Device Evaluations - Interventional Cardiology (2011) Volume 3, Issue 2
The SeQuent Please drug-coated balloon system for percutaneous transluminal coronary angioplasty
- Corresponding Author:
- Stephanie Schmitmeier
InnoRa GmbH, Robert-Koch-Platz 4
10115 Berlin, Germany
Tel: +49 302 888 3364
E-mail: stephanie.schmitmeier@innora.de
Abstract
Keywords
coronary artery disease, drug-coated balloon, paclitaxel, percutaneous coronary intervention
Rationale
Established methods for treatment of stenotic or occluded arteries are percutaneous transluminal angioplasty and percutaneous transluminal coronary angioplasty (PTCA; or in a more general sense percutaneous coronary intervention [PCI]), subspecialties of imageguided therapy. Despite revolutionizing coronary revascularization, PTCA is associated with persistent difficulties that include vessel recoil and dissection during the procedure and restenosis usually within the first 6 months post-intervention. Restenosis, which is defined as a more than 50% reduction in postprocedural luminal diameter, and its recurrence rate after PTCA has been shown to generally vary from 30 to 50% [1]. Late lumen loss following PTCA is mainly attributed to negative vascular remodeling, including vessel recoil, shrinkage and restenosis [2]. The advent of coronary bare metal stents (BMSs) coupled with antiplatelet therapy has reduced the incidence of restenosis due to limitation of the extent of elastic recoil and late vascular remodeling compared with balloon angioplasty alone [3,4]. Despite these improvements, BMSs did not prevent lumen renarrowing, typically occurring within the first 6 months after the intervention, in approximately 20–40% of cases, necessitating a repeat procedure [5]. Drug-eluting stents (DESs) have emerged as a successful strategy in the primary prevention of restenosis as documented in preclinical and clinical trials [6–8]. These stents suppress neointimal proliferation by sustained delivery of antiproliferative agents (e.g., sirolimus or paclitaxel) due to special features for slow release (mostly polymer matrixes) from the stent struts to the arterial wall. DESs are well accepted in the prevention and treatment of coronary restenosis and are currently used in more than 70% of coronary interventions in the USA, for example. The reason is a long-term clinical benefit of these devices, mainly related to reduction of cardiac events associated with restenosis. However, DESs may be accompanied by delayed and incomplete endothelialization of the stent struts [9] attributed to high drug concentrations on the struts required due to the inhomogeneous drug distribution from the stent to the vessel wall. Although rare, very late stent thrombosis is a limitation of DESs [10–12]. Approximately 85% of the stented vessel wall area is not covered by the stent struts, which may result in incomplete suppression of neointimal hyperplasia in the tissue distant from the stent struts and between the struts [13]. Furthermore, stenting in patients with diabetes mellitus [14] and for lesions with high risk for in-stent restenosis and/or thrombosis, such as lesions in small vessels [15], bifurcations [10,16] and chronic total occlusions (CTOs), is still challenging. DES and also BMS deployment requires a long-lasting dual antiplatelet regime (current standard treatment: combined therapy with aspirin and clopidogrel) to prevent late thrombotic complications [10,11,17]. In addition, stents reduce the flexibility of the vessel, thereby limiting the repeatability of stenting. Repeated use of stents appears to further exacerbate the risk of recurrence of restenosis (especially in small vessels) due to augmentation of metal burden [18]. Nevertheless, ongoing developments with the stent platform and the polymer coating are gradually improving the performance of stents in clinical practice, allowing for greater flexibility and improved deliverability of the stents. Initial DESs, which use sirolimus and paclitaxel, are now being joined by newer stents releasing drugs, such as everolimus, zotarolimus and tacrolimus. In addition, biodegradable and newer-generation durable polymers for drug release are important components in the development of ‘future generation’ DESs to reduce polymer-related adverse events.
New concepts to overcome the limitations of DESs should avoid the need for sustained drug release from stent struts to allow for earlier endothelialization and healing. Preclinical studies, including cell culture experiments [19,20] and animal studies [20–23], demonstrated that short exposure to paclitaxel can result in prolonged inhibition of cell proliferation and neointimal hyperplasia provided that the drug reaches the vessel wall in sufficient concentration. The concept of non-stent-based local paclitaxel delivery was prompted by the surprising discovery that single-dose administration of the drug is sufficient, refuting the assumption that sustained release of the drug is necessary for long-lasting inhibition of restenosis. Scheller et al. reported an efficient inhibition of neointimal formation using paclitaxel-coated balloons in the porcine model of coronary overstretch [22]. These findings were confirmed by the first successful use of a paclitaxel-coated balloon in clinical trials (PACCOCATH-ISR I and PACCOCATH-ISR II), assessing the safety and efficacy of this device in coronary in-stent restenosis [24,25]. Paccocath™ balloons are standard angioplasty balloons coated with paclitaxel at a dose of 3 μg/mm2 of balloon surface in a specific matrix coating (paclitaxel is admixed to a small amount of the x-ray contrast medium Ultravist™) [22]. Studies with Paccocath balloons in the porcine coronary overstretch model [22,23,26–28] have shown:
▪ The potential for immediate drug release without the use of a polymer that can induce chronic inflammation and late thrombosis, as observed with some drug-eluting stents;
▪ The potential for homogeneous drug distribution to the arterial wall;
▪ The superiority of drug-coated balloons (DCBs) in reducing neointimal area versus uncoated balloons and stents;
▪ The option of using balloon catheters alone or in combination with a bare-metal stent if necessary;
▪ The feasibility of the procedure, in which a segment of the diseased artery can repeatedly be treated with DCBs in a row;
▪ Balloon inflation time is not critical for effective inhibition of neointimal proliferation, an issue that is important for critically ill patients who do not tolerate prolonged ischemia time due to long-lasting balloon inflation.
