Editorial - International Journal of Clinical Rheumatology (2023) Volume 18, Issue 12
The impact of biological therapy on bone in rheumatoid arthritis after three years: data from the Moroccan RBSMR registry
Laila Taoubane1*,Abdellah El Maghraoui2,Abderrahim majjad1,Najlae El Ouardi1,Hamza taoufik1,Ihsan Hmamouchi3,Radouane Abouqal3,Rachid Bahiri4,Fadoua Allali5,Imane El Bouchti6,Imad Ghozlani7,Hasna Hassikou8,Taoufiq Harzy9,Linda Ichchou10,Oufae Mkinsi11,Radouane Niamane12,Ahmed bezza1
1Department of Rheumatology, Military Hospital Mohammed V, Ibn Sina University Hospital, Rabat, Morocco
2Private Medical Office, Rabat, Morocco
3Laboratory of Biostatistical, Clinical and Epidemiological Research, Faculty of Medicine and Pharmacy, Mohammed V University, Rabat, Morocco
4Department of Rheumatology A, El Ayachi Hospital, Ibn Sina University Hospital, Salé,Morocco
5Department of Rheumatology B, El Ayachi Hospital, Ibn Sina University Hospital, Salé, Morocco
6Department of Rheumatology, Arrazi University Hospital, Marrakech, Morocco
7Department of Rheumatology, University Hospital of Agadir,Agadir, Morocco
8Department of Rheumatology, Military Hospital Moulay Ismail, Hassan II University Hospital, Meknès, Morocco
9Department of Rheumatology, Hassan II University Hospital,Fes, Morocco
10Department of Rheumatology, Mohammed VI University Hospital, Oujda, Morocco
11Department of Rheumatology, Ibn Rochd University Hospital, Casablanca, Morocco
12Department of Rheumatology, Military Hospital Avicenne, Mohammed VI University Hospital, Marrakech, Morocco
- *Corresponding Author:
- Laila Taoubane
Department of Rheumatology, Military Hospital Mohammed V, Ibn Sina University Hospital, Rabat, Morocco
E-mail: drtaoubanelaila@gmail.com, a.elmaghraoui@ um5r.ac.ma
Received: 03-Dec-2023, Manuscript No. fmijcr-23-121930; Editor assigned: 06- Dec-2023, Pre QC No. fmijcr-23-121930; Reviewed: 20-Dec-2023, QC No. fmijcr-23-121930; Revised: 23-Dec-2023, Manuscript No. fmijcr-23-121930 (R); Published: 30-Dec-2023, DOI: 10.37532/1758- 4272.2023.18(12).370-377
Abstract
Rheumatoid Arthritis (RA) is the most prevalent chronic inflammatory rheumatic disease, characterized by functional impairment, which leads to sedentary lifestyle and chronic inflammation. These factors are intertwined with corticosteroid use, which can induce osteoporosis, a significant extra-articular manifestation. Studies have shown controversial results regarding the impact of biologic therapies (bDMARDs) on bone protection, whether through direct or indirect effects on cytokines, ultimately leading to inflammation attenuation and improved quality of life. Consequently, a reduction in degradation factors has been described in groups receiving both bDMARDs and anti-osteoporotic treatments.
Objective: Our primary objective is to determine the prevalence of osteoporosis in RA patients from the Moroccan biologic therapy registry at baseline and after 36 months of biologic therapy in the general population. We also aim to explore various factors associated with osteoporosis based on the type of biologic therapy, including rituximab, anti-TNF, or tocilizumab.
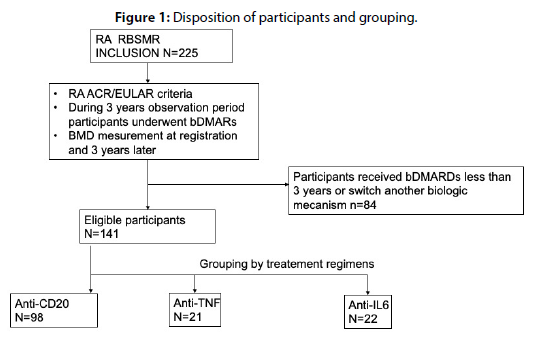
Patients and methods: This is a prospective analytical multicenter study based on data from the Moroccan biologic therapy registry, including 225 patients with RA who meet ACR-EULAR criteria. Osteoporosis status was assessed at baseline (M0) and after 36 months of biologic therapy (M36).
