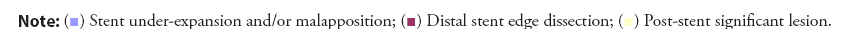Research Article - Interventional Cardiology (2022)
The use of Fractional Flow Reserve (FFR) in assessment of residual ischemia
- Corresponding Author:
- Moataz Elsanan
Department of Cardiology,
Zagazig University,
Zagazig,
Egypt,
E-mail: moatazelsanan@gmail.com
Received date: 26-Dec-2022, Manuscript No. FMIC-22- 87001; Editor assigned: 28-Dec-2022, PreQC No. FMIC-22-87001 (PQ); Reviewed date: 11-Jan-2023, QC No. FMIC-22-87001;Revised date: 18-Jan-2023, Manuscript No. FMIC-22- 87001 (R);Published date: 27-Jan-2023, DOI: 10.37532/1755-5310.2023.14 (S14). 346
Abstract
Introduction: Despite coronary angiography still being the gold standard for anatomical delineation of coronaries, it actually has a limited usefulness to assess the true functional relevance of coronary arterial stenosis. On contrary, Fractional flow reserve “FFR” is an accurate and specific index to determine whether a particular stenosis can be held accountable for ischemia.
Aim: We aimed to assess the residual ischemia post percutaneous coronary intervention “PCI” for all ischemic patients either acute or chronic by FFR.
Methodology: We recruited 100 patients with significant coronary artery disease (Angiographically and FFR<0.8) and planned for PCI at Zagazig University Hospital and National Heart Institute. FFR was performed, only patients with FFR<0.8 were included. The patients were divided randomly into two groups, according to FFR performed or not after stenting, into Group I; 50 patients, after stenting, FFR was done. It was further subdivided into Subgroup A (FFR<0.8) and Subgroup B (FFR>0.8). If FFR<0.8, IVUS had been done to assess the mechanism of residual ischemia then correction of the cause till FFR exceeded 0.8. Group II; 50 patients, after stenting, No FFR was done. All patients were followed up during hospital stay and after 3 months.
Results: During hospital Follow up, there was significant difference between both groups regarding chest pain (26% in Group II, versus 4% in Group I) and heart failure (28% in Group II versus 0% in Group I). These results were comparable to three months follow up, chest pain was (41.7% in Group II versus 0% in Group I), heart failure was (20.8% in Group II versus 0% within Group I) and occurrence of sudden cardiac death or arrhythmias (occurred only in Group II; 2.1% and 12.5% respectively). There was also a significant difference concerning in-stent thrombosis, it was recorded in 20.8% within survivors of Group II versus 0% within Group I). In Group I, 14 patients (28% of patients) had FFR<0.8 after coronary stenting and 36 patients (72% of patients) had FFR>0.8. There was significant difference between both subgroups regarding smoking, diabetes, hypertension (all were higher in those with FFR<0.8 “Subgroup A”), resting ECG findings (Acute STEMI occurred in 27.8% in Subgroup B versus 50% within subgroup A), HbA1c, total Cholesterol and Triglycerides (higher in Subgroup A), HDL-cholesterol (lower in Subgroup A). There was statistically significant positive correlation between stent size and Pre-dilatation FFR. On the other hand, there is significant negative correlation between stent length and Pre-dilatation FFR. Regarding Multivariate regression, increasing HbA1c, and increasing stent length increase risk of FFR to be<0.8 by 16.402 and 1.356 folds respectively. Increasing stent size protect against FFR<0.8.
Conclusion: Post-PCI FFR has a great potential to direct the quality of PCI, optimize PCI result, and improve prognosis by detection and subsequently correction of residual ischemia. Residual ischemia is one of a major factor that cause many post-stenting complications either immediately or during 3 months follow up. Routine FFR guided PCI is associated with less cardiovascular adverse events but is rarely performed in clinical practice due to limited data and lack of any specific guideline recommendations.
Keywords
Fractional flow reserve • Percutaneous coronary intervention • Angiographically
Introduction
The treatment of symptomatic Coronary Artery Disease (CAD) depends on the hemodynamic impairment of flow, a physiologic parameter that is not captured with coronary angiography alone [1].
Since coronary angiography has little usefulness in determining the functional relevance of coronary artery stenosis, it continues to be a key component of invasive imaging of the coronary arteries. Determining whether a stenosis causes reversible ischemia or not is of utmost importance since the existence and severity of inducible ischemia, which affects outcomes, is the most crucial component [2].
Fractional Flow Reserve (FFR) is an accurate and specific index to determine whether a particular stenosis or coronary segment can be held accountable for ischemia. Traditional noninvasive tests like the exercise test and technetium 99 m sestamibi single photon emission computed tomography fail to distinguish the specific ischemic territories and responsible stenosis [3]. Due to its continuous and independent association to outcomes, FFR can be considered as both a physiologic biomarker and a target for treatment. FFR has predictive value immediately after PCI either with pre-PCI assessment or by themselves [4].
The FFR cutoff value is clearly defined, and there is just a small grey area between 0.75 and 0.8. FFR provides unparalleled spatial resolution and directly links the severity of the stenosis to the quantity of tissue to be perfused [5].
The clinical results are significantly influenced by FFR post-PCI assessment since lower post-PCI fractional flow reserve values have been linked to an increased risk of MI, revascularization, and death [6]. Accumulating evidence suggests that significant residual ischemia after angiographically successful PCI (defined as FFR 0.80) may occur in some patients (10%) and is associated with a worse prognosis [7]. Despite this evidence, there are no specific guideline recommendations for routine post-PCI FFR assessment, and clinical adoption thus remains limited.