SeQuent™ Please balloon catheter: the next generation drug-coated balloon for coronary artery disease
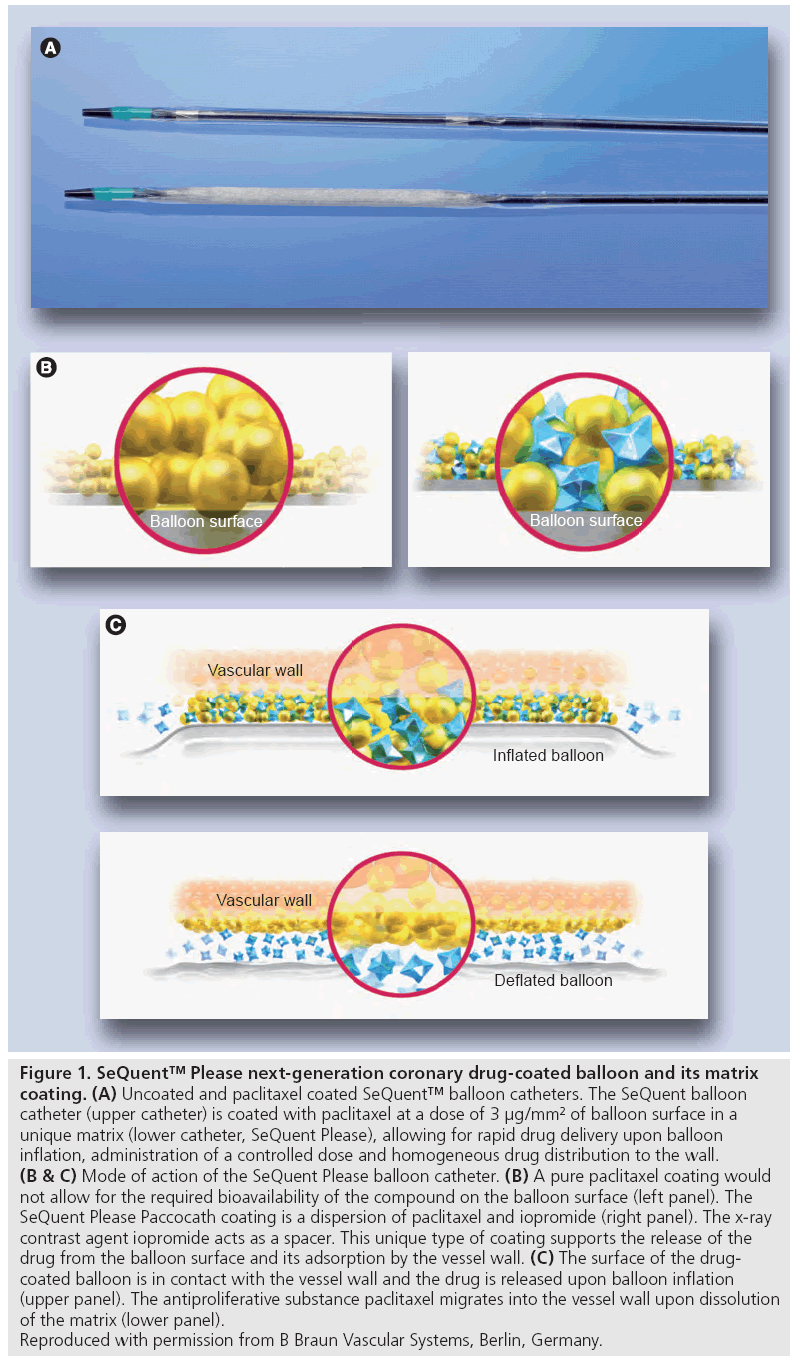
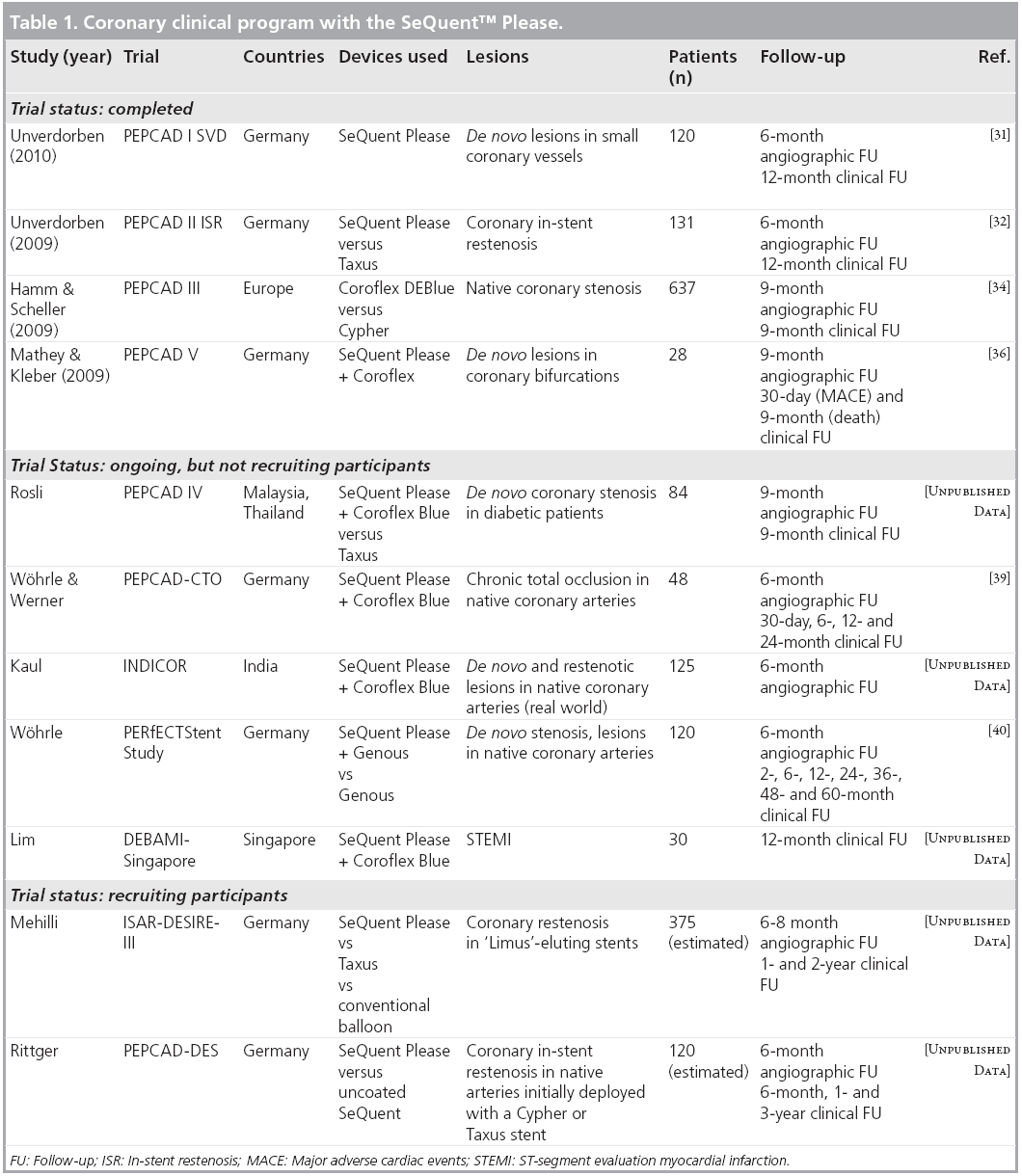
The development of next-generation DCBs was aimed at combining a state-of-the-art balloon catheter with the proven paclitaxel matrix coating technology used for Paccocath balloons in an automated coating process that guarantees a precise and uniform coating with a high reproducibility. The SeQuent™ PTCA balloon catheter (B Braun Melsungen AG, Germany) serves as an uncoated basis for the Paccocath coating since it assures with its mid-shaft helix design element a balanced force transmission allowing a sensitive catheter navigation. The balloon with its low profile and the sequential tip accurately follows even extremely curved vessels without tip flaring due to its tip material mix. The SeQuent Please balloon (coating licensed by B Braun from Charité, Berlin, Germany) is a paclitaxel-eluting rapid exchange balloon catheter for PTCA (Figure 1A). A small amount of the contrast agent iopromide (Ultravist), added to the coating, improves the solubility of paclitaxel and leads to enhanced dissolution of the drug [21], thus enabling a reliable transfer of sufficient amount of paclitaxel to the vessel wall during balloon inflation for 30–60 s (Figure 1B and C). Paclitaxel is the pharmacologically active substance, whereas iopromide, a well-tolerated nonionic x-ray contrast agent, acts as a release-supporting additive. Embedding of paclitaxel in a specif ically designed matrix provides an optimal release behavior of the drug during the coronary intervention. It has been shown that the major part of paclitaxel (~80% of the initial dose) is released during balloon inflation [22]. SeQuent Please balloons are available in diameters from 2.0 to 4.0 mm and lengths of 10 to 30 mm.
