Review Article - Interventional Cardiology (2013) Volume 5, Issue 5
The role of adjunctive imaging in chronic total occlusions
- Corresponding Author:
- Jonathan Hill
Cardiac Department, King’s College Hospital, King’s Health Partners, Denmark Hill, London, SE5 9RS, UK
Tel: +44 773 998 8822
E-mail: jmhill@nhs.net
Abstract
It has been estimated that chronic total occlusions (CTOs) are observed in up to 35% of patients with suspected coronary artery disease who undergo coronary angiography. Despite being quite common, CTO percutaneous coronary intervention (PCI) is not commonly attempted.
Keywords
coronary;CTO;imaging;nPCI.
Introduction
It has been estimated that chronic total occlusions (CTOs) are observed in up to 35% of patients with suspected coronary artery disease who undergo coronary angiography. Despite being quite common, CTO percutaneous coronary intervention (PCI) is not commonly attempted [1,2]. This is thought to be primarily owing to poor CTO PCI success rates [3], which have led to the development of novel techniques, equipment, devices and imaging. There are a multitude of different imaging modalities available that can help with the success of CTO PCI. Angiographic imaging in CTO will remain fundamentally central to procedural success. However, the rapid developments of adjunctive forms of imaging have become increasingly important in all the phases of CTO PCI patient management, including patient selection, procedural planning and follow-up [4]. This review will outline the types of imaging modalities available and will propose how they can be used in CTO PCI, allowing each operator to tailor his imaging requirements to the individual patient.
Adjunctive CTO imaging in case selection
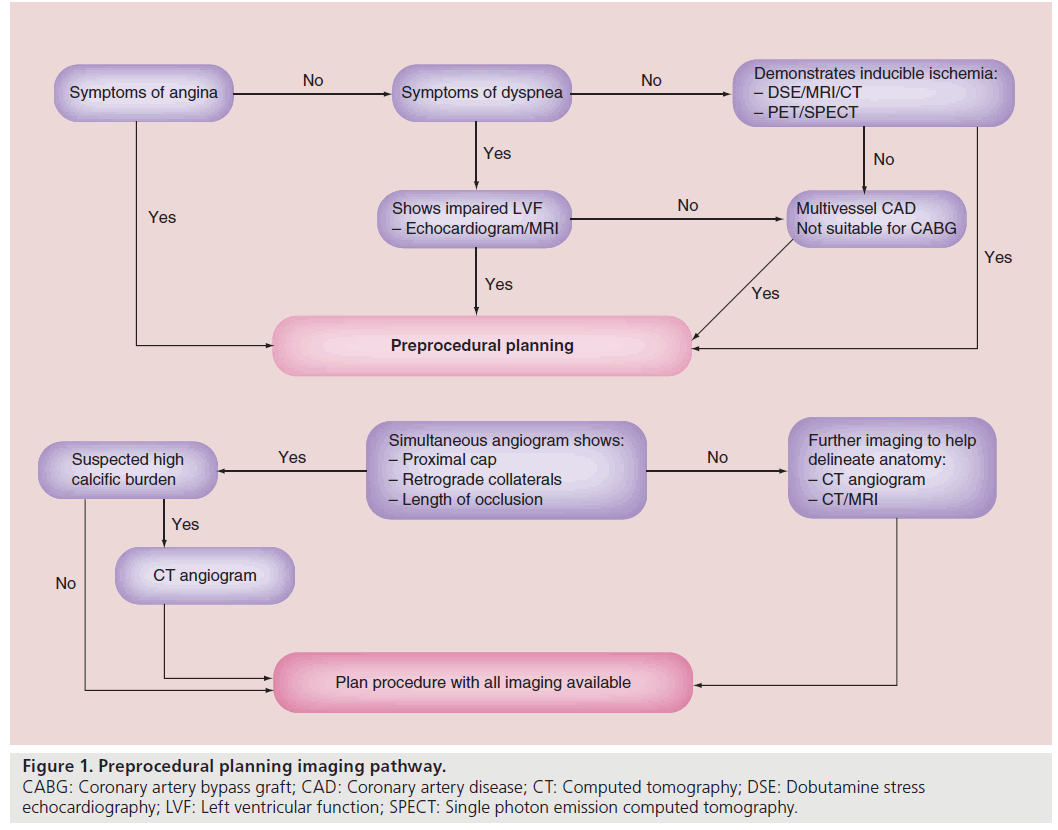
Case selection is of paramount importance in CTO PCI. Figure 1 shows a flow chart that helps characterize decision pathways involved in adjunctive CTO imaging, helping to decide on patient selection and to improve overall success. The decision to proceed onto CTO PCI should be based on either symptoms control, improvement in left ventricular function (LVF) or improved survival [5]. Common symptoms of angina and dyspnea can be discerned at the time of patient review and restoration of antegrade flow can help alleviate those symptoms. Successful treatment of a CTO has been shown to improve angina events in patients [6]. Nevertheless, adjunctive imaging in CTO has been used more in relation to determination of improvements of LVF and survival by determination of hibernating and ischemic myocardium [6]. Despite CTOs having collateral flow, allowing sufficient myocardial perfusion under basal conditions, perfusion may not be adequate, resulting in ischemia when metabolic needs increase [7]. This can result in hibernating myocardium, which is a state of persistently impaired LVF at rest owing to reduced coronary blood flow that can be partially or completely restored to normal by improving blood flow [8]. There are a multitude of different modalities that can help assess both ischemic or hibernating myocardium in patients considered for CTO PCI.
▪ Echocardiography
Classically, exercise stress echocardiography has been used to determine left ventricular ischemia in coronary artery disease. However, in patients unable to exercise, pharmacological stress agents (e.g., dobutamine, adenosine and dipyridamole) have been substituted effectively [9]. Regardless, stress echocardiography is a widespread and relatively inexpensive method to assess myocardial viability and ischemia. In relation to viability, dobutamine stress echocardiography (DSE) is one of the most common modalities used. However, even a simple resting echocardiograph can give an impression. The presence of pronounced end diastolic wall thinning virtually excludes the presence of viable tissue [10]. In patients without a clear thinning of the dysfunctional area, low-dose dobutamine echocardiography can be performed to evaluate myocardial viability. Dysfunctional, but viable myocardium is characterized by preserved contractile reserve during DSE [11]. This response is highly predictive of recovery of function after revascularization. The combination of DSE with concomitant echocardiography modalities, including the use of contrast, also helps to diagnose viable myocardium [12].
▪ Nuclear techniques: single photon emission computed tomography
Single photon emission computed tomography (SPECT) can also be used in the assessment of both ischemia and viable myocardium [13]. Two main tracers can be used: thallium-201 or technetium-99m. Prior clinical studies suggested that myocardial perfusion imaging with either thallium-201 or technetium-99m sestamibi can provide clinically important information pertaining to the status of myocardial viability when systolic dysfunction exists in the setting of severe coronary artery disease or after an acute myocardial infarction [14]. This ability to identify ischemic or viable myocardium can help with identification of suitable patients to undergo CTO PCI.