Results: A total of 141 RA patients completed 36 months of treatment with the same biologic medication and were divided into three groups: Group I (n= 98, anti-CD 22), Group II (n= 21, anti-TNFi including infliximab, etanercept, adalimumab, and golimumab), and Group III (n=22, tocilizumab). 15.7% of patients received anti-osteoporotic treatment (AOT) with bisphosphonates, with no significant differences between the three groups (p=0.94). At baseline, 23.6% of patients had osteoporosis, with rates of 25.8%, 19%, and 18.2% in Groups I, II, and III, respectively (p=0.652). Four fracture cases were recorded. FRAX fragility factors were comparable at baseline. Corticosteroid use was observed in 74.7% of patients, with no differences between the groups (p=0.5). Disease Activity Score 28 based on C-reactive protein (DAS28CRP) was higher at M0 compared to M36 (3.40 +/- 2.05) vs. (2.35 +/- 1.47); (p<0.001). Comparing osteoporosis rates between baseline (24.1%) and 3 years (5.7%), a statistically significant decrease in osteoporosis was observed (p<0.001), more pronounced in the AOT group. This decrease was noted within the groups, although the difference was significant only in the rituximab Group I (p<0.001). Multivariate analysis, after adjusting for confounding factors, revealed that osteoporosis was only associated with disease duration (OR=1.002; CI [1.001-1.003]; P<0.004).
Conclusion: Long-term biologic therapy has a beneficial effect on rheumatoid arthritis and, consequently, on bone health. This effect is more pronounced in the group receiving antiosteoporotic treatment. Patients on rituximab showed a significant reduction in osteoporosis.
Introduction
Rheumatoid arthritis (RA) is the most common among chronic inflammatory rheumatic diseases. It is a systemic autoimmune disease characterized by functional impairment, a chronic inflammatory state, and corticosteroid therapy. The combination of these factors can lead to bone loss, making osteoporosis screening essential [1][2]. Osteoporosis is the most frequent among the extra-articular manifestations of RA. Bone loss ranges from 5.5 to 10% in patients with active disease at 2 years [3][4], with fracture being the major complication. VF are detected in 36% of RA patients. Studies have shown controversial results regarding the impact of biological therapies (bDMARDs) on bone protection [5]. Biologic therapy acts directly or indirectly on cytokines and halts the inflammatory cascade, resulting in the attenuation of clinical symptoms, improved quality of life, and slowed joint damage. Some studies have reported improvements in the formation/ resorption marker ratio, suggesting a reduction in bone loss in these patients [6]. Furthermore, this reduction in degradation factors was observed in groups receiving bDMARDs in addition to anti-osteoporotic treatments, preserving bone density [7][8]. Our main objective is to determine the prevalence of osteoporosis in RA patients from the Moroccan biologic therapy registry at baseline and after 36 months of biologic therapy in the general population, stratified by type (rituximab, anti-TNF, or tocilizumab), and to investigate various associated factors.
Materials and Methods
Data source
The data for this study were sourced from the Registry of Biologic Therapies in Rheumatic Diseases of the Moroccan Society of Rheumatology (RBSMR). The RBSMR is a historical, prospective, multicenter registry comprising ten rheumatology services in medicaluniversity centers across Morocco. It enrolled patients aged 18 years and above with rheumatoid arthritis (RA) or spondylarthrosis (SpA) who were receiving biologic therapy (both initiation and ongoing treatment). The inclusion period in the registry was from May 2017 to January 2019, with a follow-up duration of 3 years. The registry enrolled 441 patients, of which 419 were eligible (225 with RA and 194 with SpA). The primary objective of the RBSMR registry was to assess the tolerance of patients with RA or SpA receiving biologic therapy in rheumatology. The Secondary objectives included evaluating the effectiveness of biologic therapies in rheumatology, assessing their impact on patients' quality of life, and monitoring and evaluating real-life patient management practices (including monitoring and pretherapeutic assessments) [9][10].
