Review Article - Clinical Investigation (2020) Volume 10, Issue 2
Selectively targeting TGF-β with Trabedersen/OT-101 in treatment of evolving and mild ards in COVID-19
- Corresponding Author:
- Fatih M Uckun
Immuno-Oncology Program, Oncotelic Inc., Agoura Hills, CA 91301, USA
E-mail: fatih.uckun@worldwide.com
Submitted: 19 May 2020; Accepted: 26 May 2020; Published online: 31 May 2020
Abstract
Based on the role of TGF- β in the immunopathology of ARDS, we and others have proposed the use of TGF-β inhibitors for the treatment of COVID-19 pneumonia and ARDS. TGF-b targeting is employed as a strategy to stimulate the immune system of advanced-stage cancer patients in an attempt to overcome the immunosuppression and T-cell exhaustion within the tumor microenvironment. Nevertheless, we do not anticipate any worsening of existing ARDS or Cytokine Storm/Cytokine Release Syndrome (CRS) of COVID-19 patients as a treatment-emergentt complication with our contemplated use of the anti-TGF-β RNA therapeutic OT-101. That is because (i) inhibitors of TGF-β signaling are not associated with ARDS, Cytokine Storm/CRS, or systemic capillary leak, (ii) OT-101 did not cause any pulmonary toxicity, non-infectious pneumonitis, CRS, systemic or pulmonary capillary leak or ARDS in any of the 61 patients with advanced solid tumors enrolled in Phase I/II study (ClinicalTrials.gov identifier: NCT00844064) who received much longer periods of OT-101 therapy, and (iii) OT-101 did not cause in human subjects an elevation of TNF-α, IL-6 or IL-10 levels associated with CRS and ARDS in COVID-19 patients - likewise, OT-101 did not induce production of these inflammatory cytokines in cultures of human white blood cells. We postulate that because of the significance of the TGF-β pathway on the development of ARDS and T cell exhaustion, treatment with OT-101 may prevent the progression of evolving or mild ARDS and help facilitate the recovery of lymphocytopenia and T-cell exhaustion in COVID-19 patients.
Keywords
COVID-19 • TGF-β • ARDS • cytokine release syndrome
Introduction
Clinical use of TGF-β pathway inhibitors are not associated with the systemic capillary leak, cytokine release syndrome/cytokine storm, or ARDS
The signaling of TGF-β can be modulated through three distinct strategies: using antisense nucleotides that block TGF-β an mRNA (Trabedersen/AP 12009-also known as OT-101), using monoclonal antibodies to block TGFβ isoforms (lerdelimumab, metelimumab) or using inhibitors of the TGF-β receptor. Several small molecules as well as large molecule inhibitors (e.g. neutralizing anti- TGF antibodies) of TGF-β signaling have been and are being evaluated in clinical trials. None of these drug candidates have caused CRS/Cytokine Storm, systemic capillary leak, or ARDS [1].
Fresolimumab is a pan-specific, recombinant, fully human anti- TGF-β monoclonal antibody. No patient experienced a systemic capillary leak, cytokine release syndrome/cytokine storm, or ARDS when treated in Phase 1 clinical trials for renal cell carcinoma, melanoma, or glioma [1]. Galunisertib is a small-molecule selective inhibitor of TGFβR1 (ALK5), the receptor that binds both TGF-β1 and TGF-β2. No patient enrolled in the open-label Phase II study in hepatocellular carcinoma (ClinicalTrials. gov: NCT01246986), experienced a systemic capillary leak, cytokine release syndrome/cytokine storm, or ARDS [2]. The observed treatment-emergent adverse events included fatigue, anemia, peripheral edema, and abdominal pain and neutropenia, anemia, embolism, high bilirubin, and low albumin levels were encountered as Grade 3-Grade 4 treatment-related Adverse Events (AEs). In another Phase II clinical study of Galunisertib in patients with myelodysplasia (ClinicalTrials.gov, number NCT02008318), fatigue, diarrhea, fever, and emesis (5/41, 12%) were observed as treatment-emergent AEs. No patient experienced a systemic capillary leak, cytokine release syndrome/ cytokine storm, or ARDS [3]. AVID200 is a rationally designed first-in-class receptor ectodomain trap that inhibits TGF-β1 and TGF-β3. Phase I studies of AVID200 were performed in patients with advanced cancers (ClinicalTrials.gov Identifiers: NCT03834662; NCT03094169). Diarrhea and elevation of the pancreas enzyme lipase were reported as drug-related Grade 3 AE but no patient experienced a systemic capillary leak, cytokine release syndrome/ cytokine storm, or ARDS [4]. Pliant Therapeutics’ proprietary small molecule PLN-74809 is an oral dual selective inhibitor of the tissue-specific αVβ6 and αVβ1 integrins blocking integrin-mediated stimulation of the TGF-β pathway that is currently in Phase 2 testing for lung fibrosis. The compound was well-tolerated in a randomized, double-blind, placebo-controlled study with no evidence of CRS/ cytokine storm, systemic capillary leak, or ARDS [5].