Figure 1: SeQuent™ Please next-generation coronary drug-coated balloon and its matrix
coating. (A) Uncoated and paclitaxel coated SeQuent™ balloon catheters. The SeQuent balloon
catheter (upper catheter) is coated with paclitaxel at a dose of 3 μg/mm2 of balloon surface in a
unique matrix (lower catheter, SeQuent Please), allowing for rapid drug delivery upon balloon
inflation, administration of a controlled dose and homogeneous drug distribution to the wall. (B & C) Mode of action of the SeQuent Please balloon catheter. (B) A pure paclitaxel coating would
not allow for the required bioavailability of the compound on the balloon surface (left panel). The
SeQuent Please Paccocath coating is a dispersion of paclitaxel and iopromide (right panel). The x-ray
contrast agent iopromide acts as a spacer. This unique type of coating supports the release of the
drug from the balloon surface and its adsorption by the vessel wall. (C) The surface of the drugcoated
balloon is in contact with the vessel wall and the drug is released upon balloon inflation
(upper panel). The antiproliferative substance paclitaxel migrates into the vessel wall upon dissolution
of the matrix (lower panel).
Reproduced with permission from B Braun Vascular Systems, Berlin, Germany.
The SPECTARIS study in 2008 calculated the potential cost savings of SeQuent Please balloon catheters in the coronary application for the treatment of in-stent restenosis to be approximately €59 million per year, based on the number of BMS and DES interventions in in-stent restenosis performed in Germany in 2007 [101]. The cost savings based on the combination of a shortened drug therapy with platelet aggregation inhibitors (~€27.5 million for 50,000 in-stent restenosis (ISR) patients treated with DCBs instead of DESs) and a reduced number of re-interventions after failed revascularization (~€31.6 million for ~7400 patients due to the difference in the reintervention rate of 14.8% between DES (18%) and SeQuent Please (3.2%) in a total of 50,000 ISR patients). However, this assessment focuses on the cost savings for the treatment of in-stent restenosis only.
The SeQuent Please does not require additional devices, such as perfusion catheters, or additional steps to treat a lesion compared with plain balloon angioplasty. There is also no significant difference in handling of the SeQuent™ Please to a regular uncoated balloon catheter, except that: first, select a balloon long enough to provide overlap beyond lesions and between balloons; second, do not touch/ bend the coated balloon more than necessary; third, keep time to lesion/inf lation short; fourth, make sure that the balloon membrane gets into close contact with the vessel wall (e.g., predilate with smaller diameter) and keep inflation pressure for 30–60 s if tolerated by the patient; and fifth, following drug release after the first inflation, the balloon may be used like an uncoated balloon for postdilatation but no additional drug will be released and no additional restenosis inhibition may be expected. Furthermore, there are no deviations from standard patient care/therapies when using SeQuent Please balloons. All patients independent of the actual treatment are assigned to treatment with antiplatelet aggregation inhibitors, anticoagulants and/or vasodilators during PCI.
Clinical trials with the SeQuent Please balloon
▪ PACCOCATH ISR-I/II studies
The first-in-man trial with a paclitaxel-eluting catheter was reported in 2006 [24]. The aim of this German, randomized, blinded, multicenter study (PACCOCATH-ISR I) with 52 patients enrolled was to investigate for the first time in humans the use of a paclitaxel-eluting balloon for the treatment of coronary in-stent restenosis. The subsequent PACCOCATHISR II trial with an additional 56 patients was conducted with an identical protocol to increase the probability of detecting coatingrelated adverse events and to test the reproducibility of the results of ISR I. These trials have clearly shown the superiority of DCBs versus uncoated balloons with respect to in-segment late lumen loss (at 6 months angiography: ISR-I: 0.74 ± 0.86 mm vs 0.03 ± 0.48 mm; p < 0.002 and ISR-II: 0.80 ± 0.79 mm vs 0.011 ± 0.44 mm; p < 0.001) and clinical outcomes. No adverse events were shown to be attributable to the Paccocath coating, and a shorter postprocedural therapy with dual platelet aggregation regimen was feasible (4 weeks instead of at least 6 months to even lifelong for DESs). Also, a sustained clinical effect of DCB was noted at 24 months with significant reduction in target lesion revascularization (37 vs 6%; p = 0.001) and major adverse cardiac events (MACE).
▪ PEPCAD studies
The SeQuent Please balloon has been clinically studied in the Paclitaxel-Eluting PTCA-Catheter in Coronary Artery Disease (PEPCAD) and other clinical trial programs (Table 1) [29]. The PEPCAD program is aimed at studying different indications with this device and focuses on criteria that are lesion-related (e.g., in-stent restenosis, small vessel disease, bifurcations and chronic total occlusions), procedure- related (e.g., sequence of using the drugcoated balloon and a BMS), and device related (DCB combined with a BMS or the SeQuent Please balloon with premounted BMS). All studies are prospective with late lumen loss of the target lesion as the primary end point. At present, some PEPCAD studies are completed, and their angiographic and clinical results are presented in Table 2 [30].
Table 2: Clinical trials of the SeQuent™ Please in coronary artery disease, published or presented at conferences.