▪ Nuclear techniques: PET
PET is a nuclear imaging technique that can detect viable myocardium with the use of a radionuclear tracer fluorodeoxyglucose. PET is often considered the reference technique for the assessment of myocardial viability owing to the quantitative results, high spatial resolution and good correlation with outcome after coronary revascularization [15]. Several studies have demonstrated that SPECT and fluorodeoxyglucose-PET imaging have a comparable diagnostic accuracy for the assessment of myocardial viability [16]; however, its cost–effectiveness in this role is unclear.
▪ Cardiac MRI
Cardiac MRI (CMR) is a versatile noninvasive imaging modality providing accurate and reproducible assessment of global and regional ventricular function, blood flow, myocardial perfusion and myocardial scar [17]. Spatial and temporal resolution have been improved with techniques such as ECG gating, acquisition during breath holds and fast data readout by means of gradient echo. This allows visualization of myocardial perfusion at rest and at stress [18,19]. With the use of stress dobutamine or adenosine, CMR can also accurately identify areas of myocardial ischemia and associated changes in myocardial contractility [20], which can indicate coronary artery stenosis. CMR can detect hibernating myocardium with either the use of dobutamine or gadolinium contrast [21,22]. Patients with dysfunctional, but viable, myocardium benefit from revascularization; the identification and quantification of the extent of myocardial viability is an important part of the preprocedural work-up of CTO patients. In fact, CMR has shown improvement of LVF after successful reopening of CTO [23]. Thus, overall, CMR is a useful tool in helping to determine which patients are likely to benefit most from successful CTO PCI in the preprocedural work-up phase [24].
▪ Preprocedural planning
After the identification of patients who require CTO PCI, the accurate identification of coronary anatomy is the next step. Visualization of the critical aspects of the CTO allow for initial treatment strategies to be formulated. Identification of the proximal cap, length of occlusion, distal vessel bed and retrograde collaterals helps to facilitate procedural strategies and improved success rates [25]. Classically, all of these can usually be identified with the use of an angiogram. However, if there are difficulties in identification, there are other imaging modalities that can be used to help further identify these key areas.
▪ Multislice computerized tomography
The use of computerized tomography coronary angiography (CTCA) for the diagnosis and evaluation of coronary artery disease has significantly progressed over the last decade. Improvement in temporal resolution and reduction in radiation doses now enable the CTO operator to integrate CTCA into preprocedural planning [26]. The ability to manipulate the rendered 3D image can provide high quantitative and qualitative diagnostic accuracy of coronary disease without the visual–spatial limitations that occur with conventional coronary angiography, for example foreshortening [27]. This less invasive method also provides information about coronary anatomy in areas of no-flow (e.g., CTOs) and can even help characterize the coronary plaque characteristics in high resolution [28]. The ability to visualize vessel course is generally related to calcium burden, and is a significant advantage of CTCA over conventional angiography [29]. The identification of calcium burden within the CTO plaque is accurate with computed tomography (CT) and is a strong positive predictor of CTO failure [30].
In addition, the incorporation of 3D imaging techniques helps to identify and accurately assess occlusion length, stump shape and vessel tortuosity [31]. These morphological characteristics, along with vessel calcification, are closely linked to procedural success [32]. This illustrates the role of CT in preprocedural work-up to help predict favorable outcomes in CTOs undergoing PCI. Adjunctive use of preprocedural CTCA can lead to procedural success of over 90% [33]. However, the use of CTCA in the preprocedural work-up of CTO patients is usually performed by experienced operators and teams, and this experience may actually be the reason for improved success rates. Another significant concern remains regarding adding to cumulative radiation exposure, particularly with poorly controlled heart rates when prospective gating protocols cannot be used [34]. Thus, CTCA should be considered on a case-by-case basis.
▪ MRI for coronary anatomy
Apart from assessment of the myocardium, the direct visualization of coronary vessels has been achieved with magnetic resonance angiography (MRA) and has been shown to be superior to standard angiography in determining the course of anomalous coronary vessels [35]. With 3D visualization this modality has been used to identify coronary stenosis [36]. However, the use of MRA was only sensitive in those patients with left main or triple vessel disease and did not help to differentiate those with CTOs [37]. Thus, overall, MRI at this stage does not have the image quality to replace standard angiography or CTCA.
CTO treatment guiding
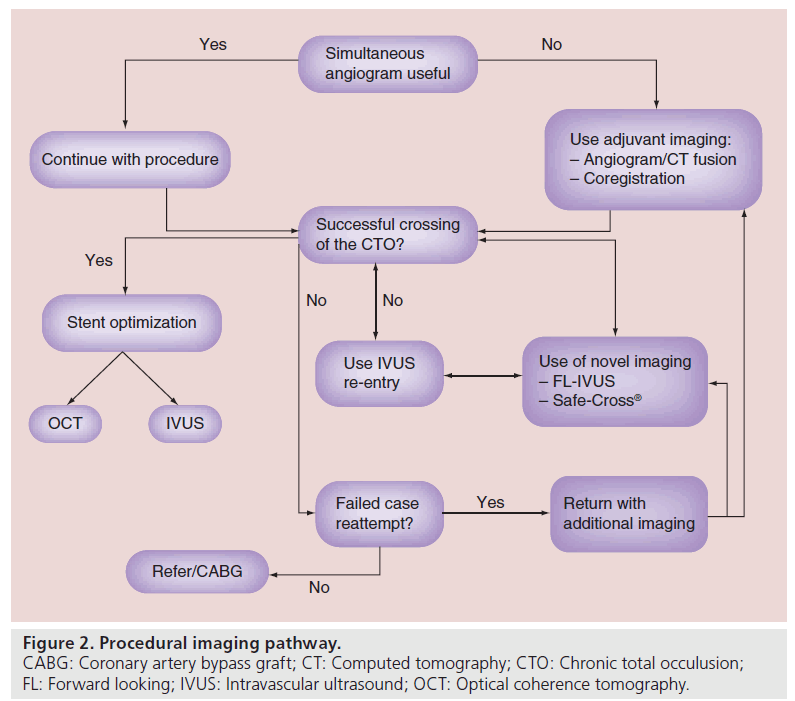
Once the decision to perform CTO PCI has been made, the use of imaging during the procedure can also help with success rates. Apart from the identification of coronary anatomy during the case, certain imaging modalities can be used to help with proximal cap localization, subintimal wire identification and stent optimization. Figure 2 demonstrates a typical flow chart that can be adopted during a procedure in which adjunctive imaging can be used. The use of these imaging techniques are commonly used by experienced CTO operators.
▪ Intravascular ultrasound
Adjunctive use of intravascular ultrasound (IVUS) in CTO PCI [38], as with conventional PCI, can provide valuable information regarding vessel size, lesion length, plaque morphology and intracoronary calcium distribution [39], and is also helpful for stent optimization [40]. Nevertheless, there are novel uses of IVUS that are particular to CTO PCI. These include IVUS-guided wire penetration from a subintimal space into the true lumen [41,42] and proximal cap identification, which can be performed by standard side-view IVUS or forward-looking (FL)-IVUS.