The study received ethical approval from the Ethics Committee of the Faculty of Medicine at Mohammed V University in Rabat, as well as from the National Committee for Personal Data Protection. All patients included in the study provided their prior written consent.
Study design and population
We conducted a multicenter analytical study using the RBSMR database, which included 225 RA patients meeting the 2010 ACR-EULAR criteria. Bone status (osteoporosis) was assessed at baseline (M0) and after 36 months of biologic treatment (M36). Bone mineral density was measured by dual-energy X-ray absorptiometry of the femoral neck and lumbar spine (L1-4), using different equipment at each center. The physician performing the examination indicated yes if the diagnosis of osteoporosis was accepted, and no if not. The diagnosis of osteoporosis was defined according to the World Health Organization (WHO) criteria from 1994, with a T-score of <-2.5. The objectives of this study were to describe and analyze our patient population at baseline and after 3 years, compare the prevalence of osteoporosis after 36 months of biologic therapy, identify associated factors, investigate the bone impact of each biologic therapy, and finally, assess the effect of combination with anti-osteoporotic treatment and compare our results to previous published studies
Statistical analysis
The statistical analysis was based on the RBSMR registry database, using jamovi software, version 2.3.21. Data were presented as mean and standard deviation or median and quartile for continuous variables, and as numbers and percentages for categorical variables. The categorical variables were analyzed using the t-student and Mann- Whitney tests, while chi-squared tests were employed for qualitative variables. Changes in osteoporosis prevalence between groups were assessed using the McNemar test. Correlations were examined using the Spearman test, with p-values < 0.05 considered statistically significant. Factors associated with osteoporosis were identified through logistic regression. Only characteristics frequently reported in the literature and those with a p-value < 0.05 in univariate analysis were included in the multivariate analysis.
Results
A total of 225 patients with RA were included in the RBSMR registry. Among them, 135 (Group I), 37 (Group II), and 53 (Group III) patients received Rituximab, anti-TNF, and tocilizumab, respectively. Considering the type of biologic therapy and the completion of the 36-month follow-up period without switching or discontinuation, which was the main inclusion criterion for our study, 84 patients were excluded, as shown in Table 1. Meanwhile, 39 patients did not receive 3 years of treatment due to either unavailability or insurance issues, 25 discontinued treatment due to side effects, including 6 cases of neoplasia, 9 cases of infections, 6 cases of anaphylactic shock, 2 cases of cholestasis, and 2 cases of cytopenia; 4 patients discontinued treatment due to inefficacy, and 1 patient due to pregnancy with one fatality. The remaining patients were excluded due to switching to a biologic with a different mode of action or other reasons. The demographic and clinical characteristics of patients are presented in Table 2. Eligible patients were grouped into Group I (n=98, anti-CD 22), Group II (n=21, anti-TNF including infliximab, etanercept, adalimumab, and golimumab), and Group III (n=22, tocilizumab). A total of 141 patients were the focus of our study. The mean age of patients was 53 (+/-7.8) years, with 87.1% being female. The disease duration was 15 years, and there were no significant differences in demographic data among the three biologic therapy groups. Clinical and Para-clinical characteristics of rheumatoid arthritis showed no differences between the three groups. Notably, there was a decrease in DAS28 CRP at 3 years compared to baseline (2.35 +/- 1.47 vs. 3.4 +/- 2.05; p<0.001). As for osteoporosis risk factors, they were comparable between the groups; 18.6% of women were postmenopausal (p=0.196); 2.9% had a history of fractures (p=0.08); the majority of patients were on corticosteroid therapy (72.9%). Furthermore, 15.7% of patients received anti-osteoporotic treatment with bisphosphonates, with no significant differences between the three groups. At baseline, 23.6% had osteoporosis, with 25.8%, 19%, and 18.2% in Groups I, II, and III, respectively (p=0.652). The FRAX fracture risk factors were also comparable at baseline. Comparing osteoporosis between baseline (24.1%) and 3 years (5.7%) for all patients (Table 3), a statistically significant decrease in osteoporosis was observed (p<0.001). This decrease was more pronounced in the group receiving anti-osteoporotic treatment (Table 4). In each of the three groups, a decrease in osteoporosis was observed, although the difference was significant only in Group I (rituximab) (p<0.001). In multivariate analysis and after adjusting for confounding factors, osteoporosis was only associated with disease duration (OR=1.002; 95% CI [1.001-1.003]; p<0.004) (Table 5).