The safety of the anti-TGF-β anti-sense Oligonucleotide Trabedersen (OT-101) was evaluated in Phase 1 and Phase 2 studies in cancer patients. In Phase I/II study (ClinicalTrials.gov identifier: NCT00844064), a total of 61 patients were treated with continuous intravenous (IV) infusion of OT-101 as 2nd to 4th-line therapy in escalating doses (40 mg/m2/day up to 330 mg/m2/day). Overall, OT-101 was well-tolerated and no patient experienced a systemic capillary leak, cytokine release syndrome/ cytokine storm, non-infectious pneumonitis, or ARDS [6]. Thrombocytopenia, gastrointestinal hemorrhage, and fever observed as related/possibly related treatment-emergent AEs.
Interleukin 6 (IL-6) as the signature proinflammatory cytokine associated with cytokine storm/cytokine release syndrome (CRS) and ARDS in COVID-19
IL-6 is a pro-inflammatory cytokine that plays a pivotal role in the development, progression, and severity of CRS as well as its complications, including Disseminated Intravascular Coagulopathy (DIC) and multiorgan failure [7-10]. It is the main signature cytokine implicated in COVID-19 associated CRS and ARDS [7]. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with markedly elevated IL-6 levels in critically ill COVID-19 patients [11]. Diao et al. reported the serum cytokine concentration from inpatient data of patients with COVID-19 in Wuhan, admitted to hospital from December 2019 to January 2020, and healthy controls, who came to the hospitals for routine evaluation. IL-6 levels, along with IL-10 and TNF-α levels were markedly elevated [12]. Xu et al. also reported that IL-6 and TNF-α levels were increased in COVID-19 patients [13]. Subsequently, several additional groups likewise reported that IL-6 and TNF-α levels were increased in COVID-19 patients [14-19]. Early data from a single-arm, 21-patient Chinese trial indicated an anti-IL6 receptor monoclonal antibody may have significant clinical benefit in COVID-19 pneumonia patients. China has approved the use of that antibody in severe forms of COVID-19. In an open-label Phase 2 study (ClinicalTrials.gov Identifier: NCT04317092), Tocilizumab is being evaluated in patients with COVID-19 pneumonia and a related randomized study (ClinicalTrials.gov Identifier: NCT04306705), it is being evaluated for its efficacy in CRS associated with COVID-19. It is also being evaluated in combination with Favipiravir (ClinicalTrials.gov Identifier: NCT04310228). Likewise, Sarilumab (Kevzara), another monoclonal antibody that binds to the IL-6 receptor, is being evaluated in a Phase 2/3 study in hospitalized COVID-19 patients (ClinicalTrials.gov Identifier: NCT04315298). Based on the role of TGF-β in the immunopathology of ARDS, we and others have proposed the use of TGF-β inhibitors for the treatment of COVID-19 pneumonia and ARDS [20-22].
OT-101 does not increase IL-6 or TNF-α levels in human subjects when used at doses comparable to those proposed for the COVID-19 patient population
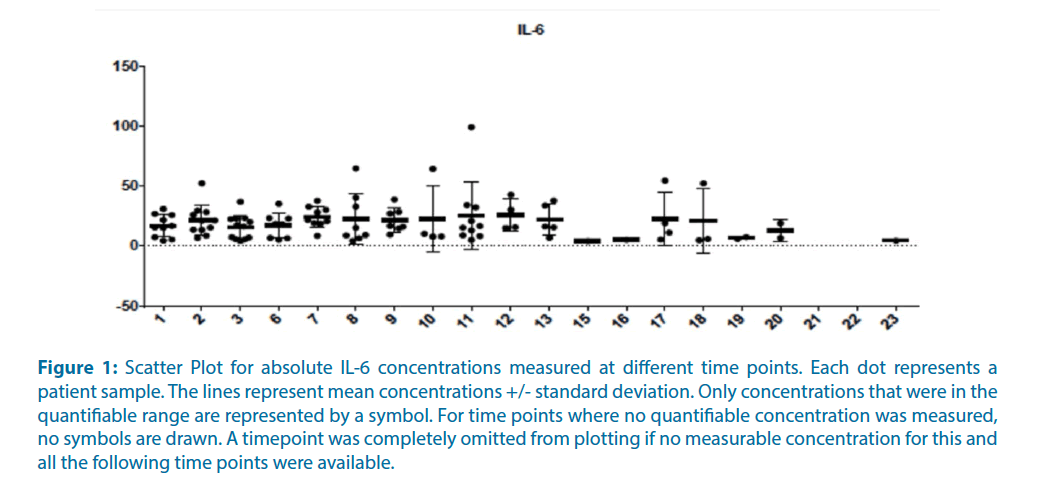
Cytokine levels of clinical plasma samples of 12 pancreatic cancer patients of the P001 study of OT- 101 in advanced solid tumor patients were measured. These 12 pancreatic cancer patients were from the last cohort of the P001 study, with OT-101 treatment at 140 mg/m2/day on 4-days-on, 10-days-off schedule. The daily dose level of OT-101 was comparable to the contemplated dose level of OT-101 for the proposed COVID-19 study (viz: 150 mg/m2 on day 1, 100 mg/m2 on days 2-7; the selected dose level of 150 mg/m2 on day 1 and 100 mg/m2 on days 2-7 is lower than the MTD of 160 mg/m2/day when OT-101 was administered via intravenous infusion according to a 7 day on, 7 day off schedule in the P001 patient population and caused no SAEs). The evaluated cytokine panel included IL-6, TNFα, IFNγ, MIP- 1α, IL-10, IL-1β, IL-12p40, IL-17A, IL-2, and IL- 8. Samples analyzed were acquired before the onset of OT-101 therapy and at selected 23-time points during the therapy. Most on-therapy samples were obtained at the following time points: Cycle 1 day 2 (time point 2), Cycle 1 day 5 (time point 3), Cycle 2 day 1 (time point 6), Cycle 2 day 2 (time point 7), Cycle 2 day 5 (time point 8), final visit (time point 9), exit period Cycle 3 day 5 (time point 11). As shown in Table 1, displaying the scatter plots for absolute IL-6 concentrations measured at different time points, OT- 101 therapy was not associated with any significant treatment-emergent increase IL-6 levels. Examining the parameter estimates of mixed ANCOVA model across time points to identify individually affected cytokines showed that only Epidermal Growth Factor (EGF) increased significantly across time. In particular, neither IL-6 or TNF-α levels showed an increase in response to OT-101 therapy (Figure 1).
