeks among Egyptian population .Thereby reducing confounding variables caused by ethnic and racial differences.
Another group of researchers observed and conducted a research study to precisely evaluate and explore clinical reliability and precision to reflect in a professional means the accuracy of fetal transcerebellar diameter normogram in the evaluation and sonographic assessment for guidance and prediction of gestational age in singleton gestation at the second and the third trimesters of singleton pregnancy. Revealing that the TCD measurement appears to be an accurate measurement aiding in calculation of the fetal gestational age, even in the third trimester of pregnancy. It is was recommended to use TCD as a biometric parameter in normal singleton for the prediction of gestational age [15,16].
A retrospective, cross-sectional analytic study of normally developing fetuses in the from 17 to 34 weeks and 73 fetuses with IUGR between 24 and 34 weeks gestation was done, researchers found that the TCD measurements are spared in cases of IUGR [14].
Another study conducted to expose the significance of fetal transverse cerebellar diameter measurement for the prediction of gestational age in growth restricted fetuses. The study sample size was applied on 100 mothers in the third trimester fulfilling the eligibility criteria, 50 were fetuses with normal fetal growth and fifty fetuses with IUGR. The data extracted from this research study demonstrated that the mean transverse cerebellar diameter in the fetuses showing normal growth was not statistically different from the mean transverse cerebellar diameter of that in the growth restricted fetus. They stated that fetal TCD measurements seem to link well with the gestational age in both normal and growth restricted fetuses as there was no statistically considerable difference in TCD measurements in normal and growth restricted fetuses. Transverse cerebellar diameter measurement could be used reliably for accurate calculation of gestational age in growth restricted fetuses [10].
Another group of researchers stated that TCD measurement was both reliable and accurate in determining gestational age even in extremes of fetal growth. While majority of data suggests that the TCD is extremely valuable when the gestational age is unknown or IUGR is suspected [3].
Another study displayed that the TCD was within two standard deviations in only 40% of IUGR cases, nevertheless they included only 44 IUGR fetuses and symmetrical IUGR were not excluded [3].
Some studies stated that the TCD was a useful sonographic measurement aiding in calculation and predicton of gestational age for fetuses with asymmetric but not symmetric growth retardation [6].
Previous research has uncovered a close correlation between cerebellar dimensions and GA using fetal growth parameters including BPD, head circumference, FL, and estimated fetal weight, this relationship had been found to be independent on fetal gender [7-16].
Correlation of the cerebellar circumference and area with GA and illustration of its usefulness in cases of unilateral cerebellar agenesis and hypoplasia was established in a study done in 2007. Accordingly, measurement of cerebellar dimensions, including the TCD might be a useful guidance in detecting cerebellar malformations, if nomograms of TCD were accessible for a selected population [5].
In our present research study, our results obtained displayed that the transcerebellar diameter is the nearly the most precise measurement in accurately detecting the GA in all means during gestation (in uncomplicated pregnancies, in hypertension with pregnancy and in pre-eclamsia associated with either symmetrical or a symmetrical IUGR, also in pregnant diabetic women with fetuses showing macrosomia in which all parameters of fetal biometry obtained are above the normal average range of measurements for gestational age (i.e., above the 95th percentile for GA) and in pregnancies having morphological abnormalities other than cerebellar anomalies. The only exceptional situation in the accuracy of transcerebellar diameter measurement in which TCD is not considered precise with low value of accuracy as it has a value less than average in cases of structural abnormalities of the cerebellum (e.g. Dandy Walker malformation).
All our data and findings were fulfilled by the Doppler study of the umbilical and Middle cerebral artery formulas that deep-rooted normal uteroplacental circulation that displayed and clearly revealed normal PI and RI numerical values of umbilical and MCA Doppler or uteroplacental insufficiency in clinical situations of pregnancy induced hypertension, preeclampsia and IUGR that showed amplified PI and RI numerical values of umbilical artery Doppler above the 95th percentile for gestational age and showed pathologically reduced PI and RI values of MCA Doppler below the 5th centile for GA.
Conclusion
From our Research we concluded that (TCD) measurement is a precise parameter in accurately calculating fetal gestational age as it comes in preference to other fetal biometric measurements (BPD, HC, AC, FL) which are classically used in pregnant women with no medical disorders and also our research revealed the high value and precision superiority of the TCD measurement in cases where other biometric parameters are altered causing false sonographic estimation of the GA as in hypertensive gestations that have IUGR fetuses, diabetic mothers with macrosomic fetuses and in fetuses with congenital abnormalities but the only drawback of TCD measurement is in presence of cerebellar abnormalities as it becomes pathologically small and not a precise tool for calculation and estimation of fetal gestational age.