Conventional side-viewing IVUS
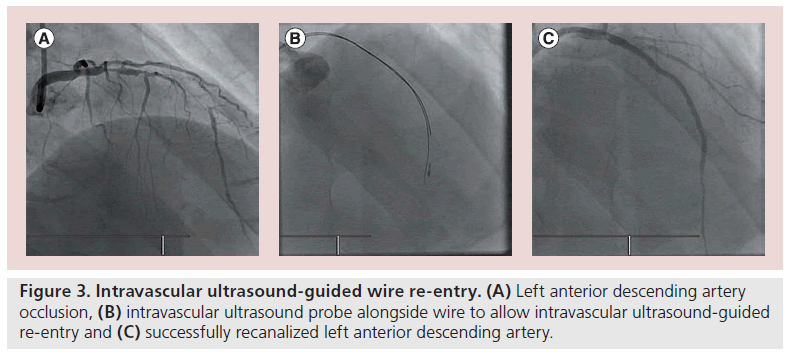
In relation to the proximal fibrous cap, an IVUS catheter can be used to determine the entry point in vessels with a flush occlusion. Case reports depict the use of an IVUS catheter to locate the proximal fibrous cap, by placing the catheter into an adjacent patent artery and allowing the image to localize the cap [43]. With the development of novel subintimal wire techniques in CTO PCI including subintimal tracking and re-entry (STAR) and controlled antegrade and retrograde tracking (CART) the use of IVUS has become more common [44]. IVUS can be used to localize wire position in the subintimal space or true lumen, but can also facilitate wire re-entry into the true lumen (Figure 3) [45,46]. This is particularly helpful when the case does not facilitate antegrade or retrograde use of contrast injection (Figure 4). This technique may well be superseded by the increased use of dissection re-entry using the CrossBoss™ catheter (Boston Scientific, MA, USA) and Stingray™ balloon (Boston Scientific) [47].
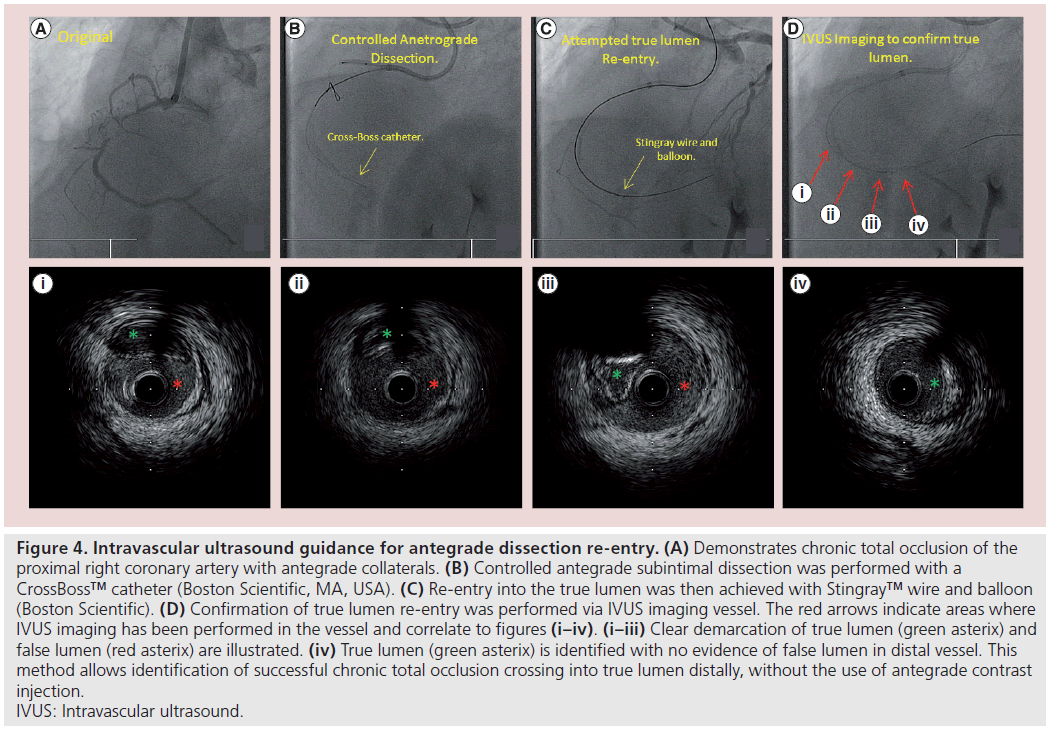
Figure 4: Intravascular ultrasound guidance for antegrade dissection re-entry. (A) Demonstrates chronic total occlusion of the proximal right coronary artery with antegrade collaterals. (B) Controlled antegrade subintimal dissection was performed with a CrossBoss™ catheter (Boston Scientific, MA, USA). (C) Re-entry into the true lumen was then achieved with Stingray™ wire and balloon (Boston Scientific). (D) Confirmation of true lumen re-entry was performed via IVUS imaging vessel. The red arrows indicate areas where IVUS imaging has been performed in the vessel and correlate to figures (i–iv). (i–iii) Clear demarcation of true lumen (green asterix) and false lumen (red asterix) are illustrated. (iv) True lumen (green asterix) is identified with no evidence of false lumen in distal vessel. This method allows identification of successful chronic total occlusion crossing into true lumen distally, without the use of antegrade contrast injection. IVUS: Intravascular ultrasound.
The traditional use of IVUS in relation to approximation of vessel size and stent optimization can also be translated into CTO work. Once the occlusion has been opened the estimation of vessel size may be difficult by quantitative angiography, due to dissection or the presence of subintimal hematoma [48], at which point IVUS can be deployed [49] to assess the vessel size. Stent optimization in CTO has been facilitated by IVUS; however, in the AVIO trial there was no statistical difference between stent optimization by IVUS or by angiography [38].
Forward-looking IVUS
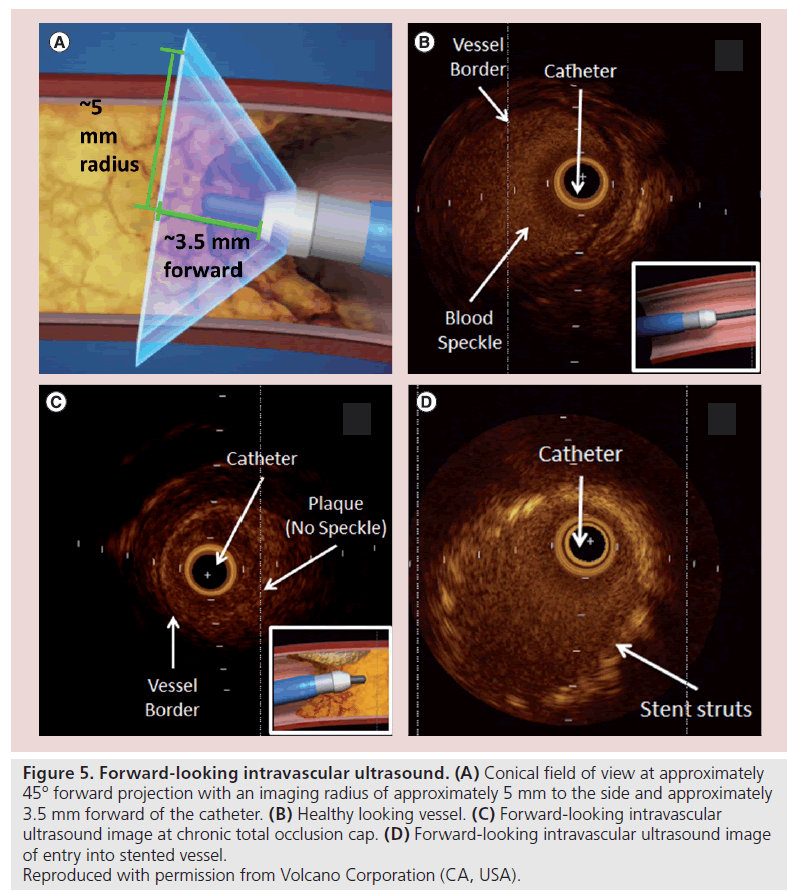
Classically, with CTO PCI, the ultimate procedural goal is to remain within the true lumen throughout the length of the occlusion. Previous IVUS studies have shown that subintimal guidewire passage may be associated with reduced procedural success rates [50,51]. Therefore the ability to precisely steer a wire to remain within the true lumen throughout the length of the occluded segment is the basis of FL-IVUS. This uses an alternative projection of the ultrasound image that is obtained by antegrade (FL) rather than axial reflection of the ultrasound signal. This enables the area of vessel distal to the catheter tip to be visualized. This offers the potential advantage of imaging the proximal cap in cases of plaque cap ambiguity and can facilitate ultrasound-guided wire progression such that the wire remains within the true vessel lumen at all times. Theoretically, the intraluminal passage of the wire reduces the risk of perforation and can be used to facilitate true lumen re-entry. FL-IVUS systems can also be combined with ablation or other strategies for altering vascular tissues (Figure 5) [52].