Figure 1: Scatter Plot for absolute IL-6 concentrations measured at different time points. Each dot represents a patient sample. The lines represent mean concentrations +/- standard deviation. Only concentrations that were in the quantifiable range are represented by a symbol. For time points where no quantifiable concentration was measured, no symbols are drawn. A timepoint was completely omitted from plotting if no measurable concentration for this and all the following time points were available.
OT-101 does not increase IL-6 levels in non-human primates (NHP) when used at doses comparable to as well as doses much higher than those proposed for the COVID-19 patient population
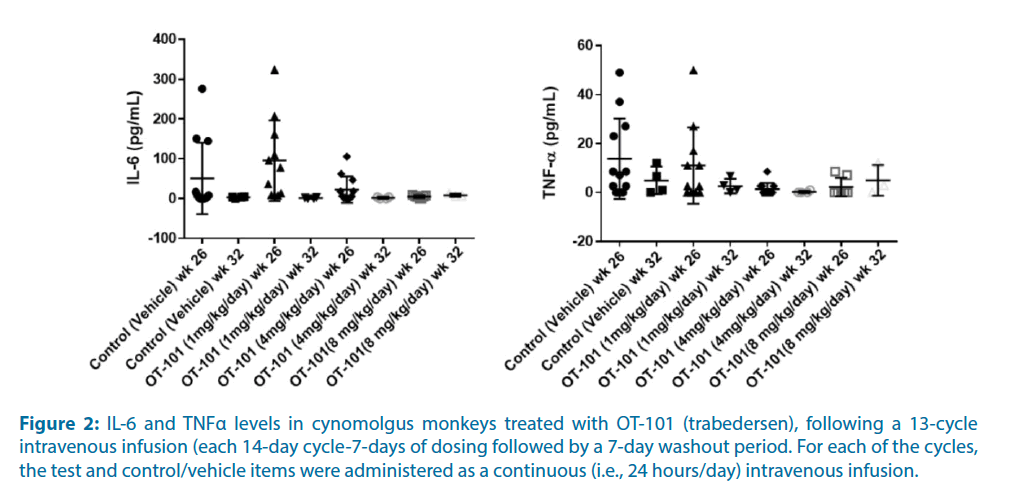
The objective of the NHP study was to determine the toxicity and toxicokinetic profile of the test item, OT-101 (Trabedersen), following a 13-cycle intravenous infusion (each 14-day cycle being 7-days of dosing followed by a 7-day washout period) to Cynomolgus monkeys and to allow assessment of reversibility of any changes following a 6-week recovery period. For each of the cycles, the test and control/vehicle items were administered as a continuous (i.e., 24 hours/day) intravenous infusion.
Notably, IL-6 or TNF-α levels showed no dose-response relationship suggestive of an OT- 101 effect (Figure 2). There was no OT-101-related complement activation (i.e., C3a values), no changes in absolute counts of T cells, activated T helper and activated T cytotoxic T-cells, activated monocytes, B cells, or NK cells.
Figure 2: IL-6 and TNFα levels in cynomolgus monkeys treated with OT-101 (trabedersen), following a 13-cycle intravenous infusion (each 14-day cycle-7-days of dosing followed by a 7-day washout period. For each of the cycles, the test and control/vehicle items were administered as a continuous (i.e., 24 hours/day) intravenous infusion.