Therefore, this study aimed to assess the residual ischemia post PCI for all ischemic patients either acute or chronic by fractional flow reserve.
Materials and Methods
This cohort study recruited 100 patients that had a significant coronary artery disease (evident angiographically and had FFR<0.8) and planned for percutaneous coronary intervention at Zagazig University Hospital and National Heart Institute, Egypt.
While, patients with PCI to a coronary bypass graft, PCI of an in-stent restenosis lesion, PCI to a target artery providing Rentrop grade 2 or 3 collateral blood supply to another vessel, Inability to receive adenosine (e.g., severe reactive airway disease, marked hypotension), Advanced atrioventricular block without pacemaker) or any arrhythmia such as atrial fibrillation, ventricular tachycardia, supraventricular tachycardia or atrial flutter, Severe cardiomyopathy (left ventricular ejection fraction <40%), Renal insufficiency and advanced hepatic diseases, Chronic total occlusion coronary artery disease or ostial lesions were excluded.
All patients were subjected to complete history taking, full clinical examination. Patient’s participation was volunteer, and the subjects could stop their attendance at any time. Informed written consent was obtained before the subject entered the study. Laboratory investigation: Complete blood picture, renal function test, lipid profile, coagulation profile, troponin, total creatinine kinase and MB creatinine kinase, D. Dimer and INR. Twelve leads resting ECG to determine type of underlying ischemia either stable angina, unstable angina, non-STEMI or STEMI. Echocardio-graphic study including the following parameters such EDD, ESD, EF, diameter of LA, and aortic root.
Coronary angiography: We performed coronary artery angiography to all cases to determine which artery is affected and site of affection, type of lesion and who is suitable for percutaneous intervention. In some cases, such as STEMI in time window coronary angiograms are performed on an emergency basis. Angiograms are performed in the catheterization lab of a hospital. We provided the patient with detailed instructions and discussed any medications they were taking.
FFR: FFR was performed for all patients before stenting to ensure significance (only patients with FFR<0.8 included in the study). The patients were divided randomly into two groups, according to FFR was performed or not after PCI;
Group I (Post-PCI FFR group) included 50 patients had been undergone percutaneous coronary intervention & post percutaneous coronary intervention fractional Flow Reserve (FFR) was done. It was further subdivided into Subgroup A (FFR<0.8) and Subgroup B (FFR>0.8).
Group II (Non Post-PCI FFR group) included 50 patients had been undergone percutaneous coronary intervention with no post percutaneous coronary intervention Fractional Flow Reserve (FFR).
In Group 1 (Post-PCI FFR group), patients with FFR<0.8 (Subgroup A), IVUS had been done to assess the mechanism of residual ischemia then correction of the cause till FFR became more than 0.8.
All patients in both groups were followed up during hospital stay and after 3 months to look for complications such the onset of symptomatic heart failure, angina, arrhythmias, in-stent restenosis (diagnosed clinically by recurrence of chest pain, laboratory by elevation of cardiac markers and Electrocardiographically by ST segment elevation synchronized with stent place), or sudden cardiac death.
Statistical analysis: Using Microsoft Excel, then, data were imported into SPSS version 20.0, Statistical Package for the Social Sciences for analysis. Qualitative data are represented as numbers and percentages depending on the type of data, whereas quantitative data are grouped and represented by means plus standard deviation. P value was set at 0.05 for significant outcomes and 0.001 for extremely significant outcomes.
Results
The study included 100 patients that had a significant coronary artery disease (evident angiographically and had FFR<0.8) and planned for percutaneous coronary intervention. Those patients were divided randomly and equally into two groups. The patients on these two groups were homogenously distributed with no significant difference between the two groups regarding Demographic (age, gender, and family history), Clinical (heart rate, systolic and diastolic blood pressure), Resting Electrocardiographic, Laboratory (including total cholesterol, triglycerides, LDL cholesterol, HDL-cholesterol, glycated hemoglobin, C reactive protein, troponin and CK-MB) Echocardiographic parameters (aortic root diameter, left atrium diameter, LVEDD, LVESD, EF or FS) and angiographic data (number of vessels affected, extent of obstruction in LCX, RCA or LAD, size or length of stent, site of stent in affected arteries or TIMI flow) (Table 1).