Figure 5: Forward-looking intravascular ultrasound. (A) Conical field of view at approximately 45° forward projection with an imaging radius of approximately 5 mm to the side and approximately 3.5 mm forward of the catheter. (B) Healthy looking vessel. (C) Forward-looking intravascular ultrasound image at chronic total occlusion cap. (D) Forward-looking intravascular ultrasound image of entry into stented vessel.
▪ Optical coherence tomography
Coronary optical coherence tomography (OCT) is a method of high-definition intravascular imaging that uses light instead of ultrasound [53]. The axial resolution ranges from 5 to 10 μm compared with 100 to 150 μm for IVUS [54]. This provides excellent luminal definition but is offset by limited vessel wall penetration compared with IVUS. There is also a need to completely remove blood from the imaging area by either contrast or saline [55]. Therefore, periprocedural use of OCT is largely confined to the optimization of stent placement and to stent apposition and postprocedural follow-up. OCT is not a useful modality prior to stent deployment because of the need to clear the lumen of blood. This is a particular disadvantage during antegrade dissection and re-entry where prior to lesion crossing and stent deployment antegrade dye injection should be avoided to prevent hydraulic dissection of the subintimal space. This is also coupled with the fact that there are no current clinical trials confirming the utility of OCT in CTO PCI.
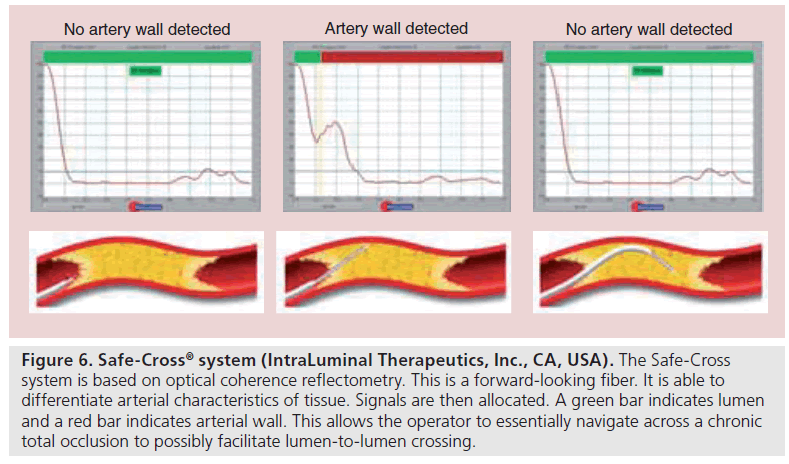
In relation to CTO PCI, once again there have been experimental uses of FL-OCT catheters in ex vivo benchtop work, but this has not translated into a clinically useful tool [56]. There has also been advancement of the use of optical coherence reflectometry in CTOs. This is a FL fiber optic guidance system to help navigate through CTOs. It is able to differentiate the tissue characteristics of arterial tissue including the calcified or noncalcified plaque and atherosclerotic lesions from arterial wall [57]. The signals are then interpreted on a monitor in real time in which a green bar indicates lumen and a red bar indicates arterial wall. This serves as the platform from which the Safe-Steer™ total occlusion (IntraLuminal Therapeutics, Inc., CA, USA) and the Safe-Cross® device (Intra- Luminal Therapeutics, Inc.) work [58]. The Safe-Cross device incorporates radiofrequency ablation technology to the wire tip, in order to penetrate through the CTO (Figure 6). The Safe- Cross system has been shown to be a successful alternative to conventional CTO PCI in cases that have been unsuccessful [59,60]. Despite this system having been available for some time, its potential use still remains controversial.
Figure 6: Safe-Cross® system (IntraLuminal Therapeutics, Inc., CA, USA). The Safe-Cross system is based on optical coherence reflectometry. This is a forward-looking fiber. It is able to differentiate arterial characteristics of tissue. Signals are then allocated. A green bar indicates lumen and a red bar indicates arterial wall. This allows the operator to essentially navigate across a chronic total occlusion to possibly facilitate lumen-to-lumen crossing.
▪ CTO follow-up
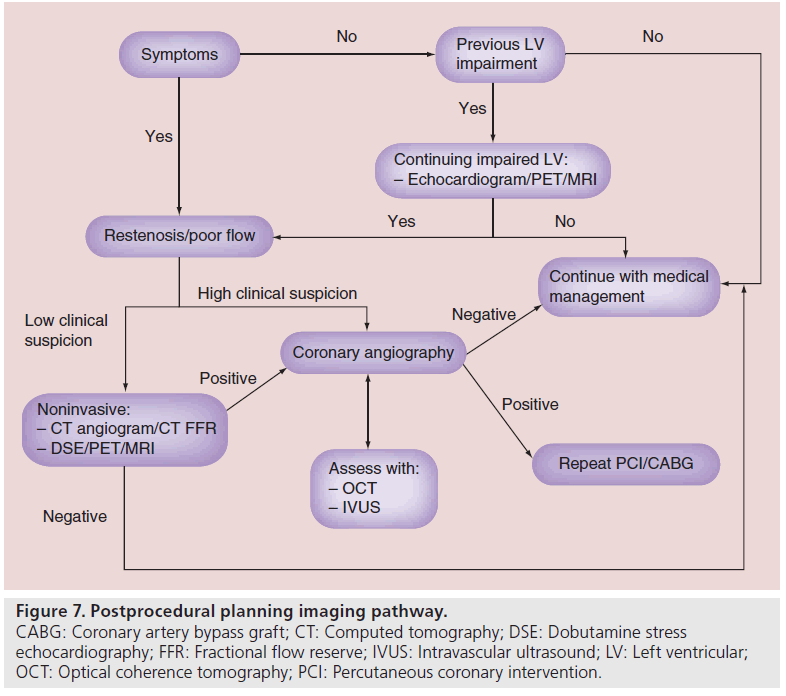
One of the most important aspects of CTO PCI is that procedural success correlates with clinical improvement. Follow-up should be focused on either improvement of symptoms or LVF. As is shown in Figure 7, if patients have no discernible change in symptoms or previously were shown to have impaired LVF, then adjunctive imaging is required post CTO PCI. Restenosis, poor flow or even stent thrombosis are issues that should be diagnosed on follow-up. If flow has been restored to the distal vessel bed, then symptoms should be improved and LVF should improve [61]. There are many different modalities available including DSE, PET and MRI; the availability of which is dependent on each center. As mentioned previously, these modalities have their advantages and disadvantages; however, being able to pair the same test preprocedurally with follow-up would provide the best objective evidence.