OT-101 does not cause IL-6 production at clinically relevant concentrations, and it does not amplify IL-6 production in stimulated whole blood cultures
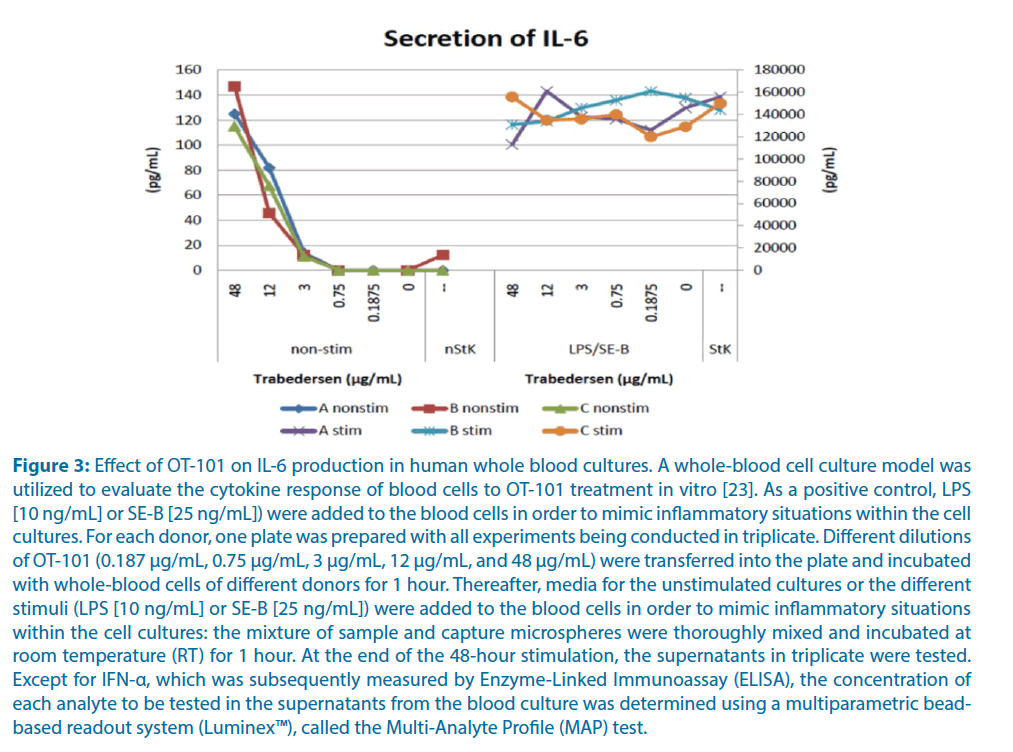
At clinically meaningful concentrations of <5 μg/mL (Cmax range in P001 study=1.46 μg/mL at 80 mg/m2 OT-101-4.45 μg/mL at 240 mg/m2 OT-101) [16], OT-101 did not induce above background IL-6 production. Even at clinically not applicable very high concentrations of 12 μg/mL or 48 μg/mL, the measured IL-6 concentrations in OT-101 treated cultures were ~0.1% of the IL-6 concentrations in positive control cultures stimulated with LPS/SE-B (Figure 3). Notably, the addition of OT-101 to cultures stimulated with LPS/SE-B did not result in amplified IL-6 production. These results demonstrate that OT-101 does not cause IL-6 production at clinically relevant concentrations, and it does not amplify IL-6 production in stimulated whole blood cultures [23].
Figure 3: Effect of OT-101 on IL-6 production in human whole blood cultures. A whole-blood cell culture model was utilized to evaluate the cytokine response of blood cells to OT-101 treatment in vitro [23]. As a positive control, LPS [10 ng/mL] or SE-B [25 ng/mL]) were added to the blood cells in order to mimic inflammatory situations within the cell cultures. For each donor, one plate was prepared with all experiments being conducted in triplicate. Different dilutions of OT-101 (0.187 μg/mL, 0.75 μg/mL, 3 μg/mL, 12 μg/mL, and 48 μg/mL) were transferred into the plate and incubated with whole-blood cells of different donors for 1 hour. Thereafter, media for the unstimulated cultures or the different stimuli (LPS [10 ng/mL] or SE-B [25 ng/mL]) were added to the blood cells in order to mimic inflammatory situations within the cell cultures: the mixture of sample and capture microspheres were thoroughly mixed and incubated at room temperature (RT) for 1 hour. At the end of the 48-hour stimulation, the supernatants in triplicate were tested. Except for IFN-α, which was subsequently measured by Enzyme-Linked Immunoassay (ELISA), the concentration of each analyte to be tested in the supernatants from the blood culture was determined using a multiparametric beadbased readout system (Luminex™), called the Multi-Analyte Profile (MAP) test.