| Parameter | Groups | Test | ||
|---|---|---|---|---|
| Group 1 (Post PCI FFR group) | Group 2 (Non-Post PCI FFR group) | t/÷2 | p | |
| N (%)=50(%) | N (%)=50(%) | |||
| Mean ± SD | Mean ± SD | |||
| Age (year) | 57.7 ± 11.8 | 61.88 ± 9.51 | -1.951 | 0.054 |
| Gender | ||||
| Male | 18 (36%) | 26 (52%) | 2.597 | 0.107 |
| Female | 32 (64%) | 24 (48%) | ||
| Family history | ||||
| Negative | 24 (48%) | 24 (48%) | 0 | >0.999 |
| Positive | 26 (52%) | 26 (52%) | ||
| Smoking | ||||
| Negative | 21 (42%) | 25 (50%) | 0.644 | 0.422 |
| Positive | 29 (58%) | 25 (50%) | ||
| Hypertension | 34 (68%) | 34 (68%) | ||
| Diabetes | 27 (54%) | 26 (52%) | 0.04 | 0.841 |
| Systolic blood pressure | 128.72 ± 12.58 | 131.28 ± 14.57 | -0.934 | 0.353 |
| Diastolic blood pressure | 73.26 ± 11.3 | 69.34 ± 12.29 | 1.661 | 0.1 |
| Heart rate (b/m) | 78.5 ± 11.52 | 76.38 ± 10.05 | 0.981 | 0.329 |
| Resting ECG | ||||
| Acute STEMI | 17 (34%) | 16 (32%) | 1.562 | 0.211 |
| Non-STEMI | 19 (38%) | 11 (22%) | ||
| Unstable angina | 6 (12%) | 11 (22%) | ||
| Chronic stable angina | 8 (16%) | 12 (24%) | ||
| HbA1c (%) | 7.32 ± 1.47 | 7.28 ± 1.08 | 0.178 | 0.859 |
| ESR | 34.16 ± 10.05 | 31.22 ± 9.15 | 1.464 | 0.146 |
| CRP | 11.64 ± 3.09 | 10.68 ± 4.83 | 1.183 | 0.12 |
| Total cholesterol (mg/dl) | 221.06 ± 28.36 | 213.14 ± 30.03 | 1.356 | 0.178 |
| Triglycerides (mg/dL) | 198.58 ± 30.59 | 189.44 ± 29.22 | 1.528 | 0.13 |
| HDL cholesterol (mg/dl) | 48.54 ± 5.19 | 49.44 ± 6.33 | -0.778 | 0.439 |
| LDL cholesterol (mg/dl) | 137.5 ± 24.82 | 134.24 ± 21.8 | 0.698 | 0.487 |
| Troponin | 21.5 (0 – 74) | 11.5 (0.01 – 54) | -1.822 | 0.068 |
| CK-MB | 32 (10 – 60) | 27.5 (8 – 72) | -1.957 | 0.05 |
| Echo data | ||||
| Aortic root (mm) | 32.78 ± 2.19 | 32.63 ± 2.99 | 0.798 | 0.428 |
| Left atrium (mm) | 35.6 ± 2.9 | 36.12 ± 2.85 | -0.905 | 0.358 |
| LVEDD (mm) | 51.55 ± 6.24 | 50.88 ± 6.21 | 0.538 | 0.592 |
| LVESD (mm) | 36.47 ± 7.29 | 37.14 ± 5.26 | -0.529 | 0.598 |
| EF (%) | 50.87 ± 8.62 | 50.48 ± 7.57 | 0.244 | 0.808 |
| FS (%) | 26.58 ± 5.24 | 26.2 ± 5.02 | 0.368 | 0.713 |
| Angiographic data | ||||
| LAD | N=8 | N=19 | ||
| Mean ± SD | 88.13 ± 4.58 | 89.42 ± 6.56 | -0.507 | 0.617 |
| LCX | N=10 | N=18 | ||
| Mean ± SD | 90.0 ± 5.77 | 88.33 ± 5.42 | 0.762 | 0.453 |
| RCA | N=34 | N=17 | ||
| Mean ± SD | 88.38 ± 6.12 | 85.88 ± 5.07 | 1.451 | 0.153 |
| No. of vessels affected | ||||
| One | 48(96%) | 46(92%) | 0.57 | 0.678 |
| Two | 2(4%) | 4(8%) | ||
| Size of stent | 2.922 ± 0.249 | 2.95 ± 0.214 | -0.603 | 0.548 |
| Length of stent | 28.8 ± 5.51 | 27.66 ± 5.37 | 1.047 | 0.298 |
| Site of stent (LAD) | N=8 | N=19 | ||
| Distal | 0(0%) | 1(5.3%) | 0.297 | 0.585 |
| Mid | 4(50%) | 10(52.6%) | ||
| Proximal | 4(50%) | 8(42.1%) | ||
| Site of stent (LCX) | N=10 | N=19 | ||
| OM | 5(50%) | 2(10.5%) | 0.466 | 0.495 |
| Mid | 0(0%) | 11(57.9%) | ||
| Proximal | 5(50%) | 6(31.6%) | ||
| Site of stent (RCA) | N=34 | N=16 | ||
| Distal | 4(11.8%) | 2(12.5%) | 1.346 | 0.246 |
| Mid | 21(61.8%) | 6(37.5%) | ||
| Proximal | 9(26.5%) | 8(50%) | ||
| TIMI flow | 3 | 3 | ||
Note: t independent sample t test χ2 chi square test P ≤ 0.05 is statistically significant
Table 1: Comparison between the studied groups regarding demographic, Clinical, Electrocardiographic, Echocardiographic and Angiographic data.
During hospital Follow up, there was significant difference between the studied groups regarding occurrence of chest pain (26% in Group II, versus 4% in Group I) and heart failure (28% in Group II versus 0% in Group I). While there were no difference regarding occurrence of arrhythmia, sudden cardiac death or in- stent thrombosis (occurred only in Group II; 8%, 4% and 6% respectively) (Table 2).
| Parameter | Groups | Test | |
|---|---|---|---|
| Group 1(Post PCI FFR group) | Group 2(Non post PCI FFR group) | P | |
| N (%) =50(%) Mean ± SD | N (%) =50(%) Mean ± SD | ||
| Arrhythmias | 0 (0%) | 4 (8%) | 0.117 |
| Chest pain | 2 (4%) | 13 (26%) | 0.004* |
| Heart failure | 0 (0%) | 14 (28%) | <0.001** |
| Sudden cardiac disease | 0 (0%) | 2 (4%) | 0.495 |
Note: t independent sample t test χ2 chi square test , **P ≤ 0.05 is statistically significant
Table 2: Immediate (in hospital) follow up of the studied groups.