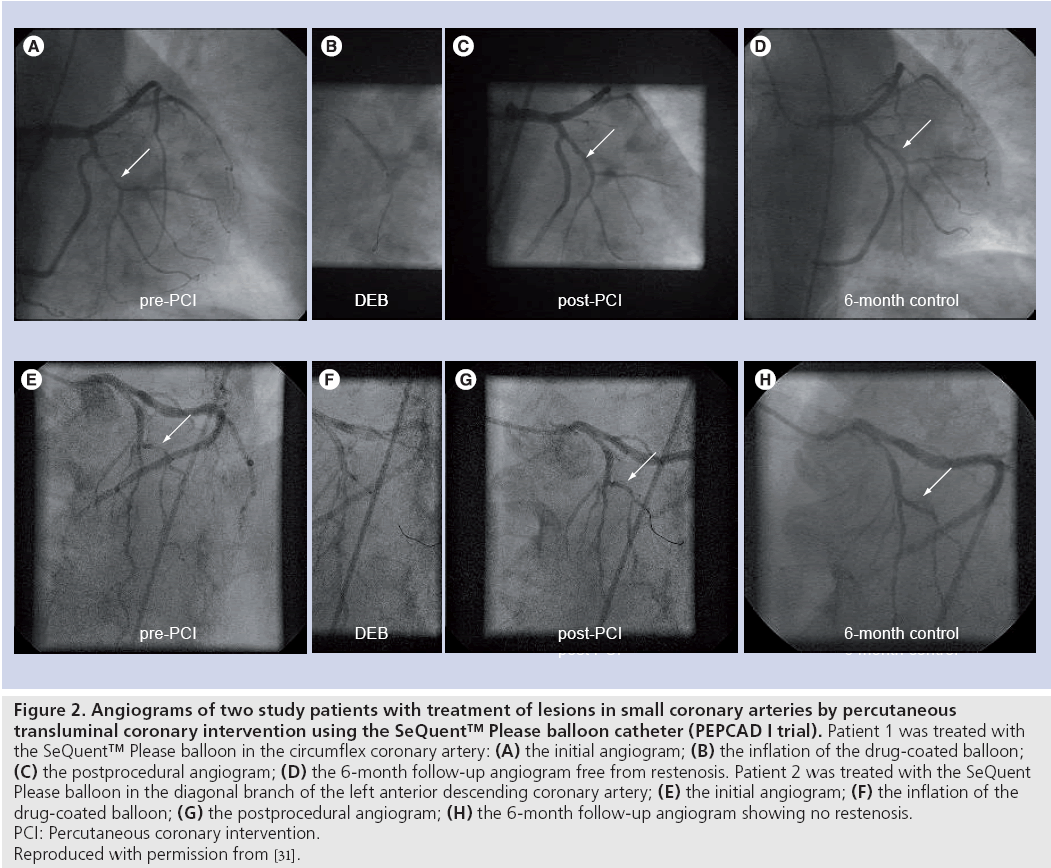
PEPCAD I was a German, non-randomized, single-arm, multicenter study investigating the safety and efficacy of the SeQuent Please balloon in small-vessel de novo lesions in 120 patients [31]. Most of the patients (70%) could be treated with the SeQuent Please balloon only, while 27% of the patients required additional BMS implantation due to acute elastic recoil or severe dissections, and in 3% of the patients the lesion could not be crossed by the study balloon. Patients treated with the DCB only demonstrated a very low in-segment late lumen loss (0.16 ± 0.38 mm) and a binary restenosis rate in the single digits (5.5%). However, in those patients with additional BMS implantation geographical mismatch between coated balloon dilatation and stent implantation was frequently associated with the occurrence of restenosis. Angiographic examples of two small-vessel disease patients treated with the SeQuent Please balloon in the PEPCAD I study are presented in Figure 2.
Figure 2: Angiograms of two study patients with treatment of lesions in small coronary arteries by percutaneous transluminal coronary intervention using the SeQuent™ Please balloon catheter (PEPCAD I trial). Patient 1 was treated with the SeQuent™ Please balloon in the circumflex coronary artery: (A) the initial angiogram; (B) the inflation of the drug-coated balloon; (C) the postprocedural angiogram; (D) the 6-month follow-up angiogram free from restenosis. Patient 2 was treated with the SeQuent Please balloon in the diagonal branch of the left anterior descending coronary artery; (E) the initial angiogram; (F) the inflation of the drug-coated balloon; (G) the postprocedural angiogram; (H) the 6-month follow-up angiogram showing no restenosis. PCI: Percutaneous coronary intervention. Reproduced with permission from [31].
PEPCAD II was a German, randomized, non-blinded, two-arm, multicenter trial of the SeQuent Please balloon versus the clinically established paclitaxel-coated Taxus™ Liberté stent (Boston Scientific, USA) in 131 patients with coronary in-stent restenosis. Compared with the DES, the DCB induced statistically significantly less in-segment late lumen loss (0.17 ± 0.42 mm vs 0.38 ± 0.61 mm; p = 0.03), resulting in a binary restenosis rate of 7 versus 20% (p = 0.06) at 6‑month follow-up and improved event-free survival (9 vs 22%; p = 0.08) at the 12‑month visit [32]. The results of this study are in good agreement with both the PACCOCATH ISR studies and the randomized Intracoronary Stenting and Angiographic Results: Drug-Eluting Stents for In-Stent Restenosis (ISAR-DESIRE) study comparing the sirolimus-eluting (Cypher™, Cordis Corp., USA) and paclitaxel-eluting (Taxus) stents with plain balloon angioplasty in the treatment of in-stent restenosis [33].
PEPCAD III was a European, randomized, single-blind (subject), two-arm, multicenter study, which compared an experimental device consisting of a cobalt–chromium stent premounted on the SeQuent Please balloon with the sirolimus-eluting Cypher stent in 637 patients with native coronary stenosis. Clinical end points were analyzed according to intention-to-treat. For the per protocol analysis of the primary end point 477 patients (75%) were available. Angiography at 9‑month followup indicated that this prototype DCB-stent system is effective in inhibiting restenosis but not to the same extent and at the high level of safety of the Cypher stent [34]. In-segment late lumen loss did not differ significantly (0.20 ± 0.52 mm vs 0.11 ± 0.40 mm; p = 0.07), whereas in-stent analysis was significantly in favor of the eluting stent (0.41 ± 0.51 mm vs 0.16 ± 0.39 mm; p < 0.001). Total MACE rate was 18.5% in the DCB plus BMS group and 15.4% in the DES group (p = 0.16). The DES group presented with a lower incidence of myocardial infarction (3.8 vs 0.6%; p < 0.01). Overall, the preliminary results of this study show that DCB plus BMS device did not meet the noninferiority criteria versus the sirolimuseluting stent presenting with exceptionally favorable results. Further design evolution is warranted to improve this new approach. In any case, DCBs are not a replacement for DESs but may provide a new platform in interventional cardiology to reduce the need for stents [35].
PEPCAD V was a small, German, prospective, single-arm, dual-center study including 28 patients with de novo bifurcational coronary artery lesions. The aim of this study was to investigate the feasibility of angioplasty using the SeQuent Please balloon in the main and the side branch of the bifurcation, followed by BMS deployment in the main branch. All procedures (equal to primary end point in this study) had been successful. In-segment late lumen loss at 9 months was 0.38 mm in the main branch and only 0.21 mm in the side branch. No MACE at 30‑day follow-up and no death at 9‑month follow-up were reported. Restenosis with target lesion revascularization (TLR) occurred in only one patient. However, in the main branch where a DCB was used in combination with BMS two patients experienced late stent thrombosis [36].
These clinical studies have shown so far (also shown in the Thunder [37] and FemPac [38] trials of the PACCOCATH balloon in peripheral arterial disease):
▪ High procedural success rate with DCBs, handling of DCBs similar to uncoated catheter;
▪ Safety of the PACCOCATH coating;
▪ The potential for improving treatment of coronary in-stent restenosis, lesions in small vessels and bifurcations, or other cases where stenting is not desirable or possible;
▪ The superiority of DCBs for treatment of instent restenosis compared with stand-alone balloon angioplasty;
▪ The potential for reducing anti-platelet therapy (few months therapy for DCBs instead of long-lasting anti-platelet regime for DESs);
▪ No observation of coating-related adverse events;
▪ The potential for avoiding the stent-in-stent approach with a second layer of metal in a native coronary artery.