The rationale for using OT-101 in COVID-19 patients with respiratory failure requiring noninvasive positive pressure ventilation (NIPPV) or mild ARDS requiring Mechanical ventilation (MV)
We postulate that the anti- TGF-β RNA therapeutic OT-101 will accelerate the resolution of respiratory failure of COVID-19 patients requiring NIPPV or Mild ARDS Requiring MV. Notably, TGF-β levels in Bronchoalveolar Lavage Fluid (BAL) samples from ARDS patients are inversely correlated with ventilator-free days and ICU-free days [22]. Furthermore, patients with higher TGF-β levels may have higher and faster case mortality [22]. Sequestering TGF-β has effectively attenuated the severity of pulmonary edema in experimental models of ARDS [22]. Furthermore, we do not anticipate any worsening of existing ARDS or Cytokine Storm/Cytokine Release Syndrome (CRS) of COVID-19 patients due to 7 days of OT-101 treatment. That is because no CRS or ARDS was observed as a treatment-emergent complication in a Phase I study of 61 patients with advanced solid tumors who were treated with more extended periods of OT-101 therapy. Additionally, OT-101 did not cause in human subjects an elevation of TNF-α, IL- 6, or IL-10 levels associated with CRS and ARDS in COVID-19 patients. Likewise, OT-101 did not induce the production of these inflammatory cytokines in cultures of human white blood cells. Inhibition of the TGF-β signaling pathway also has the potential to prevent the development of pulmonary fibrosis following ARDS and improve the pulmonary healing process
TGF-β increases capillary permeability and impairs alveolar fluid reabsorption in ARDS patients, contributing to pulmonary edema’s persistence
ARDS is characterized by severe pulmonary edema and an impaired alveolar fluid clearance incapable of removing the edema from alveoli caused by a functional defect of the Epithelial Sodium Channel (ENaC) [22]. Alveolar barrier dysfunction in ARDS leads to more pulmonary edema and the systemic release of biological mediators from the lung, contributing to the failure of other organs and potentially a multiorgan failure [24]. TGF-β is an important proinflammatory cytokine that affects the inflammatory process resulting from an acute lung injury, which contributes to the development of persistent and severe pulmonary edema in ARDS patients [25-30]. TGF-β can increase alveolar epithelial permeability [29] and pulmonary endothelial permeability by promoting adherens junction disassembly [31] as well as inhibiting pulmonary endothelial proliferation [32]. A recent study showed that TGF-β profoundly impacts alveolar ion and fluid transport by regulating the Epithelial Sodium Channel (ENaC) activity [33].
Elevated TGF-β levels in ARDS associated with SARS-CoV or SARS-CoV-2
Lavage fluid (BAL) samples from ARDS patients showed higher TGF-β levels when compared to BAL samples from non-ARDS controls [31]. The TGF-β levels in Bronchoalveolar Lavage Fluid (BAL) samples from ARDS patients were inversely correlated with the number of ventilator-free days or ICU-free days during hospitalization. Lower TGF-β levels were associated with better survival outcomes [34].
SARS-CoV up-regulates TGF-β expression, and TGF-β levels were markedly elevated in SARS patients with ARDS [35]. SARS-associated Coronavirus (SARS-CoV) nucleocapsid (N) protein potentiates TGF-β signaling via a Smad3-dependent induction of TGF-β1 expression [36]. Further, the papain-like protease (PLpro) of SARS-CoV, a deubiquitinating enzyme and virulent factor in SARS pathogenesis has been reported to trigger TGF-β production via p38 MAPK, and ERK1/2-mediated signaling [37,38]. Xiong et al. showed that TGFβ genes are up-regulated in COVID-19 [39].
The contributing factors for the increased TGF-β levels in ARDS include the activation of the complement signaling pathway with the production of the complement cleavage product, C5a, that triggers the formation of Neutrophil Extracellular Traps (NETs). NETs are extracellular webs of chromatin, microbicidal proteins, and oxidant enzymes that are released by neutrophils to contain infections. However, when not appropriately regulated, NETs have the potential to propagate inflammation and microvascular thrombosis. That is because they are capable of activating platelets to release TGFβ. Recent studies have shown that sera from individuals with COVID-19 triggered NET release from control neutrophils in vitro, and high levels of NETs in many patients with COVID-19 may contribute to cytokine release and respiratory failure. Zuo et al. reported that sera from patients with COVID-19 have elevated levels of cellfree DNA, Myeloperoxidase (MPO)-DNA, and citrullinated histone H3 (Cit-H3); the latter two are highly specific markers of NETs [40]. Highlighting the potential clinical relevance of these findings, cell-free DNA strongly correlated with acute phase reactants including C-reactive protein, D-dimer, and lactate dehydrogenase, as well as absolute neutrophil count [40,41].
Sequestering TGF-β has effectively attenuated the severity of pulmonary edema in experimental models of ARDS [29,41]. Likewise, the antiinflammatory isoflavone Puerarin ameliorates the ARDS-associated inflammatory process in the lungs by inhibiting the expression of TGF-β [42].
Contribution of TGF-β to coagulopathy in COVID-19 associated ARDS. As a platelet activator
TGF-β has been implicated in the pathophysiology of thrombosis and disseminated intravascular coagulopathy [43]. It is noteworthy that autopsies in COVID-19 cases have revealed microthrombi [44]. Stafford et al. postulated that TGFβ is a key molecule, along with TNFα, in the pathogenesis of severe COVID-19 [45].
Contribution of TGF-β to lung fibrosis post-ARDS
TGF-β is also involved in the pathogenesis of lung tissue remodeling and lung fibrosis that follows ARDS [22]. TGF-β stimulates lung fibroblasts and causes collagen production in the pulmonary interstitial and alveolar space, leading to the occurrence and development of pulmonary fibrosis [46,47].
Wang et al. reported that miR-425 reduction in lung fibroblasts contributes to the development of lung fibrosis post-ARDS through activation of the TGF-β signaling pathway [48]. Therefore, inhibition of the TGF-β signaling pathway could prevent the development of pulmonary fibrosis post-ARDS and improve the pulmonary healing process [42].