These results were comparable to three months follow up results, chest pain was (41.7% in Group II versus 0% in Group I), heart failure was (20.8% in Group II versus 0% within Group I) and occurrence of sudden cardiac death or arrhythmias (occurred only in Group II; 2.1% and 12.5% respectively). There was also a significant difference concerning in-stent thrombosis, it was recorded in 20.8% within survivors of Group II versus 0% within Group I) (Table 3).
| Parameter | Groups | Test | |
|---|---|---|---|
| Group 1 | Group 2 | P | |
| (Post PCI FFR group) | (Non post PCI FFR group) | ||
| N (%) =50(%) | N (%) =50(%) | ||
| Mean ± SD | Mean ± SD | ||
| Arrhythmia | 0 (0%) | 6 (12.5%) | 0.012 |
| Chest pain | 0 (0%) | 20 (41.7%) | <0.001** |
| Heart failure | 0 (0%) | 10 (20.8%) | <0.001** |
| Sudden cardiac disease | 0 (0%) | 1 (2.1%) | 0.49 |
| In-stent thrombosis | 0 (0%) | 10 (20.8%) | <0.001** |
Note: t independent sample t test χ2 chi square test **P ≤ 0.05 is statistically significant.
Table 3: Follow up (after 3 months) of the studied groups.
| IVUS Findings | Subgroup A patients (FFR<0.8) (14 patients) | |
|---|---|---|
| Number (no) | Percentage (%) | |
| Stent under-expansion and/or malapposition | 9 | 64.3 |
| Distal stent edge dissection | 3 | 21.4 |
| Post-stent significant lesion | 2 | 14.3 |
Table 4: IVUS findings in Subgroup A patients in FFR.
| Parameter | Group 1 (post PCI FFR group) | Test | |
|---|---|---|---|
| Subgroup A (FFR<0.8) | Subgroup B (FFR>0.8) | p | |
| N=14 (%) Mean ± SD | N=36 (%) Mean ± SD | ||
| Age(year) | 58.71 ± 13.39 | 57.31 ± 11.3 | 0.709 |
| Gender | |||
| Male | 7 (50%) | 11 (30.6%) | 0.198 |
| Female | 7 (50%) | 25 (69.4%) | |
| Family history | |||
| Negative | 6 (42.9%) | 18 (50%) | 0.65 |
| Positive | 8 (57.1%) | 18 (50%) | |
| Smoking | |||
| Negative | 2 (14.3%) | 19 (52.8%) | 0.013* |
| Positive | 12 (85.7%) | 17 (47.2%) | |
| Hypertension | 13 (92.9%) | 21 (58.3%) | 0.021* |
| Diabetes | 13 (92.9%) | 14 (38.9%) | <0.001** |
| Systolic blood pressure | 128.72 ± 12.58 | 131.28 ± 14.57 | 0.804 |
| Diastolic blood pressure | 73.26 ± 11.3 | 69.34 ± 12.29 | 0.905 |
| Heart rate(b/m) | 78.5 ± 11.52 | 76.38 ± 10.05 | 0.483 |
| Resting ECG | |||
| Acute STEMI | 7 (50%) | 10 (27.8%) | 0.027* |
| Non-STEMI | 6 (42.9%) | 11 (36.1%) | |
| Unstable angina | 1 (7.1%) | 5(13.9%) | |
| Chronic stable angina | 0 (0%) | 8(22.9%) | |
| HbA1c (%) | 7.32 ± 1.47 | 7.28 ± 1.08 | <0.001** |
| ESR | 34.16 ± 10.05 | 20.82 ± 8.8 | 0.715 |
| CRP | 11.64 ± 3.09 | 10.68 ± 4.83 | 0.618 |
| Total cholesterol (mg/dl) | 240.57 ± 33.02 | 213.47 ± 22.57 | 0.002* |
| Triglycerides (mg/dL) | 216.29 ± 25.64 | 191.69 ± 29.86 | 0.009* |
| HDL cholesterol (mg/dl) | 44.5 ± 5.29 | 50.11 ± 4.28 | <0.001** |
| LDL cholesterol (mg/dl) | 159.43 ± 24.16 | 128.97 ± 19.46 | <0.001** |
| Troponin | 21.5 (0 – 50) | 11.5 (0–74) | 0.545 |
| CK-MB | 32 (10 – 60) | 32 (10–54) | 0.965 |
| Aortic root (mm) | 32.14 ± 2.54 | 33.03 ± 2.02 | 0.202 |
| Left atrium (mm) | 35.36 ± 2.87 | 35.69 ± 2.95 | 0.716 |
| LVEDD (mm) | 52.35 ± 8.15 | 50.23 ± 8.83 | 0.576 |
| LVESD (mm) | 33.61 ± 9.23 | 37.58 ± 6.18 | 0.084 |
| EF (%) | 52.54 ± 8.15 | 50.23 ± 8.83 | 0.4 |
| FS (%) | 27.81 ± 5.02 | 26.1 ± 5.23 | 0.305 |
| LAD | N=2 | N=6 | |
| Mean ± SD | 92.5 ± 3.54 | 86.67 ± 4.08 | 0.214 |
| LCX | N=2 | N=8 | |
| Mean ± SD | 87.5 ± 10.61 | 90.63 ± 4.96 | 0.526 |
| RCA | N=11 | N=23 | |
| Mean ± SD | 86.36 ± 5.05 | 89.35 ± 6.45 | 0.188 |
| No. of vessels affected | |||
| One | 13 (92.9%) | 35 (97.2%) | 0.486 |
| Two | 1 (7.1%) | 1 (2.8%) | |
Note: t independent sample t test χ2 chi square test **P ≤ 0.05 is statistically significant.