Further PEPCAD studies are ongoing. PEPCAD IV is an Asian, randomized, twoarm, multicenter study comparing the efficacy of the SeQuent Please balloon followed by cobalt–chromium stent (Coroflex™ Blue, B Braun Melsungen AG) deployment versus paclitaxel-eluting Taxus Liberté stent in the treatment of de novo coronary stenosis in 84 diabetic patients. The primary end point is late lumen loss in target vessels at 9 months. This study is expected to be completed in 2011. PEPCAD-DES is a German, randomized, single-blind (subject), two-arm, multicenter, eff icacy study that is investigating vessel patency following treatment with either an uncoated balloon or a SeQuent Please balloon in patients initially treated with a Cypher or Taxus DES and is currently recruiting participants (estimated number to be enrolled: 120). PEPCAD-CTO is a German, singlearm, dual-center study assessing the safety and efficacy of the SeQuent Please balloon after bare metal stenting of a CTO in a native coronary artery in 48 patients. The outcome of this study is also being compared with that of a historical population of patients with CTO treated with the Taxus stent. In both trials, the primary end point is late lumen loss at 6 months. PEPCAD-DES and PEPCADCTO are expected to be completed in 2011 and 2014, respectively. For the ongoing PEPCADCTO trial the first clinical data from 6 months follow-up are now available (Table 2) showing that both the SeQuent Please balloon in combination with a BMS and the Taxus stent were similarly effective and safe in the treatment of CTO [39]. The SeQuent Please balloon is also being investigated in ISAR-DESIRE-III. This German, randomized, single-blind (outcome assessor), three-arm, dual-center study with an estimated 375 patients to be enrolled aims to determine, which treatment option, either SeQuent Please balloon, Taxus stent or plain balloon angioplasty is the most effective in the treatment of restenosis after initial implantation of a ‘Limus’-eluting stent (the ‘Limus’-family of drugs comprises rapamycin and its derivatives). INDICOR is an Indian, randomized, single-blind (subject), two-arm, multicenter real-world study investigating the acute 6‑month, 12‑month and 3-year outcome of the sequence of using the SeQuent Please balloon and the bare metal Coroflex Blue stent for the treatment of de novo and restenotic lesions in native coronary arteries in an estimated 125 patients. The primary end point in ISAR-DESIRE-III is percent insegment diameter at 6–8 months, while that of INDICOR is late in-segment and in-stent lumen loss at 6 months. The ISAR-DESIREIII and INDICOR studies are expected to be completed in 2014 and 2012, respectively. Another German, randomized, single-blind (subject), two-arm trial (the PERfECT Stent Study) has completed the enrollment of 120 patients for comparison of the combination of the SeQuent Please balloon and additional implantation of the Genous™ stent (OrbusNeich, Hong Kong) and the Genous stent alone in the prevention of restenosis in native coronary arteries. The primary end point of the PERfECT trial is late lumen loss at 6 months. The first promising clinical data of this study (Table 2) showed the superiority of the combination of the SeQuent Please balloon plus the Genous stent versus the Genous stent alone [40]. In-stent late lumen loss at 6 months was significantly lower in DCB plus stent compared with the stent alone (0.34 ± 0.45 mm vs 0.88 ± 0.48 mm; p < 0.001), resulting in significantly lower in-stent binary restenosis of 5% versus 21% (p = 0.009) and MACE (5 vs 17%; p = 0.03). However, this study is still ongoing and is expected to be completed in 2014. DEBAMI-Singapore is a Singapore single-arm, safety and efficacy study of PCI in 30 patients with ST-segment evaluation myocardial infarction (STEMI) using the SeQuent Please balloon and Coroflex Blue BMS. In this trial, critical aspects for the procedure such as the DCB diameter and stent size, as well as the length of both DCB and stent to be deployed, are considered. The primary end point of this trial is TLR at 12 months. The study is expected to be completed in 2011.
Other drug-coated balloons
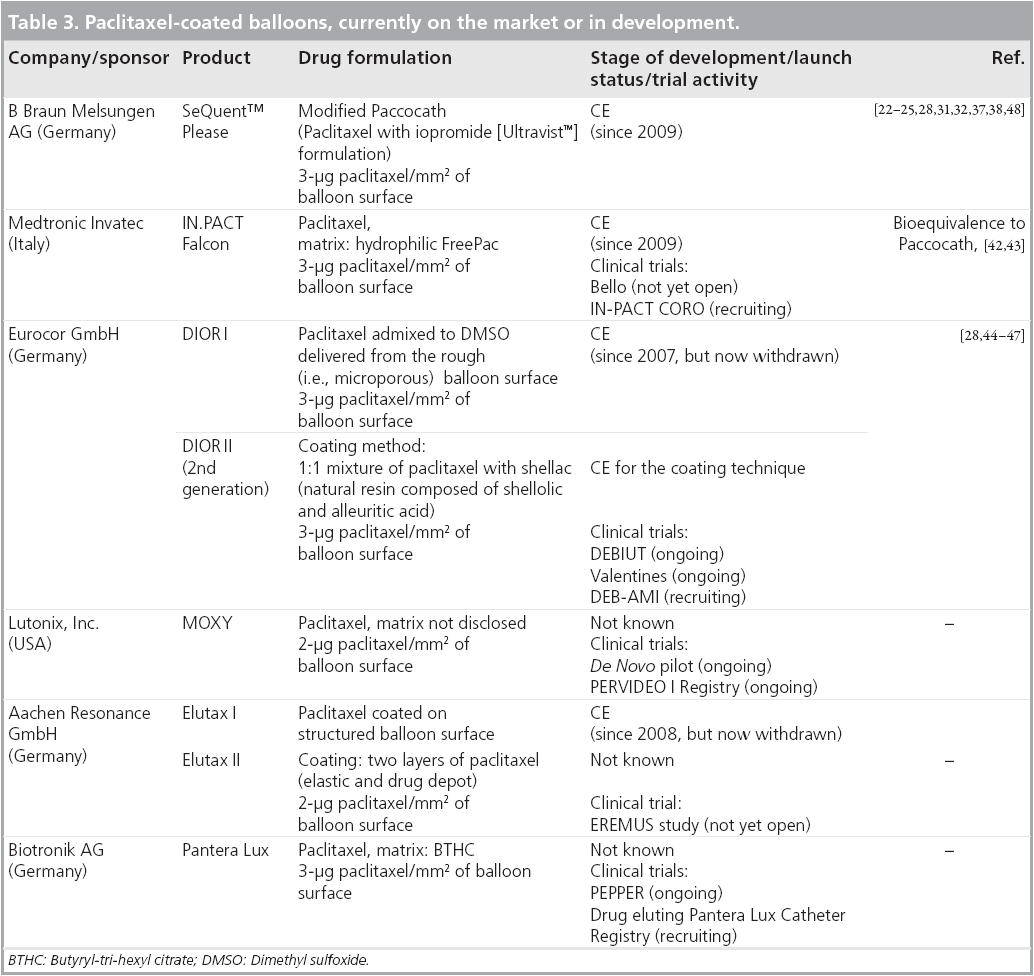
Stimulated by the initial studies on Paccocathcoated balloons several alternative DCBs for coronary artery disease, having different compositions and produced by different coating methods, have become available on the market or are in development (Table 3) [30,41]. Some of the currently available DCBs seem to be similar, but clinical data are still scarce or completely missing. Obviously, the various DCB catheters are not equally effective [28]. Several DCBs are currently being evaluated in clinical trials.