Figure 7: Postprocedural planning imaging pathway. CABG: Coronary artery bypass graft; CT: Computed tomography; DSE: Dobutamine stress echocardiography; FFR: Fractional flow reserve; IVUS: Intravascular ultrasound; LV: Left ventricular; OCT: Optical coherence tomography; PCI: Percutaneous coronary intervention.
▪ Minimally invasive
Patients with continuing symptoms with low clinical suspicion of restenosis or poor flow should undergo minimally invasive evaluation. This includes the classic modalities including extra slice spin tagging/DSE/PET/MRI, which can be sufficient in demonstrating coronary ischemia or continuing impaired LVF. If these are positive then progress onto invasive coronary angiography is warranted (Figure 7).
▪ High-definition CT
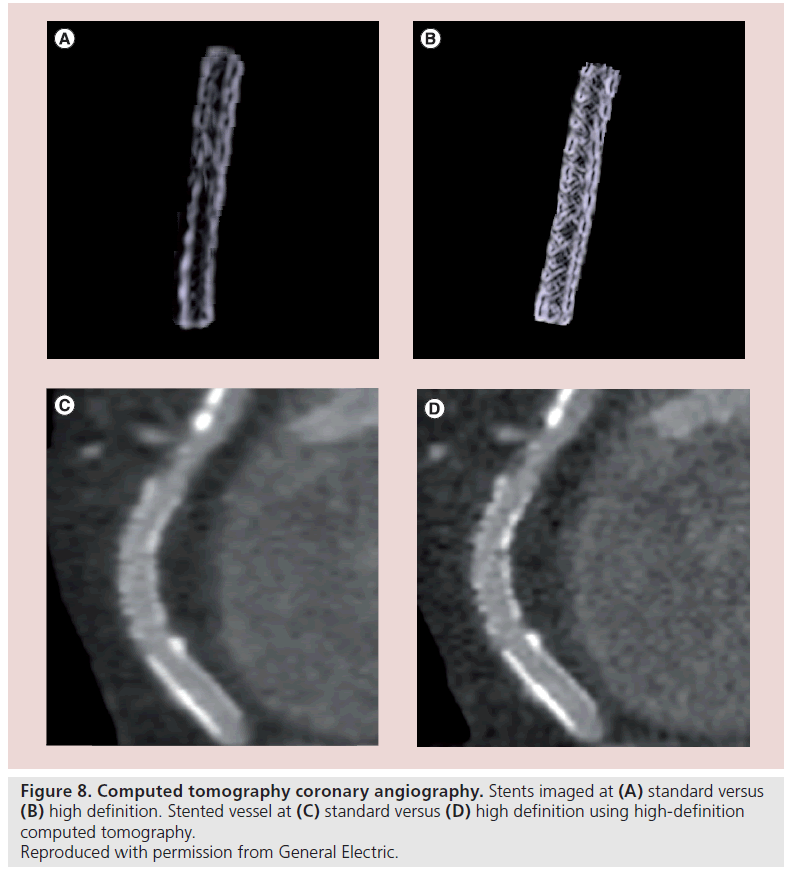
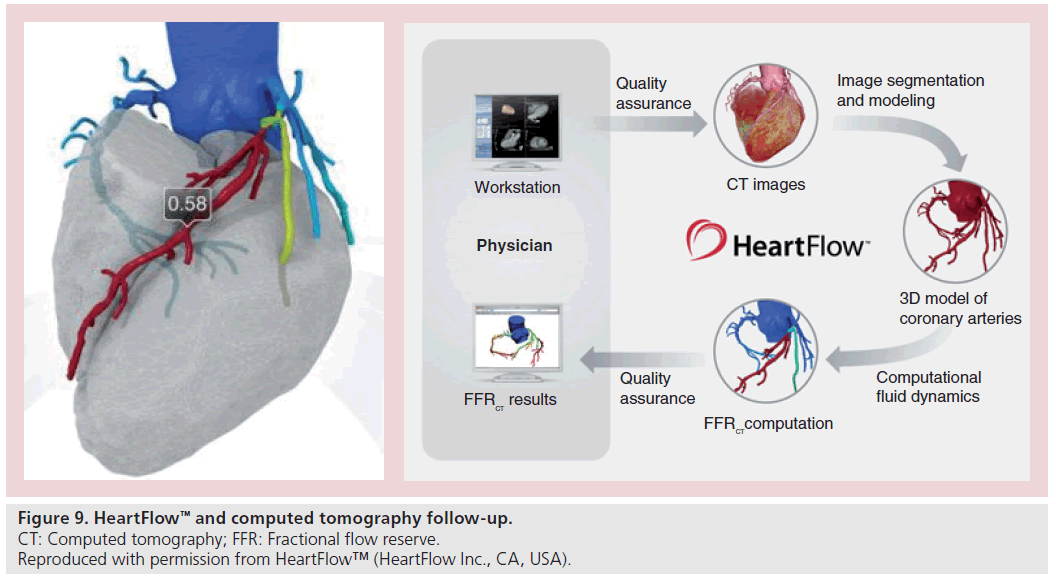
CTCA is an imaging modality that can be used in both the pre- and post-procedural stages of CTO PCI. High-definition and dual-energy CT has now negated the blooming artefact often associated with stent imaging. Increased spatial resolution and use of dual-energy scanners now allows for stent struts to be clearly visualized, as well as imaging tissue within the stented segment itself (Figure 8). If restenosis is being considered in patients who remain symptomatic at follow-up, then CTCA can offer a useful adjunct imaging modality rather than proceeding directly to repeat coronary angiography. Further advances may now allow the integration of physiological data such as fractional flow reserve into the anatomical data set (Figure 9) [62].
Figure 9: HeartFlow™ and computed tomography follow-up.
▪ Invasive
If there is a high clinical suspicion of restenosis or objective evidence of left ventricular ischemia then progression onto invasive coronary angiography is warranted. A simple angiogram can identify restenosis, poor distal bed flow or even stent thrombosis. The determination of significant restenosis may require adjunctive assessment with the use of possible fractional flow reserve wires, IVUS or OCT. The use of IVUS and OCT can help to diagnose the root of the problem and facilitate stent deployment. Once again, these are also dependant on availability.