Table 5: Comparison between both subgroups A and B.
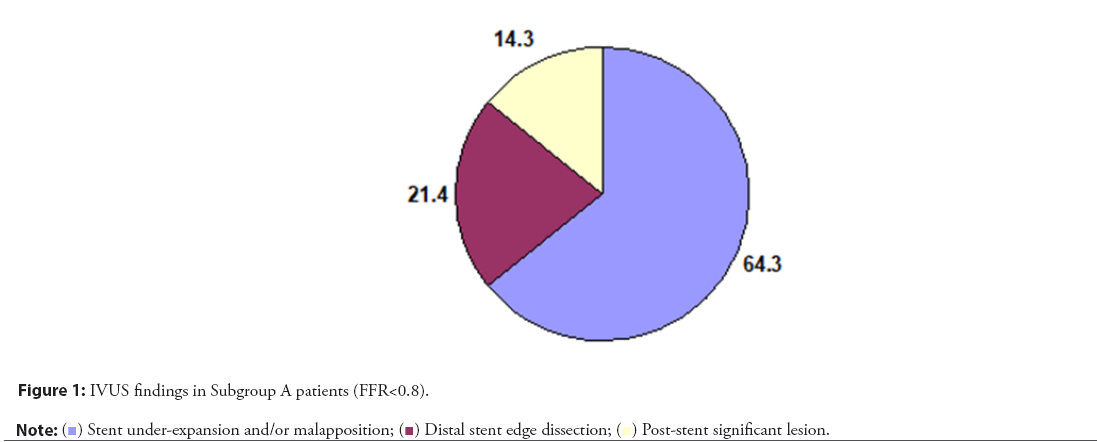
In Group I, 14 patients (28% of patients) had FFR<0.8 after coronary stenting and 36 patients (72% of patients) had FFR>0.8. IVUS has been done for those patients with FFR<0.8, revealed that “9 patients had stent under-expansion and/or malapposition, 3 patients had distal edge dissection of the stent and 2 patients had post stent significant lesion”. Patients with stent under-expansion and/or malapposition had been corrected by balloon dilatation using non-compliant balloons till FFR became>0.8, while patients with distal edge dissection and post-stent significant lesion had been treated by another stent inflation then FFR had been repeated till became>0.8 (Figure 1).
There was non-significant difference between FFR<0.8 (subgroup A) and FFR>0.8 (subgroup B), regarding age, gender, family history, heart rate, systolic or diastolic blood pressure, ESR level, C-reactive protein level, CK-MB level, troponin level, aortic root diameter, left atrium Diameter, LVEDD, LVESD, EF or FS.
Also there was no significant difference between both subgroups regarding number of vessels affected and Extent (severity) of vessel obstruction.
On contrary; there was significant difference between both subgroups regarding smoking, diabetes, hypertension (all were higher in those with FFR<0.8 “Subgroup A), resting ECG findings (Acute STEMI occurred in 27.8% in subgroup B versus 50% within subgroup A), HbA1c, total Cholesterol and Triglycerides (higher in subgroup A), HDL-cholesterol (lower in subgroup A).
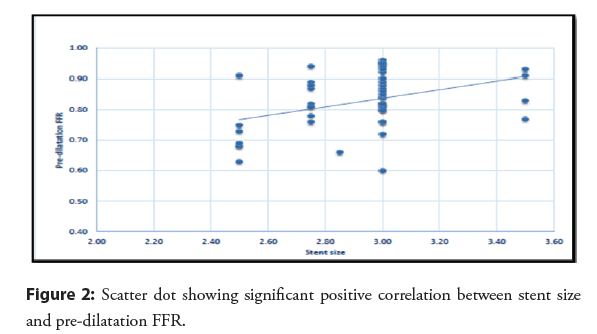
Also there was significant difference between both subgroups regarding size, and length of stent (Tables 4 and 5). There was statistically significant positive correlation between stent size and Pre-dilatation FFR. On the other hand, there is significant negative correlation between stent length and Pre-dilatation FFR (Figures 2 and 3). Regarding Multivariate regression, increasing HbA1c, and increasing stent length increase risk of FFR to be less than 0.8 by 16.402 and 1.356 folds respectively. Increasing stent size protect against FFR<0.8 (Tables 6 and 7).
| Parameter | Group 1 (post PCI FFR group) | Test | |
|---|---|---|---|
| Subgroup A (FFR<0.8) | Subgroup B (FFR>0.8) | p | |
| N=14 (%) Mean ± SD | N=36 (%) Mean ± SD | ||
| Size of stent | 2.775 ± 0.302 | 2.979 ± 0.202 | 0.032* |
| Length of stent | 32.43 ± 5.09 | 27.39 ± 5.06 | 0.003* |
| Site of stent (LAD) | N=2 | N=6 | |
| Mid | 0 (0%) | 4 (66.7%) | 0.429 |
| Proximal | 2 (100%) | 2 (33.3%) | |
| Site of stent (LCX) | N=2 | N=8 | |
| OM | 0(0%) | 5 (62.5%) | >0.999 |
| Proximal | 2 (100%) | 3 (37.5%) | |
| Site of stent (RCA) | N=11 | N=23 | |
| Distal | 2 (11.8%) | 2 (12.5%) | 0.819 |
| Mid | 5 (61.8%) | 16 (37.5%) | |
| Proximal | 4 (26.5%) | 5 (50%) | |
Note: t independent sample t test χ2 chi square test **P≤0.05 is statistically significant
Table 6: Comparison between both subgroups A and B regarding PCI data.