Contribution of TGF-β to lymphopenia and immune exhaustion in COVID-19
A consistent finding in COVID-19 is lymphopenia with low CD4 counts [49]. TGFβ has been shown to reduce T-cell numbers in disease states [50,51]. Xu et al. reported that a significant decrease of the T lymphocyte subset is positively correlated with in-hospital death and severity of illness based on the data from a total of 187 COVID-19 patients [52]. All patients exhibited a significant drop of T lymphocyte subsets counts with remarkably increasing concentrations of SAA, CRP, IL-6, and IL-10 compared to normal values. The median concentrations of SAA and CRP in critically-ill patients were nearly 4- and 10-fold than those of mild-ill patients, respectively. As the severity of COVID-19 getting worse, the counts of T lymphocyte dropped lower. 28 patients died in hospital, the median lymphocyte, CD3+ T-cell, CD4+ T-cell, CD8+ T-cell, and B-cell were significantly lower than other patients. Lower counts (/μL) of T lymphocyte subsets lymphocyte (<500), CD3+ T-cell (<200), CD4+ T-cell (<100), CD8+ T-cell (<100) and B-cell (<50) were associated with higher risks of inhospital death of CIVID-19 [52]. TNF-α, IL-6, and IL-10 have been implicated in COVID-19 associated lymphocytopenia and T-cell reduction [52].
Similarly, Chen et al. [53] reported that compared with moderate cases, severe cases more frequently had lymphopenia with higher levels of alanine aminotransferase, lactate dehydrogenase, C-reactive protein, ferritin, and D-dimer as well as markedly higher levels of IL-2R, IL-6, IL-10, and TNF-α. Absolute numbers of T lymphocytes, CD4+ T cells, and CD8+ T cells were decreased in nearly all the patients and were markedly lower in severe cases (294.0, 177.5, and 89.0 × 106/L, respectively) than moderate cases (640.5, 381.5, and 254.0 × 106/L, respectively). Diao et al. [54] reported that the T cell numbers in COVID-19 patients are inversely correlated with serum IL-6, IL-10, and TNF-α concentrations. T cells from COVID-19 patients also show increased expression of programmed cell Death Marker-1 (PD-1) and T cell Immunoglobulin and Mucin domain 3 (TIM-3) consistent with “exhaustion” [6]. Likewise, profound T cytopenia was reported by Chiappelli et al. [55]. The reduction of the T-cell numbers, as well as the expression of the T-cell exhaustion markers PD-1 and TIM-3, correlated with the progression of the disease from early stages to advanced ARDS requiring ICU management.
TGF-β has been implicated in regulating the size of the pathogen-specific T-cell responses and the propensity of these cells to undergo apoptosis [55-58]. Enhanced and sustained TGF-β/Smad signaling is a distinctive feature of virus-specific CD8 T cells during chronic versus acute viral infections in vivo. Selective attenuation of TGF-β signaling on T cells increases the function of CD8 T cells indirectly, rapidly eradicates the persistenceprone virus, and enables the generation of an effective memory response. Therefore, targeting TGF-β may favorably affect the immune function of host T-cells in COVID-19 patients.
OT-101 was evaluated in Phase I/II study (ClinicalTrials.gov identifier: NCT00844064), involving a total of 61 patients with advanced solid tumors [22]. OT-101 was well tolerated and had no significant effect on the lymphocyte counts and it did not cause lymphocytopenia. The results for 2 months of therapy, i.e., 14-day cycles × 4 cycles are shown in Table 1. Based on these findings, we postulate that because of the significance of the TGF-β pathway on the development of T cell exhaustion, treatment with OT-101 may help facilitate the recovery of lymphocytopenia and T-cell exhaustion in COVID-19 patients.
| Laboratory value | Mean (STD) | |||||
|---|---|---|---|---|---|---|
| Screening/Baseline | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | ||
| Day 8 | Day 8 | Day 8 | Day 8 | |||
| Hematocrit (L/L) | 0.36 | 0.35 | 0.34 | 0.34 | 0.33 | |
| 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | ||
| Erythrocytes (10^12/L) | 3.94 | 3.83 | 3.84 | 3.89 | 3.89 | |
| 0.53 | 0.5 | 0.44 | 0.49 | 0.57 | ||
| Hemoglobin (g/L) | 119.12 | 114.95 | 112.12 | 110.99 | 110.12 | |
| 15.31 | 14.18 | 13.15 | 13.36 | 13.5 | ||
| Platelets (10^9/L) | 268.18 | 192.18 | 188.81 | 201.86 | 212.24 | |
| 108.88 | 127.36 | 82.8 | 105.83 | 108.49 | ||
| Leukocytes (10^9/L) | 6.97 | 6.61 | 6.77 | 6.6 | 7.42 | |
| 2.04 | 2.73 | 3.44 | 2.75 | 4.32 | ||
| Lymphocytes (%) | 19.62 | 20.39 | 20.74 | 21.21 | 21.82 | |
| 8.84 | 8.47 | 9.85 | 7.5 | 10.26 | ||
| Absolute neutrophils count | 4.52 | 4.1 | 4.37 | 4.43 | 4.94 | |
| 1.6 | 2.1 | 2.18 | 2.5 | 3.47 | ||
Table 1: Hematology laboratory values, by visit (FAS)
Conclusion
TGF-β inhibitors show potential for the treatment of COVID-19 pneumonia and ARDS. Inhibitors of TGF-β pathway may prevent or reduce the risk of development of ARDS and T cell exhaustion, halt or reverse the progression of evolving or mild ARDS and help facilitate the recovery of lymphocytopenia in COVID-19 patients.