Variables |
N=84 |
|---|---|
| bDMARDs Discontinuation (n, %): | 68 (80,9) |
| Insurance issues or indisponibility | 39 |
| Loss of efficacy | 4 |
| Side Effects: | |
| neoplasia | 6 |
| Infection: | |
| Septic arthritis | 1 |
| Sore throat | 2 |
| Abscess | 1 |
| Tuberculosis | 1 |
| Shingles | 1 |
| COVID-19 | 3 |
| Anaphylactic shock | 6 |
| Cholestasis | 2 |
| Neutropenia | 2 |
| Paradoxical Effects | |
| lupus | 1 |
| uveitis | 3 |
| Death | 1 |
| Pregnancy | 1 |
| bDMARDs switch or others n(%) | 10(11,9) |
Table 1: Causes of exclusion
group |
all n=141 | I n=97 | II n=21 | III n=22 | Pf |
|---|---|---|---|---|---|
| age(year) | 53+/-7,8 | 52+/-11,9 | 53,71+/-7,94 | 55,95+/-6,75 | 0,3 |
| female n(%) | 122(87,1%) | 85(87,6) | 18(85,7) | 19(86,4) | 0,965 |
| body weight(kg) | 72,12+/-13,93 | 70,92+/-13,38 | 79,18+/-18,28 | 71,55+/-10,73 | 0,078 |
| body height (cm) | 162,9+/-7,6 | 162,26+/-7,2 | 164,3+/-7,9 | 162,9+/-8,9 | 0,589 |
| BMI(kg/cm2) | 26,93+/-6,2 | 27,11+/-5,42 | 29,37+/-6,3 | 24,14+/-8,8 | 0,039 |
| Factors Associated With RA | |||||
| disease duration (years) | 15 | 14 | 15 | 20 | 0,106 |
| baseline DAS28-CRP | 3,40+/-2,05 | 3,58+/-2,00 | 2,70+/-2,26 | 3,27+/-1,99 | 0,196 |
| 3-year mean DAS28-CRP | 2,35+/-1,47 | 2,48+/-1,60 | 2,18+/-1,34 | 2,07+/-1,01 | 0,471 |
| rheumatoid factors+, (%) | 115(82,1) | 80(82,5) | 17(81) | 18(81,8) | 0,885 |
| ACPA,+(%) | 95(67,9) | 72(74,2) | 10(47,6) | 13(59,1) | 0,078 |
| FRAX risk factors a | |||||
| previous fracture +, n(%) | 4(2,9) | 3(3,1) | 0(0) | 1(4,5) | 0,08 |
| osteoporosis fr , n(%) | 7(5) | 5(5,2) | 0(0) | 2(9,1) | 0,512 |
| menopause | 26(18,6) | 21(21,6) | 1(4,8) | 4(18,2) | 0,196 |
| Glucocorticoid b | |||||
| baseline exposure + ,n(%) | 102(72,9) | 77(79,4) | 15(71,4) | 10(45,5) | 0,5 |
| 3-year exposure,n(%) | 73(53,7) | 66(68) | 9(43) | 6(27) | <0,001 |
| baseline dose (mg/day) | 6,91+/-5,17 | 7,32+/-5,75 | 5,91+/-2,02 | 5,14+/-2,61 | 0,46 |
| cumulative exposurec,n(%) | 54(38) | 42(43) | 8(38) | 4(18) | 0,77 |
| mean dose d (mg/day) | 6,91+/-5,17 | 7,32+/-5,75 | 15(71,4) | 10(45,5) | 0,005 |
| Parent Fractured Hip +, N(%) | |||||
| Baseline osteoporosis e, n(%) | 33(23,6) | 25(25,8) | 4(19) | 4(18,2) | 0,652 |
| current smoking + , n(%) | 3(2,1%) | 2(2,1) | 0(0) | 1(4,5) | 0,586 |
| vit d25(OH) ng/ml | 25,75+/-16,71 | 20,50+/-12,09 | . | 36,25+/-21,50 | 0,129 |
| AOT + ,n(%) | 22(15,7) | 19(19,6) | 1(4,8) | 1(4,5) | 0,94 |
Table 2: demographics and clinical characteristics of patients with RA and Comparison between groups.