| p | CI | AOR | â | |
|---|---|---|---|---|
| 0.055 | 0.95–284.01 | 16.402 | 2.797 | HbA1c |
| 0.077 | 0–3.337 | 0 | -11.291 | Stent size |
| 0.044* | 1.01–1.823 | 1.356 | 0.304 | Stent length |
Note: *p<0.05 is statistically significant AOR Adjusted Odds Ratio CI confidence interval
Table 7: Multivariate regression analysis of factors associated with FFR<0.8 among studied patients.
Discussion
Regular coronary angiograms cannot always detect stenosis that is functionally meaningful. In more than one-third of patients, the original therapy choice is reclassified based on the functional assessment of intermediate lesions. Visual angiographic assessment of coronary stenosis is inferior to hemodynamic assessment utilizing Fractional Flow Reserve (FFR) [8].
A valid indicator of the functional severity of epicedial artery stenosis is FFR. This diagnostic method makes it easier to identify coronary artery disease that affects hemodynamics, which leads to more suitable interventions and better clinical results [9]. The European Society for Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines on myocardial revascularization developed strong recommendations for FFR guidance for Percutaneous Coronary Intervention (PCI) [10]. On the other hand, little research has been done on the value of FFR just after stenting to determine how the procedure affects coronary flow and any potential residual stenosis, and there is a dearth of information on this particular FFR application.
Given this background, we performed this study to Reduce post PCI complications. Up to my knowledge, this is the first study to discuss, this in Zagazig University Hospitals.
This study has three important results. First, in spite of successful percutaneous intervention angiographically but still significant numbers of patients suffering from residual ischemia and this leads to multiple of complications (recurrent angina, Arrhythmia, Restenosis, in-stent thrombosis or sudden cardiac death). Second, FFR after coronary stenting is accurate and sheep method to diagnose residual ischemia and consequently we can do IVUS to detect and correct the cause. Finally, post-stenting Non-compliant balloon dilatation should be done routinely because mal-opposed stent is the most common cause of residual ischemia.
In our study, there was statistically non-significant difference between both groups regarding Echocardiographic parameters including aortic root diameter, left atrium diameter, LVEDD, LVESD, EF or FS. In agreement to our study, Van Belle, et al. [11], studied Outcome Impact of Coronary Revascularization Strategy Reclassification With Fractional Flow Reserve at Time of Diagnostic Angiography demonstrated that independent parameter have been identified, LVEF.
We found a statistically non-significant difference between both groups regarding number of vessels affected and extent of obstruction in LCX, RCA or LAD. In agreement to our study, Van Belle, et al. [11], studied Outcome Impact of Coronary Revascularization Strategy Reclassification With Fractional Flow Reserve at Time of Diagnostic Angiography demonstrated that independent parameters have been identified, including the number of diseased vessels by angiography and lesions characteristics such as LAD location.
In our study, Among Group 1 (Post PCI FFR group), 14 patients (28% of patients) had FFR<0.8 after coronary stenting (subgroup A) and 36 patients (72% of patients) had FFR>0.8 (subgroup B). IVUS has been done for those patients with FFR<0.8, revealed that “9 patients had stent under-expansion and/or malapposition, 3 patients had distal edge dissection of the stent and 2 patients had post stent significant lesion”. Patients with stent under- expansion and/or malapposition had been corrected by balloon dilatation using non-compliant balloons till FFR became >0.8, while patients with distal edge dissection and post-stent significant lesion had been treated by another stent inflation then FFR had been repeated till became >0.8. There is significant increase in FFR in patients whose FFR pre-dilatation was <0.8 from 0.708 to 0.893 after dilatation. These results were concordant with Yang, et al. [12] reported effect of Coronary Disease Characteristics on Prognostic Relevance of Residual Ischemia after Stent Implantation and reported that the mean pre-and post-PCI FFR were 0.68 ± 0.11 and 0.87 ± 0.07, respectively (P<0.001). Agarwal, et al. [13] reported that Additional interventions were performed in 87/118 (73.7%) of these vessels which increased the final FFR to ≥ 0.80 in 58/87 (66.7%). In the overall population, additional post-dilation or stenting reduced the proportion of vessels with post-PCI FFR ≤ 0.80 from 118 (17.8%) to 63 (9.5%). In total, 137 vessels (20.6%) underwent further treatment for what were perceived to be suboptimal post-PCI FFR results with further post-dilatation (42%) and/or additional stenting (33%) or both (18%). Fractional flow reserve was repeated in all 137 lesions with an overall improvement from 0.78 ± 0.07 to 0.87 ± 0.05. Kikuta, et al. [14] noted that, a substantial number (more than one-third) of residual focal pressure gradients were found within the stented segment (despite their angiographically benign appearance), while about two thirds were present at the site of angiographically mild untreated lesions, indicating that further PCI (ideally with intravascular imaging) could lead to improved post-procedural physiology in the majority of patients. In this regard, a recent study evaluated the ability of pre-PCI-FFR to predict post-PCI physiology with high reliability.