▪ IN.PACT™ (Medtronic Invatec)
Preclinical data of the IN.PACT™ Falcon paclitaxel- coated balloon show similar efficacy in animal experiments compared with the Paccocath coating [42]. The German, first-in-man, singlearm, single-center IN.PACT CORO ISR trial yielded promising results with the paclitaxelurea coated Falcon in the treatment of coronary in-stent restenosis [43]. However, this series is limited by its small number of patients (23 patients), the absence of a control group and a short follow-up time. Several clinical trials are ongoing and planned, including randomized, controlled studies.
▪ DIOR™ (Eurocor)
Comparison of two different paclitaxel-coated balloon catheters (Paccocath and DIOR™) in the porcine coronary model showed a dependence of inhibition of neointimal proliferation by paclitaxel on the coating composition. Whereas the dose of paclitaxel is identical in the case of the DIOR balloon catheter, adherence of paclitaxel is mediated by a rough membrane of the balloon without a hydrophilic matrix (first generation of DIOR). Cremers et al. showed in a head-to-head comparison of both balloon catheters in a porcine model that not every formulation is similarly effective [28]. The second generation of DIOR with new coating composition (paclitaxel plus shellac) shows some improved features versus its predecessor model [44,45]. The Dutch, singlearm, single-center Drug-Eluting Balloon in Bifurcation Utrecht (DEBIUT) Registry with 20 patients enrolled shows, despite missing data for the efficacy of DIOR balloons, that the treatment of coronary artery bifurcation lesions with these balloons is feasible and well tolerated [46].
In the Italian, randomized, noninferiority PICCOLETO trial, enrolling 57 patients with small coronary vessel disease the DIOR balloon failed to meet the noninferiority criteria versus the Taxus stent in terms of angiographic percent stenosis. Other clinical outcomes (death, myocardial infarction) were found to be equivalent in both groups, except for a trend toward increased TLR in the DIOR group [47].
▪ MOXY™ (Lutonix)
The potential of the MOXY™ paclitaxelcoated balloon is currently being assessed in two trials (de novo Pilot Study and PREVIDEO I Registry) for the treatment of coronary de novo stenosis and in-stent restenosis in bare metal stented vessel segments. Both studies are sponsored by Lutonix, Inc. (MN, USA) and are still ongoing.
▪ Elutax™ (Aachen Resonance)
The Italian, randomized, investigator-initiated, three-arm study EREMUS trial is not yet open for recruitment. This study is aimed at determining which treatment option, either Elutax™ paclitaxel-coated balloon combined with Genous stent (OrbusNeich), uncoated balloon combined with Genous stent, or uncoated balloon combined with Taxus stent, is the safest for patients undergoing PCI. The coating of the Elutax balloon consists of paclitaxel (2 μg/mm2) bound to the surface in two layers (ICE and SNOW technology).
▪ Pantera™ Lux (Biotronik)
The German, non-randomized, open-label, single-arm, multicenter PEPPER study is currently evaluating the safety and efficacy of the Pantera™ Lux paclitaxel-coated balloon in patients with in-stent restenosis in a coronary artery.
Currently, some of the paclitaxel-coated balloon catheters are also being investigated in the treatment of peripheral arterial disease in the femoropopliteal and below the knee studies (Cotavance™/MEDRAD, MOXY™/Lutonix, IN.PACT/Medtronic Invatec, Elutax™/ Aachen Resonance, and Advance™ 18PTX™/ Cook Medical).
The immediate comparators for DCBs are DESs. Meanwhile, a number of new stents are in different stages of development, either preclinical research, clinical trials, or have achieved marketing approval inside and outside the USA. So far, the US FDA has approved the following DESs for sale in the USA:
▪ Cypher sirolimus-eluting stent from Cordis Corp. (approved in 2003)
▪ Taxus paclitaxel-eluting stent from Boston Scientific (approved in 2004)
▪ Endeavor™ and Integrity™ zotarolimuseluting stents from Medtronic (approved in 2008 and 2010, respectively)
▪ Xience™/Promus™ everolimus-eluting stent from Abbott Vascular (approved in 2008; Xience is distributed as Promus by Boston Scientific)
Status of the SeQuent Please balloon on the market
The SeQuent Please was CE marked in March 2009 for use within the coronary arteries for primary angioplasty and for the treatment of restenosis of BMSs and DESs. This DCB was launched in Europe in March 2009 and had regulatory approval in several countries in Asia (except for Japan, Korea and China) prior to CE marking. None of the currently available DCBs have yet been approved by the US FDA due to a significantly more time-consuming and costly regulatory approval process. Similar to the European CE marking, the perspective of the FDA on DCBs is that it is a combinational product of balloon catheter and drug, both of which need to be properly evaluated by the Center for Devices and Radiological Health and the Center for Drug Evaluation and Research.
Conclusion
Although the use of DCBs appears to hold promise as a viable alternative to stand-alone balloon angioplasty supplementing stent implantation for the treatment of coronary artery disease, there are still many questions to be answered and regulatory processes to be satisfied in order to find the final role of DCB in interventional cardiology. So far, data from randomized clinical trials in the coronary application have identified the DCB as a viable technology for the treatment of coronary in-stent restenosis. First encouraging results from clinical trials, evaluating DCB safety and efficacy in the treatment of de novo lesions in small vessels and bifurcations, may give rise to more indications for this technique. Importantly, it has to be pointed out that not every formulation in DCB is similarly effective even if the same drug and dose has been chosen.
Future perspective
Non-stent-dependent targeted pharmacotherapy of vascular disease is an exciting field of basic and clinical research with the very real opportunity to broaden and improve therapeutic options. DCBs represent an excellent therapeutic concept, offering a valuable therapeutic alternative in situations in which the current therapies have proven unfeasible. However, there is still a strong need for further clinical evidence of each product’s capability, eff icacy, and safety. Numerous preclinical studies and clinical trials using this technology will open up routes to new therapies. Initially, high local drug concentrations may be shown to yield persistent beneficial effects beyond restenosis inhibition with low systemic toxicity.
Information resources
▪ The Paclitaxel-coated SeQuent™ Please balloon catheter system: www.deb-bbraun.com
▪ Clinical Trials: www.clinicaltrials.org
▪ TCTMD (source for interventional cardiovascular news and education): www.tctmd.com
Financial and competing interests disclosure
B Cremers and B Scheller received speaker’s honoraria from B Braun (Melsungen, Germany). B Scheller and U Speck are co-inventors on patents for various methods of inhibiting restenosis, including the products mentioned in this review, which were supported by Charité (Berlin, Germany). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Introduction
▪ Percutaneous transluminal coronary angioplasty with plain balloons was often associated with vessel recoil, dissections and high rates of restenosis.
▪ Coronary bare metal stents coupled with antiplatelet therapy reduce restenosis incidence versus plain balloon angioplasty, but did not prevent lumen renarrowing in approximately 20–40% of cases.