References
- Batlle E, Massague J. Transforming growth factor-β signaling in immunity and cancer. Immunity 50: 924-940 (2019).
- Kelley RK, Gane E, Assenat E, et al. A phase 2 study of galunisertib (tgf-β1 receptor type i inhibitor) and sorafenib in patients with advanced hepatocellular clinical and translational gastroenterology. Clin Transl Gastroenterol 10: 1-9 (2019).
- Santini V, Valcárcel D, Platzbecker U, et al. Phase 2 study of the alk5 inhibitor galunisertib in very low-, low-, and intermediate-risk myelodysplastic syndromes. Clin Cancer Res 25: 6976-6985 (2019).
- Yap T, Araujo D, Wood D, et al. P856 avid200, first-in-class tgf-beta1 and beta3 selective inhibitor: results of a phase 1 monotherapy dose escalation study in solid tumors and evidence of target engagement in patients. J Immunother Cancer 8: A1-A12 (2020).
- Turner S, Lepist EI, Rock F, et al. Late-breaking abstract-PK/PD assessment of an oral, selective aVß6/aVß1 integrin dual antagonist, PLN-74809, for the treatment of idiopathic pulmonary fibrosis. European Resp J 54: PA1298 (2019).
- Uckun FM, Trieu V. Medical-scientific rationale for a randomized, placebo-controlled, phase 2 study of trabedersen/ot-101 in covid-19 patients with hypoxemic respiratory failure. Ann Pulm Crit Care Med 3: 01-09 (2020).
- Uckun FM. Reducing the fatality rate of COVID-19 by applying clinical insights from immuno-oncology and lung transplantation. Front Pharmacol pre-proof (2020).
- Maude SL, Barrett D, Teachey DT, et al. Managing cytokine release syndrome associated with novel t cell-engaging therapies. Cancer J 20: 119-122 (2014).
- Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6: 1-23 (2014).
- Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 121: 5154-5157 (2013).
- Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis 70: 449 (2020).
- Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of t cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol 11: 1-7 (2020).
- Xu B, Fan CY, Wang AL, et al. Suppressed T cell-mediated immunity in patients with COVID-19: A clinical retrospective study in Wuhan, China. J Infect 21: 1-10 (2020).
- Chiappelli F, Khakshooy A, Greenberg G. COVID-19 Immunopathology and Immunotherapy. Bioinformation 16: 219-222 (2020).
- Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130: 2620-2629 (2020).
- Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest 130: 2202-2205 (2020).
- Li H, Chen K, Liu M, et al. The profile of peripheral blood lymphocyte subsets and serum cytokines in children with 2019 novel coronavirus pneumonia. J Infect pre-proof (2020).
- Zhang C, Wu Z, Li JW, et al. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents pre-proof (2020).
- Moore J, June C. Cytokine release syndrome in severe COVID-19. Science 368: 473-474 (2020).
- Chen W. A potential treatment of COVID-19 with TGF-b blockade. Int J Biol Sci 16: 1954-1955 (2020).
- Stafford N, Arnold A, Jebakumar S, et al. Therapeutic strategies for COVID-19: new insights into the value of transforming growth factor beta (tgfβ) antagonists such as imatinib and other kinase inhibitors. BMJ 369: m1610 (2020).
- Uckun FM, Trieu V. Medical-scientific rationale for a randomized , placebo-controlled, phase 2 study of trabedersen/ot-101 in COVID-19 patients with hypoxemic respiratory failure. Ann Pulm Crit Care Med 3: 01-09 (2020).
- D'Cruz OJ, Qazi S, Hwang L, et al. Impact of targeting transforming growth factor β-2 with antisense OT-101 on the cytokine and chemokine profile in patients with advanced pancreatic cancer. Onco Targets Ther 11: 2779-2796 (2018).
- Imai Y, Parodo J, Kajikawa O, et al. Injurious mechanical ventilation and end organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 289: 2104-2112 (2003).
- Frank J, Roux J, Kawakatsu H, et al. Transforming growth factor-β1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem 278: 43939-43950 (2003).
- Fahy RJ, Lichtenberger F, McKeegan CB, et al. The acute respiratory distress syndrome: A role for transforming growth factor-beta 1. Am J Respir Cell Mol Biol 28: 499-503 (2003).
- Willis BC, Kim KJ, Li X, et al. Modulation of ion conductance and active transport by TGF-β 1 in alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 285: L1192-L1200 (2003).