In the current study, during hospital Follow up, there was significant difference between the studied groups regarding occurrence of chest pain (26% in Group II, versus 4% in Group I) and heart failure (28% in Group II versus 0% in Group I). While there were no difference regarding occurrence of arrhythmia, sudden cardiac death or in-stent thrombosis (occurred only in Group II; 8%, 4% and 6% respectively). Also during hospital follow up results were comparable to three months follow up results, chest pain was (41.7% in Group II versus 0% in Group I), heart failure was (20.8% in Group II versus 0% within Group I) and occurrence of sudden cardiac death or arrhythmias (occurred only in Group II; 2.1% and 12.5% respectively). The only difference was that There was also a significant difference concerning in-stent thrombosis, it was recorded in 20.8% within survivors of Group II versus 0% within Group I) Which in agreement with Tonino, et al. [15] studied Fractional flow reserve versus angiography for guiding percutaneous coronary intervention and found that FAME (fractional flow reserve versus angiography for guiding percutaneous coronary intervention) trial in 2009 demonstrated that the FFR-guided revascularization leads to lower 1-year adverse events and reduced costs compared to PCI based on angiographic findings. Also, Hakeem, et al. [16] has demonstrated an association between lower post-PCI FFR values and increased rates of major adverse cardiac events.
In the current study, there is statistically non-significant difference between Subgroup A (FFR<0.8) and Subgroup B (FFR>0.8) regarding age, gender, or family history. There is statistically significant difference between the studied sub-groups regarding, smoking, diabetes, and hypertension (all were higher in those with FFR<0.8). In agreement to our study, A study conducted by Diletti, et al. [17] studied the impact of post-stenting fractional flow reserve on long-term clinical outcomes and reported that Patients with a final post-stenting FFR<0.90 more frequently had hypertension (57% versus 51%, P=0.005) and diabetes (24% versus 17%, P=0.001).
Ojha, et al. [18] studied the clinical significance of physiological assessment of Residual Ischemia after Percutaneous Coronary Intervention agreed with our study in reporting that Diabetes mellitus patients tend to have lower post-PCI FFR, likely because of the presence of diffuse CAD in this population, while disagreed with our study in reporting that Lower post-PCI FFR has been associated with males.
In the current study, there is statistically significant difference between Subgroup A (FFR<0.8) and Subgroup B (FFR>0.8) regarding resting ECG findings. Acute STEMI occurred in 50% and 27.8% within groups of FFR<0.8 and >0.8 respectively. While, A study conducted by Diletti et al. [17] studied the impact of post- stenting fractional flow reserve on long-term clinical outcomes and reported that Patients with a final post-stenting FFR ≥ 0.90 presented more often with a STEMI (20% versus 44%, P<0.001).
In our study, there is statistically significant difference between Subgroup A (FFR<0.8) and Subgroup B (FFR>0.8) regarding HbA1c (higher in sub-group A), total cholesterol and triglycerides (higher in sub-group A) and HDL-cholesterol (which is significantly lower in sub-group A). There is statistically non- significant difference between the studied groups regarding ESR, or C reactive protein. In agreement to our study, A study conducted by Diletti et al. [17] studied the impact of post-stenting fractional flow reserve on long-term clinical outcomes and reported that Patients with a final posts-tenting FFR<0.90 more frequently had hypercholesterolemia (52% versus 44%, P=0.001).
In the current study, there is statistically non-significant difference between Subgroup A (FFR<0.8) and Subgroup B (FFR>0.8) regarding number of vessels affected and extent of obstruction in LCX, RCA or LAD.
Ojha, et al. [18] studied the clinical significance of physiological assessment of residual ischemia after percutaneous coronary intervention disagreed with our study in reporting that lower post-PCI FFR has been associated with use of multiple stents, and the presence of LAD lesion. Lower post-PCI FFR was more frequent in patients undergoing PCI to LAD when compared to the circumflex coronary artery or right coronary artery. Likely explanation is that the larger myocardium supplied by LAD which in turn leads to higher peak flow but lower post-PCI FFR with residual stenosis.
In the current study, we notice that increasing HbA1c, and increasing stent length increase risk of FFR<0.8 by 16.402 and 1.356 folds respectively. Increasing stent size protect against FFR<0.8.
The study by Kibarskis, et al. [19] which defined optimal post- PCI FFR>0.95 and found that only 12.2% had optimal post-PCI FFR when receiving long stents which was defined as stent length in between 30 and 49 mm in the study, and no patient had optimal post-PCI FFR when receiving ultra-long stents, which was defined as ≥ 50 mm and this came in contact with our study.
Although routine assessment of intermediate coronary stenoses pre-PCI is common, post-PCI physiology is rarely performed in clinical practice due to limited data Wolfrum, et al. [20] and lack of any specific guideline recommendations. With better wires and easier set up tools on the horizon, post-PCI FFR has the potential to be more widely accepted in the future, which will provide further evidence and possibly help in achieving better standardization.
This study has several limitations. The small number of patients included in the study. The follow-up period was limited to 3 months and long-term follow-up data are needed to validate our findings. Lack of any specific guideline recommendations for the use of FFR routinely post-PCI in addition to the cost.
Further, large randomized trials are needed to fully investigate the relation between suboptimal post-PCI FFR and clinical events and to elucidate PCI optimization strategies.
Conclusion
Post-PCI FFR has a great potential to direct the quality of PCI, optimize PCI, and improve prognosis by detection and subsequently correction of residual ischemia. Residual ischemia has many causes as stent under-expansion and/or malapposition, stent edge dissection and distal lesion after the stent. Early diagnosis and correction of residual ischemia will improve life style, decrease post-intervention complications (immediate and long term) and minimize re intervention as numerous studies have demonstrated that up to 20% of patients experience recurrent angina in the year following PCI, necessitating costly noninvasive and invasive testing and repeat revascularization mainly FFR. Further randomized controlled studies on larger sample and longer period of follow up are recommended to emphasize our conclusion.