▪ IVUS follow-up
IVUS has been used very successfully to help diagnose the cause of in-stent restenosis [63]. Considering some CTO PCI involves stenting in the subintimal space, appropriate stent sizing is sometimes difficult. It is well known that longer total stent length, smaller reference lumen diameter, smaller final minimal lumen diameter by angiography and smaller stent lumen cross-sectional area by IVUS are all predictors of in-stent restenosis [63]. Thus, IVUS can be a useful adjunctive modality to assist in identifying causes of stenosis and helping to ameliorate them.
▪ OCT follow-up
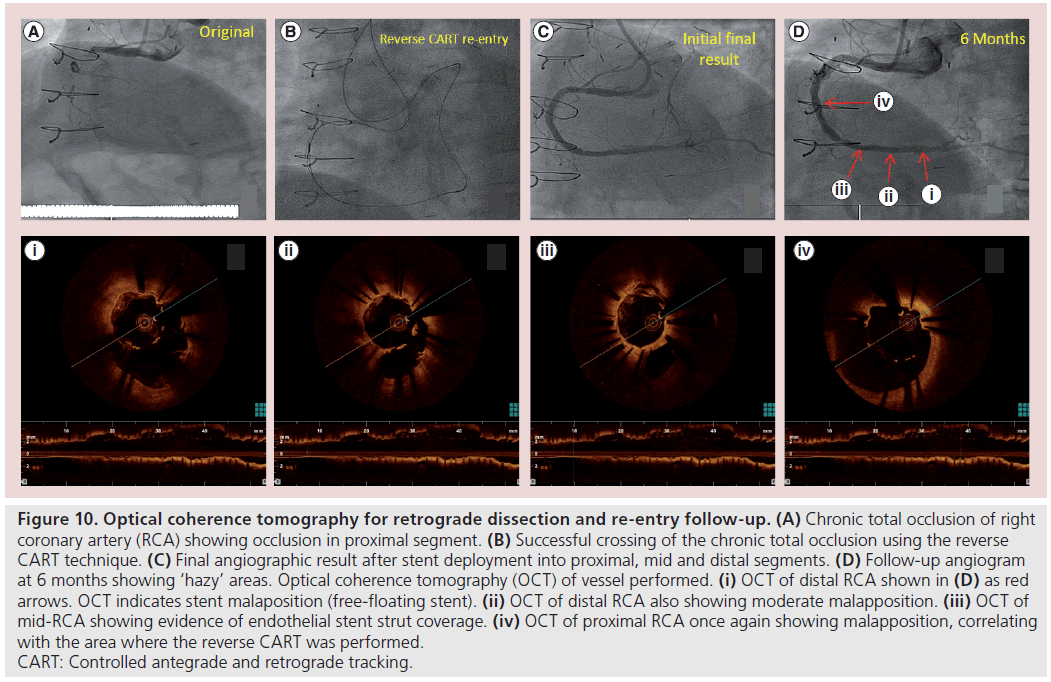
At follow-up in patients undergoing further angiography for in-stent restenosis concerns, or because of other ongoing symptoms, OCT can be extremely useful in determining the vessel healing response to stent implantation (Figure 10) [64]. It remains to be seen how the increasing use of the subintimal space with both retrograde and antegrade dissection re-entry techniques will impact on long-term CTO outcomes. However, it is likely that at postprocedural angiographic follow-up OCT will become the dominant intravascular imaging modality compared with IVUS. Certainly OCT is the optimal tool for accurately assessing the endoluminal stent restenosis although it may not have sufficient penetration to accurately assess vessel size in heavily diseased or calcificied vessels, which is very often the case with CTOs. However,the micron scale assessment of stent strut coverage and endothelial/neointimal healing is clearly in the range of OCT resolution and cannot be carried out comparably with IVUS (Figure 10) [65].
Figure 10: Optical coherence tomography for retrograde dissection and re-entry follow-up. (A) Chronic total occlusion of right coronary artery (RCA) showing occlusion in proximal segment. (B) Successful crossing of the chronic total occlusion using the reverse CART technique. (C) Final angiographic result after stent deployment into proximal, mid and distal segments. (D) Follow-up angiogram at 6 months showing ‘hazy’ areas. Optical coherence tomography (OCT) of vessel performed. (i) OCT of distal RCA shown in (D) as red arrows. OCT indicates stent malaposition (free-floating stent). (ii) OCT of distal RCA also showing moderate malapposition. (iii) OCT of mid-RCA showing evidence of endothelial stent strut coverage. (iv) OCT of proximal RCA once again showing malapposition, correlating with the area where the reverse CART was performed. CART: Controlled antegrade and retrograde tracking.
Future developments
Currently there are many adjunctive imaging modalities available to CTO operators. Some of them have specialized roles and are usually reserved for those with more experience. Nevertheless, in the future, adjunctive imaging may be available to even the most novice CTO operator in order to improve success. There are continuing developments in OCT image processing that can help render real-time 3D images at the time of angiography [66]. The calcium blooming artefact seen in CTCA can obscure the lumen and vessel detail. Latest-generation scanners, however, can overcome these limitations with use of prospective gating and new detector technology. Image fusion techniques are now possible, which allow coregistration and fusion of CTCA with coronary angiography. Along with the use of latest-generation CT scanners, which can reduce the radiation dose [67], CTCA can be a useful preprocedural tool in selected cases. Coregistration of both the angiogram and the CTCA in the catheter laboratory at the time of the procedure is currently possible but definitely not widely available. In addition, developments of rapid imaging and catheter devices visible under MRI have made real-time MRI interventions possible [68,69]. The development of MRIvisible procedural catheters, wires and sheaths have allowed the successful recanalization of CTO in a carotid artery swine model [70]. This has yet to be translated to human trials as not only can catheter materials become displaced by the magnetic fields, but the conductive materials can heat from microwave exposure during MRI [71]. However, there have been further advancements with modified catheters that can serve as MRI antennae contributing to imaging quality and yet are heat resistant [72]. Along with the development of combined x-ray and MRI imaging laboratories, this may improve the success rate of this contemporary method of CTO PCI in the future [73].
Conclusion
There are numerous different imaging modalities available to the CTO operator. There is not a universal imaging modality that does everything. Some help with patient selection and others with preventing complications or lowering procedural times. In addition, not all are available to every operator, suitable for all patients or are used in mainstream clinical work. This means that case selection is vital when searching for a specific type of imaging modality to help with CTO PCI. Particular attention needs to be taken to the type of information required, and the advantages and disadvantages associated with each type of imaging. Further development in this area will be a further step towards optimizing success rates for CTO PCI.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪▪ Coronary angiography remains the central imaging modality for chronic total occlusion (CTO) percutaneous coronary intervention (PCI).
▪▪ Adjunctive imaging has a role at all stages of CTO PCI.
▪▪ Confirmation of ischemia prior to CTO PCI is helpful.
▪▪ Periprocedural intravascular imaging is usually with intravascular ultrasound.
▪▪ Optical coherence tomography may have a role in postprocedural follow-up.
▪▪ CTO PCI adjunctive imaging utilizes all available modalities.
▪▪ An integrated imaging approach can improve CTO outcomes.
References
- Cohen HA, Williams DO, Holmes DR Jr et al. Impact of age on procedural and 1-yearoutcome in percutaneous transluminal coronary angioplasty: the NHLBI Dynamic Registry. Am. Heart J. 146, 513–519 (2003).