▪ Drug-eluting stents (DESs) significantly reduce restenosis rates, but require a long-lasting anti-platelet regime to avoid late thrombosis. DESs may not be desirable or applicable in lesions with high risk of in-stent restenosis and/or thrombosis, such as small vessels and bifurcations. Stents reduce vessel flexibility, thus limiting repeatability of stenting.
▪ Drug-coated balloons (DCBs): therapeutic alternative in cardiovascular intervention; significant reduction of in-stent restenosis by shortterm transfer of antiproliferative drugs upon balloon inflation (vs sustained release from stents). DCBs also allow for homogeneous drug distribution to the wall and do not require polymers for drug-release.
The SeQuent™ Please balloon catheter for coronary artery disease
▪ A paclitaxel-coated Rapid Exchange balloon catheter for percutaneous transluminal coronary angioplasty.
▪ Uses the Paccocath™ coating, consisting of paclitaxel at a dose of 3 μg/mm2 of balloon surface and the x-ray contrast agent iopromide (Ultravist™).
▪ Allows for reliable drug transfer to the vessel wall upon balloon inflation with an inflation time of 30–60 s.
▪ Available in different diameters (from 2.0 to 4.0 mm) and lengths (10–30 cm).
▪ Potential cost savings in the coronary application for in-stent restenosis treatment due to the combination of a shortened drug therapy with platelet aggregation inhibitors and a reduced number of re-interventions.
▪ No requirement of additional devices or additional steps.
▪ No significant difference in handling when compared with an uncoated balloon catheter.
Clinical trials with the SeQuent Please
▪ PEPCAD program of the SeQuent Please with or without bare metal stent for the treatment of in-stent restenosis, de novo lesions in small vessels and bifurcations, chronic total occlusion, native and de novo coronary stenosis (PEPCAD I-V, PEPCAD-CTO and -DES, INDICOR, ISAR-DESIRE-III, PERfECT Stent, DEBAMI-Singapore).
▪ Results from the completed clinical trials show:
– High procedural success rate with DCBs
– Safety of the PACCOCATH™ coating: no coating-related adverse events
– Improvement of treatment of coronary in-stent restenosis, de novo lesions in small vessels and bifurcations
– Superiority of DCB for treatment of in-stent restenosis versus stand-alone angioplasty
– In the treatment of in-stent-restenosis: DCB is at least as efficacious as DES
– Shorter duration of antiplatelet therapy (few months therapy instead of long-lasting dual antiplatelet regime for DESs)
Other drug (paclitaxel)-coated balloons for coronary artery disease
▪ The following DCBs, having different compositions and produced by different coating methods, have become available on the market: IN.PACT™ Falcon (Medtronic/Invatec), DIOR™ (Eurocor), MOXY™ (Lutonix), Elutax™ (Aachen Resonance) and Pantera™ Lux (Biotronik).
▪ Not all DCBs are equally effective (in respect to restenosis inhibition).
▪ Several DCBs are currently being evaluated in clinical trials.
Status of the SeQuent Please balloon system on the market
▪ CE marked in March 2009 for use within the coronary arteries for primary angioplasty and for restenosis of bare metal stents and DES.
▪ Launched in Europe in March 2009.
▪ Regulatory approval in several countries in Asia (except for Japan, Korea and China) prior to CE marking.
▪ Not yet approved by the US FDA.
Conclusion
▪ DCB is a clinically proven technology for the treatment of coronary in-stent restenosis.
▪ Positive outcome of the completed and current clinical trials may open the application of DCB to other coronary indications.
▪ DCB are not a substitute for BMS or DESss but a supplement where stenting is not necessary, not possible or not desirable.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Popma JJ, Califf RM, Topol EJ: Clinical trials of restenosis after coronary angioplasty. Circulation 84, 1426–1436 (1991).
- Mintz GS, Popma JJ, Pichard AD et al.: Arterial remodeling after coronary angioplasty: a serial intravascular ultrasound study. Circulation 94, 35–43 (1996).
- Serruys PW, de Jaegere P, Kiemeneij F et al.: A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N. Engl. J. Med. 331, 489–495 (1994).
- Fischman DL, Leon MB, Baim DS et al.: A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N. Engl. J. Med. 331, 496–501 (1994).
- Kastrati A, Mehilli J, Dirschinger J et al.: Restenosis after coronary placement of various stent types. Am. J. Cardiol. 87, 34–39 (2001).
- Heldman AW, Cheng L, Jenkins GM et al.: Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation 103, 2289–2295 (2001).
- Morice MC, Serruys PW, Sousa JE et al.: A randomized comparison of a sirolimuseluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346, 1773–1780 (2002).
- Moses JW, Leon MB, Popma JJ et al.: Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349, 1315–1323 (2003).
- Joner M, Finn AV, Farb A et al.: Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48, 193–202 (2006).
- Iakovou I, Schmidt T, Bonizzoni E et al.: Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293, 2126–2130 (2005).
- Pfisterer M, Brunner-La Rocca HP, Buser PT et al.: Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J. Am. Coll. Cardiol. 48, 2584–2591 (2006).
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL: Late thrombosis of drug-eluting stents: a metaanalysis of randomized clinical trials. Am. J. Med. 119, 1056–1061 (2006).
- Hwang CW, Wu D, Edelman ER: Physiological transport forces govern drug distribution for stent-based delivery. Circulation 104, 600–605 (2001).
- West NE, Ruygrok PN, Disco CM et al.: Clinical and angiographic predictors of restenosis after stent deployment in diabetic patients. Circulation 109, 867–873 (2004).
- Elezi S, Dibra A, Mehilli J et al.: Vessel size and outcome after coronary drug-eluting stent placement: results from a large cohort of patients treated with sirolimus- or paclitaxeleluting stents. J. Am. Coll. Cardiol. 48, 1304–1309 (2006).
- Tanabe K, Hoye A, Lemos PA et al.: Restenosis rates following bifurcation stenting with sirolimus-eluting stents for de novo narrowings. Am. J Cardiol. 94, 115–118 (2004).
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE: Stent thrombosis in randomized clinical trials of drug-eluting stents. N. Engl. J. Med. 356, 1020–1029 (2007).
- Elezi S, Kastrati A, Hadamitzky M, Dirschinger J, Neumann FJ, Schomig A: Clinical and angiographic follow-up after balloon angioplasty with provisional stenting for coronary in-stent restenosis. Catheter. Cardiovasc. Interv. 48, 151–156 (1999).
- Axel DI, Kunert W, Goggelmann C et al.: Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 96, 636–645 (1997).
- Scheller B, Speck U, Schmitt A, Bohm M, Nickenig G: Addition of paclitaxel to contrast media prevents restenosis after coronary stent implantation. J. Am. Coll. Cardiol. 42, 1415–1420 (2003).
- Scheller B, Speck U, Romeike B et al.: Contrast media as carriers for local drug delivery. Successful inhibition of neointimal proliferation in the porcine coronary stent model. Eur. Heart J. 24, 1462–1467 (2003).