Osteoporosis |
All | I(rituximab) | II(anti-TNF) | II(tocilizumab) |
|---|---|---|---|---|
| Baseline n(%) | 34(24,1) | 24(26,1) | 4(20) | 4(19) |
| After 3 years n(%) | 8(5,7) | 1(20) | 0(0) | 0(0) |
| p | <0,001 | <0.001 | 0,180 | 0,180 |
Table 3: comparison of OP between baseline and 3 years later in each treatment group of biology
N (%) |
AOT-115(81,5) | AOT+ 22(15,7) |
|---|---|---|
| Baseline n | 7 | 21 |
| After 3 years n | 6 | 2 |
Table 4: Comparison of OP between patients with RA with AOT and without AOT
| O. R | univariate model 95 % Confidence Interval | p Value | O. R | Multivariate model 95 % Confidence Interval | p Value | |
|---|---|---|---|---|---|---|
| age | 1,07 | 1,03-1,12 | 0,001 | 1,04 | 0,98-1,10 | 0,14 |
| Duration of the disease | 1,002 | 1,001-1,002 | <0,001 | 1,002 | 1,001-1,003 | 0,004 |
| Vit D 25 | 1,07 | 0,97-1,19 | 0,162 | 0,96 | 0,78-1,19 | 0,76 |
Table 5: Factors associated with the presence of osteoporosis at the base line: univariate and multivariate analysis
ACPA (anti-citrullinated protein antibody); AOT(antiosteoporosis therapy); FRAX(fracture risk assessment tool).DAS28-CRP, the disease activity score-28 for rheumatoid arthritis based on protein C reactive rate; RA(rheumatoid arthritis); TNFi , TNF-a (inhibitors). Data are presented as mean ± standard deviation, or median (interquartile range).a defined as in FRAX tool. B dose of glucocorticoid was converted to a prednisolone equivalent dose. C defined as number and proportion (%) of participants who had ever received glucocorticoid therapy during the 3-year observation period. D only for participants receiving glucocorticoid during observation period. E defined as a T-score equal to −2.5 or less at femoral neck. F comparison among group I, II, and III.
Discussion
Our study demonstrates an improvement in osteoporosis in patients with rheumatoid arthritis (RA) treated with biologic therapy. This confirms the findings of previous studies, which have shown that (bDMARDs) prevent bone loss compared to (cDMARDs) [11]. Osteoporosis is associated with an increased risk of fractures [12] [13] . Several factors contribute to systemic bone loss in patients with RA. These factors include female gender, advanced age, the use of glucocorticoids, and sedentary lifestyle due to functional impairment. Interestingly, active disease is also a major predictor of osteoporosis in rheumatoid arthritis, independently of other contributing factors [14]. In the present study, we found a statistically significant positive effect of rituximab on osteoporosis (p<0.001). A previous study investigating the influence of rituximab on bone metabolism markers in RA patients after 3 to 15 months of treatment concluded a significant decrease in the bone degradation marker, desoxypyridinoline, derived from collagen type I degradation, and a non-significant trend of decreasing RANKL. There was no significant change in the bone formation markers alkaline phosphatase and C-terminal propertied of collagen I. In contrast, it appears that rituximab reduces the activity of osteoclasts, which are often found to be increased in active rheumatoid arthritis, contributing to osteoporosis in this disease [15]. Some authors have even found a favorable effect of B-cell depletion on bone mineral density in the spine and stability in the hip after 18 months of treatment, which may confirm the pathogenic role of activated B-cells in bone loss in RA patients [16]. Several articles have demonstrated the active role of B-cells in modulating RANKL and the RANKL/osteoprotegerin (OPG) ratio. They actively participate in bone homeostasis and act as a bridge between the immune and skeletal systems [17]. Osteoprotegerin belongs to the tumor necrosis factor receptor superfamily, binds to RANKL, and prevents it from binding to its receptor RANK on the surface of osteoclasts, thereby inhibiting bone resorption. In contrast to OPG, RANK (Receptor Activator of NFκB) promotes osteoclastogenesis. The chronic inflammatory state in RA causes bone loss by promoting the secretion of RANKL by B-cells, leading to a disturbance in the RANKL/OPG ratio and consequently a decrease in bone mineral density. Demineralization in conditions such as myeloma and during human immunodeficiency virus (HIV) infection