- Anderson HV, Shaw RE, Brindis RG et al. A contemporary overview of percutaneous coronary interventions: the American College of Cardiology national Cardiovascular Data Registry (ACC-NCDR). J. Am. Coll. Cardiol. 39, 1096–1103 (2002).
- Ivanhoe RJ, Weintrub WS, Douglas JS et al. Percutaneous transluminal coronary angioplasty of chronic total occlusions. Primary success, restenosis, and long-term clinical follow-up. Circulation 85(1), 106–115 (1992).
- Newell MC, Schwart RS. Utility of CT coronary angiography for planning CTO intervention. Cardiac Interventions Today, 25–28 March 2009.
- Shah PB. Management of chronic total occlusions: clinic update. Circulation 123, 1780–1784 (2011).
- Joyal D, Afilalo J, Rinfret S. Effectiveness of recanalization of chronic total occlusions: a systematic review and meta-analysis. Am. Heart J. 160, 179–187 (2010).
- Cohen M, Renntrop KP. Limitations of myocardial ischemia by collateral circulation during sudden controlled coronary artery occlusion in human subjects: a prospective study. Circulation 74, 469–476 (1986).
- Rahimtoola SH. Coronary bypass surgery for chronic angina. A perspective. Circulation 65(2), 225–241 (1981).
- Krahwinkel W, Ketteler T, Wolfertz J et al. Detection of myocardial viability using stress echocardiography. Eur. Heart J. 18(Suppl. D), D111–D116 (1997).
- Cwajg JM, Cwajg E, Nagueh SF et al. End-diastolic wall thickness as apredictor of recovery of function in myocardial hibernation: relation to rest-redistribution Tl-201 tomography and dobutamine stress echocardiography. J. Am. Coll. Cardiol. 35, 1152–1161 (2000).
- Cigarroa CG, deFilippi CR, Brickner E et al. Dobutamine stress echocardiography identifies hibernating myocardium and predicts recovery of left ventricular function after coronary revascularization. Circulation 88, 430–436 (1993).
- Shimoni S, Frangogiannis NG, Aggeli CJ et al. Identification of hibernating myocardium with quantitative intravenous myocardial contrast echocardiography: comparison with dobutamine echocardiography and thallium-201 scintigraphy. Circulation 107, 538–544 (2003).
- Maes AF, Borgers M, Flameng W et al. Assessment of myocardial viability in chronic coronary artery disease using technetium-99m sestamibi SPECT: correlation with histologic and positron emission tomographic studies and functional follow-up. J. Am. Coll. Cardiol. 29(1), 62–68 (1997).
- Sansoy V, Glover DK, Watson DD et al. Comparison of thallium-201 resting redistribution with technetium-99m-sestamibi uptake and functional response to dobutamine for assessment of myocardial viability. Circulation 92, 994–1004 (1995).
- Tillisch J, Brunken R, Marshall R et al. Reversibility of cardiac wall motion abnormalities predicted by positron tomography. N. Engl. J. Med. 314, 884–888 (1986).
- Srinivasan G, Kitsiou AN, Bacharach SL et al. [18F]fluorodeoxyglucose single photon emission computed tomography: can it replace PET and thallium SPECT for the assessment of myocardial viability? Circulation 97, 843–850 (1998).
- Attili A, Schuster A, Nagel E, Reiber JH, Geest RJ. Quantification in cardiac MRI: advances in image acquisition and processing. Int. J. Card. Imaging 26(1), 27–40 (2010).
- Genker R, Schwitter J, Fleck E, Nagel E. Review how to perform myocardial perfusion with cardiovascular magnetic resonance. J. Cardiovasc. Magn. Res. 9(3), 539–547(2007).
- Pennell DJ, Underwood SR, Manzara CC et al. Magnetic resonance imaging duringdobutamine stress. Am. J. Card. 70, 34–40 (1992).
- West A, Kramer CM. Cardiovascular magnetic resonance imaging of myocardial infarction, viability and cardiomyopathies. Curr. Probl. Cardiol. 35(4), 176–220 (2010).
- Camici P, Prasad SK, Rimoldi OE. stunning hibernation, and assessment of myocardial viability. Circulation 117, 103–114 (2008).
- Saraste A, Nekolla S, Schwaiger M et al. Contrast-enhanced magnetic resonance imaging in the assessment of myocardial infarction and viability. J. Nucl. Card. 15, 105–117 (2008).
- Baks T, van Geuns R, Duncker DJ et al. Prediction of left ventricular function after drug-eluting stent implantation for chronic total occlusions. J. Am. Coll. Cardiol. 47, 721–725 (2006).
- Cheng A. Selvanayagam JB, Jerosch-Herold et al. Percutaneous treatment of chronictotal coronary occlusions improves regional hyperemic myocardial blood flow and contractility: insights from quantitative cardiovascular magnetic resonance imaging.Am. Coll. Cardiol. Interv. 1, 44–53 (2008).
- Brilakis ES, Grantham AJ, Rinfret S et al.percutaneous treatment algorithm for crossing coronary chronic total occlusions.Am. Coll. Cardiol. 5(4), 367–379 (2012).
- Klass O, Walker M, Seibach A et al. Prospectively gated axial CT coronary angiography: comparison of image quality and effective radiation dose between 64- and 256-slice CT. Eur. Radiol. 20(5), 1124–1131 (2010).
- Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J. Am. Coll. Cardiol. 46(3), 552–557 (2005).
- Leber AW, Becker A, Knez A et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J. Am. Coll. Cardiol. 47(3), 672–677 (2006).
- Agatston AS, Janowitz WR, Hildner FJ et al. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Card. 15(4), 827–832 (1990).
- Soon KH, Cox N, Wong A et al. CT coronary angiography predicts the outcome of percutaneous coronary intervention of chronic total occlusion. J. Interv. Cardiol. 20, 359–366 (2007).
- Schuhbaeck A, Lehnhardt S, Pflederer T et al.CT-based score to predict the success of interventional revascularization of chronic total coronary occlusions. J. Am. Coll.Cardiol. 59(Suppl. 13), E1212 (2012).
- Mollet NR, Hoye A, Lemos PA et al. Value of pre-procedure multislice computed tomographic coronary angiography to predict the outcome of percutaneous recanalization of chronic total occlusions. Am. J. Cardiol. 95, 240–243 (2005).
- Yokoyama N, Yamamoto Y, Suzuki S et al. Impact of 16-slice computed tomography in percutaneous intervention of chronic total occlusions. Catheter. Cardiovasc. Interven.68(1), 1–7 (2006).
- Scheffel H, Alkadhi H, Plass A et al. Accuracy of dual-source CT coronary angiography; first experience in a high pre-test probability population without heart rate control. Eur. Radiol. 16, 2739–2747 (2006).
- McConnell M, Ganz P, Selwyn AP, Li W, Edelman RR, Manning WJ. Identification of anomalous coronary arteries and their anatomic course by magnetic resonance coronary angiography. Circulation 92, 3158–3162 (1995).
- Stuber M, Botna RM, Danias PG et al. Double oblique free-breathing high-resolution 3D MRA. J. Am. Coll. Cardiol. 34, 524–531 (1999).
- Yong Kim W, Danias PG, Stuber M et al. Coronary magnetic resonance angiography for the detection of coronary stenoses. Engl. J. Med. 345, 1863–1869 (2001).