- Jenkins RG, Su X, Su G, et al. Ligation of protease-activated receptor 1 enhances α(v)β6 integrin-dependent TGF-β activation and promotes acute lung injury. J Clin Invest 116: 1606-1614 (2006).
- Pittet JF, Griffiths MJ, Geiser T, et al. TGF-beta is a critical mediator of acute lung injury. J Clin Invest 107: 1537-1544 (2001).
- Frank JA, Matthay MA. TGF-β and lung fluid balance in ARDS. Proc Natl Acad Sci USA 111: 885-886 (2014).
- Hurst VI, Goldberg PL, Minnear FL, et al. Rearrangement of adherens junctions by transforming growth factor-beta1: role of contraction. Am J Physiol 276: 582-595 (1999).
- Das SK, White AC, Fanburg BL. Modulation of transforming growth factor-beta 1 antiproliferative effects on endothelial cells by cysteine, cystine and N-acetylcysteine. J Clin Invest 90: 1649-1656 (1992).
- Peters DM, Vadasz I, Wujak T, et al. TGF-β directs trafficking of the epithelial sodium channel ENaC which has implications for ion and fluid transport in acute lung injury. Proc Natl Acad Sci USA 111: E374-E383 (2014).
- Budinger S, Chandel NS, Donnelly HK, et al. Active transforming growth factor-b1 activates the procollagen I promoter in patients with acute lung injury. Intensive Care Med 31: 121-128 (2005).
- Lee CH, Chen RF, Liu JW, et al. Altered p38 mitogen-activated protein kinase expression in different leukocytes with increment of immunosuppressive mediators in patients with severe acute respiratory syndrome. J Immunol 172: 7841-7847 (2004).
- Zhao X, Nicholls JM, Chen YG. Severe Acute Respiratory syndrome-associated coronavirus nucleocapsid protein interacts with smad3 and modulates transforming growth factor-signaling. J Biol Chem 283: 3272-3280 (2008).
- Li SW1, Yang TC, Wan L, et al. Correlation between TGF-β1 expression and proteomic profiling induced by severe acute respiratory syndrome coronavirus papain-like protease. Proteomics 12: 3193-3205 (2012).
- Wang CY, Lu CY, Li SW, et al. SARS coronavirus papain-like protease up-regulates the collagen expression through non-Samd TGF-β1 signaling. Virus Res 235: 58-66 (2017).
- Xiong Y, Liu Y, Cao L, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect 9: 761-770 (2020).
- Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight pre-proof: (2020).
- Bossman M, Ward PA. Protein-based therapies for acute lung injury: targeting neutrophil extracellular traps. Expert Opin Ther Targets 18: 703-714 (2014).
- Hu X, Huang X. Alleviation of inflammatory response of pulmonary fibrosis in acute respiratory distress syndrome by puerarin via transforming growth factor (TGF-b1). Med Sci Monit 25: 6523-6531 (2019).
- Lev P, Salim J, Marta R, et al. Platelets possess functional TGF-β receptors and Smad2 protein. Platelets 18: 35-42 (2007).
- Fox R, Akmatbekov A, Harbert J, et al. Pulmonary and cardiac Pathology in COVID -19; the first autopsy series from New Orleans. Medrxiv pre-proof (2020).
- Stafford N, Arnold A, Jebakumar S, et al. Therapeutic strategies for COVID-19: New insights into the value of transforming growth factor beta (TGFβ) antagonists such as imatinib and other kinase inhibitors. BMJ 369: m1610 (2020).
- Shimbori C, Bellaye PS, Xia J, et al. Fibroblast growth factor-1 attenuates TGF-beta1-induced lung fibrosis. J Pathol 240: 197-210 (2016).
- Nithiananthan S, Crawford A, Knock JC, et al. Physiological fluid flow moderates fibroblast responses to TGF-beta1. J Cell Biochem 118: 878-890 (2017).
- Wang L, Liu J, Xie W, et al. miR-425 reduction causes aberrant proliferation and collagen synthesis through modulating TGF-β/Smad signaling in acute respiratory distress syndrome. Int J Clin Exp Pathol 12: 2604-2612 (2019).
- Bourboulis E, Netea M, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host and Microbe 27: 1-9 (2020).
- Bald T, Smyth MJ. TGFβ shuts the door on T cells. Br J Cancer 119: 1-3 (2018).
- Kelly A, Houston SA, Sherwood E, et al. Regulation of Innate and adaptive immunity by TGFβ. Adv Immunol 134: 137-233 (2017).
- Xu B, Fan CY, Wang AL, et al. Suppressed T cell-mediated immunity in patients with COVID-19: A clinical retrospective study in Wuhan, China. J Infect pre-proof (2020).
- Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest pre-proof (2020).
- Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). MedRxiv pre-proof (2020).
- Chiappelli F, Khakshooy A, Greenberg G. COVID-19 Immunopathology and Immunotherapy. Bioinformation 16: 219-222 (2020).
- Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25: 455-71 (2006).
- Tinoco R, Alcalde V, Yang Y, et al. TGF-β Signaling in T cells is essential for CD8 T cell suppression and viral persistence in vivo. Immunity 31: 145-57 (2009).
- Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology 129: 474-481 (2010).