is explained by the disruption of the immune system of activated B-cell lineage [16][17]. The suppression of inflammation by biologic diseasemodifying antirheumatic drugs (DMARDs) reduces the rate of bone loss in RA [18]. However, in a cohort of RA patients treated with rituximab, a significant reduction in the inflammatory state was observed without a significant decrease in bone mineral density. There was an increase in bone formation markers P1NP and BAP and stability in resorption markers [19]. In the anti-TNF group (Group II), there was a non-significant decrease in osteoporosis (p=0.18). TNFα is responsible for the differentiation of pro-osteoclasts into osteoclasts independently of RANK [20]. This differentiation is enhanced in the presence of even low concentrations of RANKL [21]. The expression of RANKL is stimulated, along with that of M-CSF, by TNFα in several target cells, including osteoblasts, during inflammation, indirectly promoting osteoclast differentiation [22]. Furthermore, TNFα inhibits osteoclast apoptosis through the mTOR/S6 kinase pathway [23]. Collectively, these actions potentiate the survival of osteoclasts, leading to increased bone resorption in inflammatory conditions. TNFα also acts on the bone formation pathway through nuclear factor κB (NF- κB), which suppresses osteoblastic differentiation and activity [24]. This differentiation is also inhibited by the expression of osterix, an essential regulator of early osteoblast differentiation, preventing the interaction of NFATc2 with osterix and osteogenic target genes [25]. TNFα stimulates inhibitors of the Wnt pathway, a key osteoblast stimulator, mainly DKK1 [26] and sclerostin [27]. However, in an observational study conducted in America, no difference in the risk of non-vertebral fractures was found between patients on conventional DMARDs and patients on a combination of anti-TNF and methotrexate (RR=1.1, 95% CI [0.6–2.0]) or on anti-TNF monotherapy (RR=1.2, 95% CI [0.6–2.3]) [28]. This suggests that inflammation plays a primary role in bone loss in RA. The first anti-TNF, infliximab, improved bone status in the hip but not in the spine and hand [29]. One major limitation of all these studies is the small sample size. Anti-IL6 combats bone pain by suppressing Calcitonin gene-related peptide (CGRP) in neurons in osteoporotic mice [30]. Serum IL-6 is a significant predictor of total hip bone loss at 3 years, although there was no significant association in women over 10 years’ post-menopause. In women with early menopause, IL-6 was the most important predictor of total femoral bone loss in multivariable analyses, with total femoral BMD decreasing by 1.35% for each unit increase in IL-6 [31]. Inhibition of IL-6 delays the progression of bone loss independently of its impact on disease activity [32]. A one-year prospective study found a decrease in DKK-1 and an increase in bone formation markers without a significant modification of BMD in RA patients receiving tocilizumab [33]. This confirms the reduction in osteoporosis in our tocilizumab group (Group III) after 3 years of biologic therapy, but this decrease is not significant (p=0.18). Increased BMD under tocilizumab has been observed in several previous studies [34][35], or sometimes stabilization of BMD in others [36]. The strengths of our study lie in the fact that it is the first large-scale multicenter study in Morocco and North Africa, in which RA and AS patients from all regions of Morocco were enrolled with a broad age range of true life. The limitations of this study are the relatively small number of our patients, and the database did not contain information on bone mineral density.
Conclusion
Our study, conducted in real-life conditions, demonstrates that treatment with biologics has a beneficial effect on rheumatoid arthritis and, consequently, on bone health, with a decrease in the incidence of osteoporosis, which is a serious comorbidity, especially due to the risk of fractures. This is attributed to the reduction in disease activity through their antiinflammatory effects, improvement in quality of life, and reduced corticosteroid use.
Availability of data and material
The datasets are available from the RBSMR registry of the Moroccan Society of Rheumatology.