- Scheller B, Speck U, Abramjuk C, Bernhardt U, Bohm M, Nickenig G: Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation 110, 810–814 (2004).
- Speck U, Scheller B, Abramjuk C et al.: Neointima inhibition: comparison of effectiveness of non-stent-based local drug delivery and a drug-eluting stent in porcine coronary arteries. Radiology 240, 411–418 (2006).
- Scheller B, Hehrlein C, Bocksch W et al.: Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N. Engl. J. Med. 355, 2113–2124 (2006).
- Scheller B, Hehrlein C, Bocksch W et al.: Two year follow-up after treatment of coronary in-stent restenosis with a paclitaxelcoated balloon catheter. Clin. Res. Cardiol. 97, 773–781 (2008).
- Scheller B, Speck U, Bohm M: Prevention of restenosis: is angioplasty the answer? Heart 93, 539–541 (2007).
- Cremers B, Speck U, Kaufels N et al.: Drug-eluting balloon: very short-term exposure and overlapping. Thromb. Haemost. 101, 201–206 (2009).
- Cremers B, Biedermann M, Mahnkopf D, Bohm M, Scheller B: Comparison of two different paclitaxel-coated balloon catheters in the porcine coronary restenosis model. Clin. Res. Cardiol. 98, 325–330 (2009).
- Scheller B, Speck U: Potential solutions to the current problem: coated balloon. Euro- Intervention 4(Suppl. C), C63–C66 (2008).
- Tepe G, Schmitmeier S, Speck U, Schnorr B, Kelsch B, Scheller B: Advances on drugcoated balloons. J. Cardiovasc. Surg. (Torino) 51, 125–143 (2010).
- Unverdorben M, Kleber FX, Heuer H et al.: Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin. Res. Cardiol. 99, 165–174 (2010).
- Unverdorben M, Vallbracht C, Cremers B et al.: Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation 119, 2986–2994 (2009).
- Kastrati A, Mehilli J, von Beckerath N et al.: Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA 293, 165–171 (2005).
- Hamm CW, Cremers B, Moellmann H et al.: Paclitaxel-eluting PTCA-balloon in combination with the Coroflex Blue Stent vs. the sirolimus coated Cypher stent in the treatment of advanced coronary artery disease – PEPCAD III. Presented at: American Heart Association (AHA) 2009. Orlando, FL, USA, 14 November, 2009.
- Poss J, Jacobshagen C, Ukena C, Bohm M: Hotlines and clinical trial updates presented at the German Cardiac Society Meeting 2010: FAIR-HF, CIPAMI, LIPSIA-NSTEMI, Handheld-BNP, PEPCAD III, remote ischaemic conditioning, CERTIFY, PreSCD-II, German Myocardial Infarction Registry, DiaRegis. Clin. Res. Cardiol. 99, 411–417 (2010).
- Mathey DG: The PEPCAD Clinical Trial Program (oral presentation). Presented at: Transcatherter Cardiovascular Therapeutics (TCT) 2009. San Francisco, CA, USA 22 September, 2009.
- Tepe G, Zeller T, Albrecht T et al.: Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N. Engl. J. Med. 358, 689–699 (2008).
- Werk M, Langner S, Reinkensmeier B et al.: Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation 118, 1358–1365 (2008).
- Werner GS, Wöhrle J: A prospective non-randomized study of paclitaxel-eluting balloons after bare metal stent placement in successfully recanalized chronic total coronary occlusions (PEPCAD CTO) (Oral presentation). Transcatherter Cardiovascular Therapeutics (TCT) 2010. Washington, DC, USA 24 September, 2010.
- Wöhrle J: A prospective, randomized trial evaluating a paclitaxel-eluting balloon in patients treated with endothelial progenitor cell capturing stents for de novo coronary artery disease, the PERfECT Stent Study (Oral presentation). TCT 2010. Washington, DC, USA 23 September, 2010.
- Speck U, Scheller B, Tepe G: Drug-eluting balloon therapy: sustained efficacy without sustained-release formulation? Hosp. Imag. Radiol. Eur. 4, 39–42 (2009).
- Kelsch B, Scheller B, Biedermann M et al.: Dose response to paclitaxel-coated balloon catheters in the porcine coronary overstretch and stent implantation model. Invest. Radiol. 46, 255–263 (2011).
- Cremers B, Clever Y, Schaffner S, Speck U, Bohm M, Scheller B: Treatment of coronary in-stent restenosis with a novel paclitaxel urea coated balloon. Minerva Cardioangiol. 58, 583–588 (2010).
- Posa A, Hemetsberger R, Petnehazy O et al.: Attainment of local drug delivery with paclitaxel-eluting balloon in porcine coronary arteries. Coron. Artery Dis. 19, 243–247 (2008).
- Posa A, Nyolczas N, Hemetsberger R et al.: Optimization of drug-eluting balloon use for safety and efficacy: evaluation of the 2nd generation paclitaxel-eluting DIORballoon in porcine coronary arteries. Catheter. Cardiovasc. Interv. 76, 395–403 (2010).
- Fanggiday JC, Stella PR, Guyomi SH, Doevendans PA: Safety and efficacy of drug-eluting balloons in percutaneous treatment of bifurcation lesions: the DEBIUT (drug-eluting balloon in bifurcation Utrecht) registry. Catheter. Cardiovasc. Interv. 71, 629–635 (2008).
- Cortese B, Micheli A, Picchi A et al.: Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO study. Heart 96, 1291–1296 (2010).
- Albrecht T, Speck U, Baier C, Wolf KJ, Bohm M, Scheller B: Reduction of stenosis due to intimal hyperplasia after stent supported angioplasty of peripheral arteries by local administration of paclitaxel in swine. Invest. Radiol. 42, 579–585 (2007).
▪ An early key publication comparing stent implantation versus balloon angioplasty.
▪ Demontrates that paclitaxel stent coating is feasible to reduce coronary restenosis in animals.
▪ Demonstrates that sirolimus-eluting stents are effective in reducing coronary restenosis in patients with a lesion in a native coronary artery.
▪ Showed in randomized clinical trials of drug-eluting stent that the incidence of stent thrombosis is not significantly different between patients with drug-eluting stents and those with bare-metal stents.
▪ A key publication showing long-lasting inhibition of arterial smooth muscle cell proliferation by paclitaxel after a short-time exposure of cells to the drug.
▪▪ Showed for the first time that paclitaxel balloon coating is safe and efficacious in restenosis inhibition in animals.
▪▪ Showed for the first time successful treatment of in-stent restenosis with paclitaxel-coated balloon catheters in humans.
▪ Describes successful treatment of de novo lesions in small coronary vessels with paclitaxel-coated balloon catheters.
▪ Showed that the paclitaxel-coated balloon is at least as efficacious and well tolerated as the paclitaxel-coated stent for the treatment of coronary in-stent restenosis.
▪ Showed for the first time successful treatment of stenosis, restenosis and occlusion of femoropopliteal arteries with paclitaxel-coated balloon catheters.
▪ Website
- SPECTARIS: Potential cost-savings of innovative medical technology in health care www.deb-bbraun.com/documents/ Knowledge/231008_PotentialCost Savings_2008