- Chieffo A, Latib A, Caussin C et al.prospective randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am. Heart J. 165, 65–72 (2013).
- Suzuki T, Hiroaki H, Koichi Y et al. Time-dependent morphological characteristics in angiographic chronic total occlusions. Am.Cardiol. 88, 167–169 (2001).
- Nissen S, Yock P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation 103, 604–616 (2001).
- Stone GW, Colombo A, Teirstein PS et al. Percutaneous recanalization of chronically occluded coronary: procedural techniques, device, and results. Catheter. Cardiovasc.Interv. 66, 217–236 (2005).
- Matsubara T, Murata A, Kanyama H, Ogino IVUS-guided wiring technique: promising approach for the chronic total occlusion.Catheter. Cardiovasc. Interv. 61, 381–386(2004).
- Ito S, Suzuki T, Ito T et al. Novel technique using intravascular guided guidewire cross in coronary intervention. Circulation 68, 1088–1092 (2004).
- Tsuchikane E, Katoh O, Kimura M, Nasu K, Kinoshita Y, Suzuki T. The first clinical experience with a novel catheter for collateral channel tracking in retrograde approach. JACC Cardiovasc. Interv. 3, 165–171 (2010).
- Rathore S, Katoh O, Tuschikane E, Oida A, Suzuki T, Takase SA. Novel modification of the retrograde approach for the recanalization of chronic total occlusion of the coronary arteries intravascular ultrasound-guided reverse controlled antegrade and retrograde tracking. JACC Cardiovasc. Interv. 3, 155–164 (2010).
- Kimura M, Asakura Y. IVUS-guided recanalization of CTO. In: Chronic Total Occlusions: a Guide to Recanalization.Waksman R, Saito S (Eds). John Wiley & Sons Publishers, Oxford, UK, 67–78 (2013).
- Whitlow PL, Burke MN, Lombardi WL et al. Use of a novel crossing re-entry system in coronary chronic total occlusions that have failed standard crossing techniques: results of the FAST-CTOs (facilitated antegrade steering technique in chronic total occlusions) trial. JACC Cardiovasc. Interv. 5(4), 393–401 (2012).
- Fujii K, Ochiai M, Mintz GS et al. Procedural implications of intravascular ultrasound morphologic features of chronic total occlusions. Am. J. Cardiol. 97, 1455–1462 (2006).
- Mohander M, Guarinos J, Sans J, Bardaji A. Intravascular ultrasound in percutaneous coronary intervention for chronic total occlusion. Iranian Cardiovasc. Res. J. 4(3), 101–106 (2010).
- Kimura BJ, Tsimikas S, Bhargava V, DeMaria AN, Penny WF. Subintimal wire position during angioplasty of a chronic total occlusion: detection and subsequent procedural guidance by intravascular ultrasound. Cathet. Cardiovasc. Diagn. 35, 262–265 (1995).
- Gatzoulis L, Watson RJ, Jordan LB et al. Three-dimensional forward-viewing intravascular ultrasound imaging of human arteries in vitro. Ultrasound Med. Biol. 27, 969–982 (2001).
- Roger J. Forward-looking IVUS in chronic total occlusions. Cardiac Interventions Today,21 June–24 July 2009.
- Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI. Intracoronary optical coherence tomography: a comprehensive review. J. Am. Coll. Cardiol. 2(11), 1035–1046 (2009).
- Brezinski ME, Tearney GJ, Weissman NJ et al. Assessing atherosclerotic plaque morphology: comparison of optical coherence tomography and high frequency intravascular ultrasound. Heart 77, 397–403 (1997).
- Patel NA, Stamper DL, Brezinski ME. Review of the ability of optical coherence tomography to characterize plaque, including a comparison with intravascular ultrasound. Cardiovasc. Interv. Radiol. 28(1), 1–9 (2005).
- Munce NR, Yang VXD, Standish BA et al. Ex vivo imaging of chronic total occlusionsusing forward-looking optical coherence tomography. Laser Surg. Med. 39(1), 28–35 (2007).
- Yamashita T, Kasaoka S, Son R et al. Optical coherence reflectometry: a new technique to guide invasive procedures. Catheter.Cardiovasc. Interv. 54, 257–263 (2001).
- Quash HR, Heuser RR. Optical coherence reflectometry in coronary CTOs. Cardiac Interventions Today, 51–53, December 2007.
- Werner G, Fritzenwanger M, Prochnau D et al. Improvement of the primary success rateof recanalization of chronic total coronary occlusions with the Safe-Cross system after failed conventional wire attempts. Clin. Res. Cardiol. 96(7), 489–496 (2007).
- Baim DS, Braden G, Heuser R et al. Utility of the Safe-Cross-guided radiofrequency total occlusion crossing system in chronic coronary total occlusions. Am. J. Cardiol. 94, 853–858 (2004).
- Paul GA, Connelly K, Zia M et al. Impact of percutaneous coronary intervention of chronic total occlusion on left ventricular function using cardiac magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 13(1), 1532–1535 (2011).
- Min JK, Leipsic J, Pencina MJ et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 3(12), 1237–1245 (2012).
- Kasaoka S, Tobis JM, Akiyama T et al. Angiographic and intravascular ultrasound predictors of in-stent restenosis. J. Am. Coll. Cardiol. 32(6), 1630–1635 (1998).
- Tahara S, Chamié D, Baibars M, Alraies C, Costa M. Optical coherence tomography endpoints in stent clinical investigations: strut coverage. Int. J. Cardiovasc. Imaging 27(2), 271–287 (2011).
- Matsumoto D, Shite J, Shinke T et al. Neointimal coverage of sirolimus-eluting stents at 6-month follow-up: evaluated by optical coherence tomography. Eur. Heart J. 28(8), 961–967 (2006).
- Prati F, Regar E, Mintz GS et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur. Heart J. 31 (4), 401–415 (2010).
- Hatem A, Stolzmann P, Desbiolles L et al. Low-dose, 128-slice, dual-source CT coronary angiography: accuracy and radiation dose of the high-pitch and the step-and-shoot mode. Heart 96(12), 933–938 (2010).
- Yang X, Atalar E. Intravascular MR-monitored balloon angioplasty: an in vivo feasibility study. J. Vasc. Interv. Radiol. 9, 953–959 (1998).
- Razavi R, Hill DLG, Keevil SF et al. Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. Lancet 362, 1877–1882 (2003).
- Raval A, Karmarkar PV, Guttman MA et al. Real-time magnetic resonance imaging – guided endovascular recanalization of chronic total arterial occlusion in a swine model. Circulation 113, 1101–1107 (2006).
- Nitz WR, Oppelt A, Renz W et al. On the heating of linear conductive structures such as guide wires and catheters in interventional MRI. J. Magn. Reson. Imaging 13, 105–113 (2001).
- Susil R, Yeung CJ, Atalar E. Intravascular extended sensitivity (IVES) MRI antennas. Magn. Reson. Med. 50, 383–390 (2003).
- Dick A, Raman VK, Raval AN et al. invasive human magnetic resonance imaging: feasibility during revascularisation in a combined XMR suite. Catheter. Cardiovasc. Interven. 64(3), 265–274 (2005).