Funding
The ancillary study described in this manuscript was done without any type of funding.
Grant
Data collection was supported by an unrestricted grant from Pfizer, Novartis, Janssen, and Abbvie.
Authors’ contributions
LT drafted this manuscript and reviewed the literature. E.A and A.B reviewed critically the manuscript.
Acknowledgements
The authors would like to thank the scientific Committee and national principal investigators of the RBSMR study: Ahmed Bezza, Abdellah El Maghraoui, Lahsen Achemlal, Fadoua Allali, Rachid Bahiri, Redouane Abouqal, Imane El Bouchti, Imad Ghozlani, Taoufik Harzy, Ihsane Hmamouchi, Linda Ichchou, Hasna Hassikou, Ouafa Mkinsi, and Redouane Niamane; patients who agreed to participate in this study.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
The protocol for the original RBSMR study was reviewed and approved by local institutional review boards and the national ethic committee.
Consent for publication
Written informed consent for publication was obtained from the patients.
References
- McInnes B. The Pathogenesis of Rheumatoid Arthritis The New England Journal of Medicine (2011).
- Kleyer A and Schett G. Arthritis and bone loss: a hen and egg story Current Opinion in Rheumatology 26, 80-4 (2014).
- Fardellone P, Salawati E, Le Monnier L et al. Bone Loss, Osteoporosis, and Fractures in Patients with Rheumatoid Arthritis: A Review JCM 9 3361(2020).
- L. Bustamente long-term effect of biological therapies on bone mineral density (bmd) for ra patients compared to patients treated by synthetic dmard over an 8-year follow-up.
- Suzuki T, Nakamura Y, Kato H. Effects of denosumab on bone metabolism and bone mineral density with anti-TNF inhibitors, tocilizumab, or abatacept in osteoporosis with rheumatoid arthritis.14, 453-9 (2018)
- Lodder M C. Bone mineral density in patients with rheumatoid arthritis: relation between disease severity and low bone mineral density Annals of the Rheumatic Diseases.63, 1576-80 (2004).
- Mok C, Tong K H, To C H et al. Risedronate for prevention of bone mineral density loss in patients receiving high-dose glucocorticoids: a randomized double-blind placebo-controlled trial. Osteoporos Int. 19,357-64 (2008).
- Shin K, Park S-H, Park W et al. Monthly Oral Ibandronate Reduces Bone Loss in Korean Women With Rheumatoid Arthritis and Osteopenia Receiving Long-term Glucocorticoids: A 48-week Double-blinded Randomized Placebo-controlled Investigator-initiated Trial Clinical Therapeutics. 39, 268-278 (2017).
- Anon. Radiographic axial versus non-radiographic axial spondyloarthritis: Comparison of the disease activity parameters and the disease activity and functional scores: RBSMR study.(2019)
- Hmamouchi I, Abouqal R, Achemlal L et al. Registre des Biothérapies de la Société Marocaine de Rhumatologie (RBSMR)â¯: méthodes et résultats préliminaires des données à l’inclusion. (2019)
- Chen M H, Yu S F, Chen J F et al. Different Effects of Biologics on Systemic Bone Loss Protection in Rheumatoid Arthritis: An Interim Analysis of a Three-Year Longitudinal Cohort Study Front. Immunol. 12-783030 (2021).
- Woolf A D. Osteoporosis in rheumatoid arthritis—the clinical viewpoint rheumatology 30, 82-4 (1991).
- Gough A. Generalised bone loss in patients with early rheumatoid arthritis The Lancet 344, 23-7(1994).
- Kvien T K. Data driven attempt to create a clinical algorithm for identification of women with rheumatoid arthritis at high risk of osteoporosis Annals of the Rheumatic Diseases. 59, 805-11 (2000).
- Hein G, Eidner T, Oelzner P et al. Influence of Rituximab on markers of bone remodeling in patients with rheumatoid arthritis: a prospective open-label pilot study Rheumatol Int. 31, 269-72 (2011).
- Titanji K. Beyond Antibodies: B Cells and the OPG/RANK-RANKL Pathway in Health, Non-HIV Disease and HIV-Induced Bone Loss Front. Immunol. 8-1851(2017).