Conflict of Interest
None
Funding
IRB approval number ZU-IRB#6516/15-11-2020.
References
- Pijls NH, van Gelder B, van der Voort P, et al. Fractional flow reserve: A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 92(11):3183-3193 (1995).
[Crossref] [Google Scholar] [PubMed]
- Pakkal M, Raj V, McCann GP. Non-invasive imaging in coronary artery disease including anatomical and functional evaluation of ischaemia and viability assessment. Br J Radiol. 84(3):S280-S295 (2011).
[Crossref] [Google Scholar] [PubMed]
- Berry C, Layland J, Sood A, et al. Fractional flow reserve versus angiography in guiding management to optimize outcomes in Non–ST-Elevation Myocardial Infarction (FAMOUS-NSTEMI): Rationale and design of a randomized controlled clinical trial. Am Heart J. 166(4):662-668 (2013).
[Crossref] [Google Scholar] [PubMed]
- Collison D, Didagelos M, Aetesam-ur-Rahman M, et al. Post-stenting fractional flow reserve vs coronary angiography for optimization of percutaneous coronary intervention (TARGET-FFR). Eur Heart J. 42(45):4656-4668 (2021).
[Crossref] [Google Scholar] [PubMed]
- Balanescu S. Fractional flow reserve assessment of coronary artery stenosis. Eur Cardiol. 11(2):77 (2016).
[Crossref] [Google Scholar] [PubMed]
- Piroth Z, Otsuki H, Zimmermann FM, et al. Prognostic Value of Measuring Fractional Flow Reserve After Percutaneous Coronary Intervention in Patients With Complex Coronary Artery Disease: Insights From the FAME 3 Trial. Circ Cardiovasc Interv. 15(11):884-891 (2022).
[Crossref] [Google Scholar] [PubMed]
- Pijls NH, Klauss V, Siebert U, et al. Coronary pressure measurement after stenting predicts adverse events at follow-up: A multicenter registry. Circulation. 105(25):2950-2954 (2002).
[Crossref] [Google Scholar] [PubMed]
- Meijboom WB, van Mieghem CA, van Pelt N, et al. Comprehensive assessment of coronary artery stenoses: Computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. 52(8):636-643 (2008).
[Crossref] [Google Scholar] [PubMed]
- Vijayan S, S Barmby D, R Pearson I, et al. Assessing coronary blood flow physiology in the cardiac catheterisation laboratory. Curr Cardiol Rev. 13(3):232-243 (2017).
[Crossref] [Google Scholar] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 40(2):87-165 (2019).
[Crossref] [Google Scholar] [PubMed]
- Van Belle E, Rioufol G, Pouillot C, et al. Outcome impact of coronary revascularization strategy reclassification with fractional flow reserve at time of diagnostic angiography: insights from a large French multicenter fractional flow reserve registry. Circulation. 129(2):173-185 (2014).
[Crossref] [Google Scholar] [PubMed]
- Yang S, Hwang D, Lee JM, et al. Role of post-stent physiological assessment in a risk prediction model after coronary stent implantation. JACC Cardiovasc Interv. 13(14):1639-1650 (2020).
[Crossref] [Google Scholar] [PubMed]
- Agarwal SK, Kasula S, Almomani A, et al. Clinical and angiographic predictors of persistently ischemic fractional flow reserve after percutaneous revascularization. Am Heart J. 184:10-16 (2017).
[Crossref] [Google Scholar] [PubMed]
- Kikuta Y, Cook CM, Sharp AS, et al. Pre-angioplasty instantaneous wave-free ratio pullback predicts hemodynamic outcome in humans with coronary artery disease: Primary results of the international multicenter FFR GRADIENT registry. JACC: Cardiovascular interventions. 11(8):757-767 (2018).
[Crossref] [Google Scholar] [PubMed]
- Tonino PA, de Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 360(3):213-224 (2009).
[Crossref] [Google Scholar] [PubMed]
- Hakeem A, Uretsky BF. Role of post intervention fractional flow reserve to improve procedural and clinical outcomes. Circulation. 139(5):694-706 (2019).
[Crossref] [Google Scholar] [PubMed]
- Diletti R, Masdjedi K, Daemen J, et al. Impact of poststenting fractional flow reserve on long-term clinical outcomes: The FFR-SEARCH study. Circ Cardiovasc Interv. 14(3):e009681 (2021).
[Crossref] [Google Scholar] [PubMed]
- Ojha CP, Ibrahim A, Paul TK, et al. The clinical significance of physiological assessment of residual ischemia after percutaneous coronary intervention. Curr Cardiol Rep. 22(4):1-7 (2020).
[Crossref] [Google Scholar] [PubMed]
- Kibarskis A, Baranauskas A, Peace A, et al. FFR result post PCI is suboptimal in long diffuse coronary artery disease. EuroIntervention. (12):1473-80 (2016).
[Crossref] [Google Scholar] [PubMed]
- Wolfrum M, Fahrni G, de Maria GL, et al. Impact of impaired fractional flow reserve after coronary interventions on outcomes: A systematic review and meta-analysis. BMC Cardiovasc Disord. 16(1):1-9 (2016).
[Crossref] [Google Scholar] [PubMed]