Therefore after conducting the present research we concluded that TCD is the most accurate biometric measurement in both uncomplicated pregnancies and in complicated pregnancies with medical disorders such as D.M and hypertension especially when associated with macrosomic or IUGR fetuses and in structural abnormalities affecting organs other than cerebellum as the cerebellar growth is not affected by these circumstances. Moreover, dating of pregnancy can be made depending on the TCD in pregnant women with unsure of their dates as it is not affected by hypertension or diabetes mellitus or any growth disturbances. But the only pitfall of TCD measurement expected before beginning the study is in existence of cerebellar abnormalities as it becomes small and not accurate with GA.
State of Declaration
All authors declare that there are no any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
References
- Gottlieb AG, Galan HL. Nontraditional sonographic pearls in estimating gestational age. Semin. Perinatol. 154-160 (2008).
- Whitworth M, Bricker L, Neilson JP et al. Ultrasound for fetal assessment in early pregnancy. Cochrane. Database. Syst. Rev. 4 (2010).
- Ananth CV. Menstrual versus clinical estimate of gestational age dating in the United States: Temporal trends and variability in indices of perinatal outcomes. Paediatr. Perinat. Epidemiol. 21, 22-30 (2007).
- 4- Ergaz U, Goldstein I, Divon M et al. A preliminary study of three-dimensional sonographic measurements of the fetus. Rambam. Maimonides. Med. J. 6, 2-5 (2015).
- Salomon L, Alfirevic Z, Berghella V et al. Practice guidelines for performance of the routine mid‐trimester fetal ultrasound scan. Ultrasound. Obstet. Gynecol. 37, 116-126 (2011).
- Sherer D, Sokolovski M, Dalloul M et al. Nomograms of the axial fetal cerebellar hemisphere circumference and area throughout gestation. Ultrasound. Obstet. Gynecol. 29, 32-37 (2007).
- Araujo E, Pires CR, Nardozza LMM et al. Correlation of the fetal cerebellar volume with other fetal growth indices by three-dimensional ultrasound. J. Matern. Fetal. Neonatal. Med. 20, 581-587 (2007).
- Volpe JJ (2009) Cerebellum of the premature infant: Rapidly developing, vulnerable, clinically important. J. Child. Neurol. 24, 1085-1104.
- Habas PA, Scott JA, Roosta A et al. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cerebral. Cortex. 53 (2011).
- Chavez MR, Ananth CV, Smulian JC. Fetal transcerebellar diameter measurement for prediction of gestational age at the extremes of fetal growth. J. Ultrasound. Med. 26, 1167-1171 (2007).
- Goel P, Singla M, Ghai R et al. Transverse cerebellar diameter: A marker for estimation of gestational age. J. Anat. Soc. India. 59, 158-161 (2010).
- Ahmed MA Accuracy of fetal transcerebellar diameter nomogram in the prediction of gestational age in singleton gestation at the second and the third trimesters of singleton pregnancy. J. Evid. Based. Womens. Health. J. Soc. 4, 184-188 (2014).
- Afshan A, Nadeem S, Shamimasim S (2014) Fetal transverse cerebellar diameter measurement; a useful predictor of gestational age in growth restricted fetuses. Professional. Medical. Journal. 21.
- Holanda-Filho JA, Souza AI, Souza ASR et al. Fetal transverse cerebellar diameter measured by ultrasound does not differ between genders. Arch. Gynecol. Obstet. 284, 299-302 (2011).
- Bansal M, Bansal A. A study of correlation of transverse cerebellar diameter with gestational age in the normal and growth restricted foetuses in western Uttar Pradesh. People. J. Sci. Res. 7 (2014).
- Uikey PA, Kedar KV, Khandale SN. Role of trans-cerebellar diameter in estimating gestational age in second and third trimester of pregnancy. Int. J. Reprod. Contracept. Obstet. Gynecol. 5, 3411-3415 (2016).
- Goel P, Singla M, Ghai R et al. Transverse cerebellar diameter -A marker for estimation of gestational age. Journal. Of. Anatomical. Society. Of. India. 59, 158-161 (2010).
- Chavez MR, Ananth CV, Smulian JC et al. Foetal transcerebellar diameter measurement for prediction of gestational age at the extremes of foetal growth. J. Ultrasound. Med. 26, 1167-1171 (2007).
- Joshi BR. Foetal transcerebellar diameter nomogram in Nepalese population. Journal. Of. Institute. Of. Medicine. 32, 19-23 (2010).
- Gupta AD, Banerjee A, Rammurthy N et al. Gestational age estimation using transcerebellar diameter with grading of foetal cerebellar growth. Natl. J. Clin. Anatomy. 1, 115-120 (2012).
- Naseem F, Ali S, Basit U et al. Assessment of gestational age; comparison, between transcerebellar diameter versus femur length on ultrasound in third trimester of pregnancy. Professional. Medical. Journal. 21, 412-417 (2014).