- Nikolajczyk B S. B cells as under-appreciated mediators of non-auto-immune inflammatory disease Cytokine. 50, 234-42(2010).
- Haugeberg G, Helgetveit K B, Førre Ø et al. Generalized bone loss in early rheumatoid arthritis patients followed for ten years in the biologic treatment era. BMC Musculoskelet Disord. 15-289 (2014).
- Wheater G, Elshahaly M, Naraghi K et al. Changes in bone density and bone turnover in patients with rheumatoid arthritis treated with rituximab, results from an exploratory, prospective study. M Nurmohamed PLoS ONE. 13- E0201527 (2018).
- Fuller K, Murphy C, Kirstein B et al. TNFα Potently Activates Osteoclasts, through a Direct Action Independent of and Strongly Synergistic with RANKL Endocrinology. 143, 1108-18(2002).
- Lam J, Takeshita S, Barker J E et al. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand J. Clin. Invest. 106, 1481-8 (2000)
- Komine M, Kukita A, Kukita T et al. Hotokebuchi T and Kohashi O Tumor Necrosis Factor-⣠Cooperates With Receptor Activator of Nuclear Factor â¬B Ligand in Generation of Osteoclasts in Stromal Cell-depleted Rat Bone Marrow Cell Culture 28.
- Glantschnig H, Fisher J, Wesolowski G et al. TNFa and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase Cell Death and Differentiation.
- Krum S A, Chang J, Miranda-Carboni G et al. Novel functions for NFκB: inhibition of bone formation Nat Rev Rheumatol. 6, 607-11(2010).
- Barbuto R, Mitchell J. Regulation of the osterix (Osx, Sp7) promoter by osterix and its inhibition by parathyroid hormone. Journal of Molecular Endocrinology. 51, 99-108 (2013).
- Diarra D, Stolina M, Polzer K et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 13,156–63 (2007).
- Findlay D M, Atkins G J. TWEAK and TNF Regulation of Sclerostin: A Novel Pathway for the Regulation of Bone Remodelling Advances in TNF Family Research Advances in Experimental Medicine and Biology. 691,337- 48 (2011).
- Kim S Y, Schneeweiss S, Liu J et al. Effects of disease-modifying antirheumatic drugs on nonvertebral fracture risk in rheumatoid arthritis: A population-based cohort study. J Bone Miner Res. 27, 789-96 (2012).
- Haugeberg G, Conaghan P G, Quinn M et al. Bone loss in patients with active early rheumatoid arthritis: infliximab and methotrexate compared with methotrexate treatment alone. Explorative analysis from a 12-month randomised, double-blind, placebo-controlled study Annals of the Rheumatic Diseases. 68, 1898–901(2009).
- Wakabayashi H, Miyamura G, Nagao N et al. Functional Block of Interleukin-6 Reduces a Bone Pain Marker But Not Bone Loss in Hindlimb-Unloaded Mice IJMS. 21-3521(2020).
- McLean R. Proinflammatory cytokines and osteoporosis Curr Osteoporos Rep. 7, 134-9 (2009).
- Smolen J S, Avila J C M, Aletaha D. Tocilizumab inhibits progression of joint damage in rheumatoid arthritis irrespective of its anti-inflammatory effects: disassociation of the link between inflammation and destruction Ann Rheum Dis. 71,687-93 (2012).
- Briot K, Rouanet S, Schaeverbeke T et al. The effect of tocilizumab on bone mineral density, serum levels of Dickkopf-1 and bone remodeling markers in patients with rheumatoid arthritis Joint Bone Spine. 82,109-15 (2015).
- Abu M, Zisman D, Balbir-Gurman A et al. Effect of Tocilizumab on Fatigue and Bone Mineral Density in Patients with Rheumatoid Arthritis.
- Chen Y-M, Chen H-H, Huang W-N et al. Tocilizumab potentially prevents bone loss in patients with anticitrullinated protein antibody-positive rheumatoid arthritis ed S V Reddy. PLoS ONE. 12- E0188454 (2017).
- Kume K, Amano K, Yamada S et al. The effect of tocilizumab on bone mineral density in patients with methotrexate-resistant active rheumatoid arthritis Rheumatology. 53,900-3 (2014).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref