Review Article - Interventional Cardiology (2014) Volume 6, Issue 3
Right heart catheterization and other venous cardiovascular procedures from the arm
- Corresponding Author:
- Ian C Gilchrist
Pennsylvania State University, Heart & Vascular Institute, 500 University Drive, Hershey, PA 17033, USA
Tel: +1 717 531 5888
Fax: +1 717 531 7969
E-mail: icg1@psu.edu
Abstract
Access to the central venous system and the circulation of the right heart continues to evolve with improvements in technology and the needs of medical diagnosis and therapeutics. Early cardiovascular investigators first accessed the central venous system via forearm veins to understand the physiology and pathophysiology of heart disease. With time, there derived a need to access the central venous system, not only to understand the function of the cardiopulmonary system, but also to build on the developing physiologic knowledge base and to therapeutically monitor cardiovascular hemodynamics. In addition, the ability to provide therapy directly with devices that require delivery to the central venous system, such as temporary pacing, right side endomyocardial biopsy and placement of vena cava filters, has developed. This evolution continues today, with trends to move procedural entry sites to less invasive locations such as the arm versus the central venous or femoral sites, with their inherent hazards. The purpose of this article is to highlight this evolution in central venous access with an emphasis on practical advice based on personal experience of how to assess to the right heart system and to consider trends for the future.
Keywords
anatomy, cardiac catheterization, complications, endocardial biopsy, medical history, pacemaker, peripheral venous access, transradial, venous system
Access to the central venous system and the circulation of the right heart continues to evolve with improvements in technology and the needs of medical diagnosis and therapeutics. Early cardiovascular investigators first accessed the central venous system via forearm veins to understand the physiology and pathophysiology of heart disease. With time, there derived a need to access the central venous system, not only to understand the function of the cardiopulmonary system, but also to build on the developing physiologic knowledge base and to therapeutically monitor cardiovascular hemodynamics. In addition, the ability to provide therapy directly with devices that require delivery to the central venous system, such as temporary pacing, right side endomyocardial biopsy and placement of vena cava filters, has developed. This evolution continues today, with trends to move procedural entry sites to less invasive locations such as the arm versus the central venous or femoral sites, with their inherent hazards. The purpose of this article is to highlight this evolution in central venous access with an emphasis on practical advice based on personal experience of how to assess to the right heart system and to consider trends for the future.
Historical perspective
Advent of invasive peripheral venous access for right heart catheterization
The first modern reports of peripheral access for central venous cardiac procedures surfaced separately from different investigators [1–5]. Many operators initially viewed this approach as a novelty, but it actually represents a return to a forgotten, historic, vascular entry site. Advances in catheter technology and device miniaturization now permit a percutaneous venous approach, which allows operators to reach the central veins from small peripheral veins and perform right heart catheterization. In addition, the newer techniques allow preservation of the vein, whereas the pioneering venous catheterizations of the early 20th century usually result in a sacrifice of the vein.
Is right heart catheterization still needed?
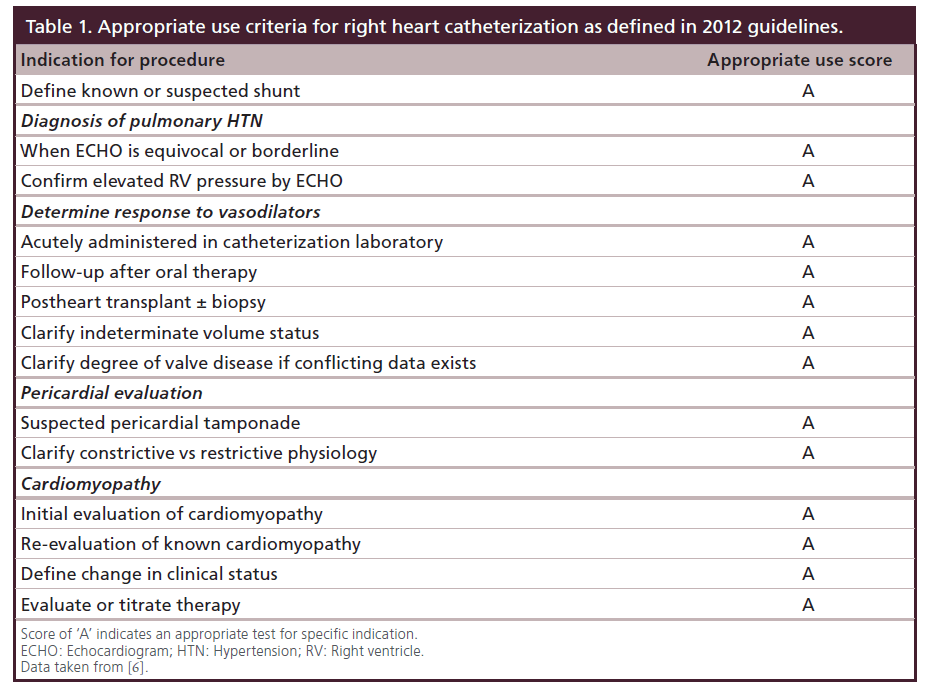
Why bother with a peripheral approach to the central venous system? After all, echocardiography, MRI and CT scanning can provide a plethora of information all via noninvasive approaches. While noninvasive techniques have supplanted many diagnostic right heart catheterizations, the need for invasive access into the central venous and pulmonary systems remain. Common modern indications for right heart catheterizations are found in recently published ‘Appropriate Use Criteria’ [6] shown in Table 1. In addition, interventional procedures such as temporary pacemakers and right ventricular biopsy cannot be done without invasive access.
Access into the central venous system is commonly obtained via direct puncture into a central vein such as the femoral, subclavian or jugular veins. These procedures have become so routine using the anatomical landmarks and techniques described several decades ago that the residual risk is often forgotten. Despite the use of ultrasound guidance, pneumothorax, retroperitoneal hemorrhage and other, misadventures still occur and these approaches using direct central vein puncture cannot be considered risk-free. In addition, certain patient characteristics such as coagulopathy (either therapeutic or due to disease) and obesity can raise the risk substantially. The need to improve patient safety at the time of central venous access is an important goal and peripheral access to the central venous system brings this procedure one step closer to being safe.
Anatomy of invasive peripheral venous access for right heart catheterization
The defining principal for peripheral venous access to the central system is the fact that all veins eventually lead to the heart. Compared with arteries, veins are far more compliant and willing to expand in order to accept relatively large catheters if needed. Many of the veins in the arm are actually quite large when compared with the size of their neighboring arteries and may accommodate devices much larger than one might place into a similar arterial tree.
Venous forearm anatomy
The venous anatomy of the arm is highly variable, although certain generalities usually hold true. The radial side of the forearm usually drains into a cephalic venous system, although in approximately 50% of people there is crossover from the lower forearm into the basilic system. The ulnar side of the forearm in a majority of people drains up the arm into the basilic venous system, which continues in combination with the deep brachial veins to form the axillary vein and then subclavian vein as it enters the thorax [7]. The cephalic vein passes up the forearm lateral to the biceps brachii then between the pectoralis major and deltoid muscles. It then pierces the clavipectoral fascia to empty into the axillary vein, often entering the main venous channel in a perpendicular intersection. At this point, the cephalic vein is usually significantly smaller in diameter than the recipient axillary vein, although both the basilic or cephalic route usually provide adequate access to the central venous system.
Antecubital region
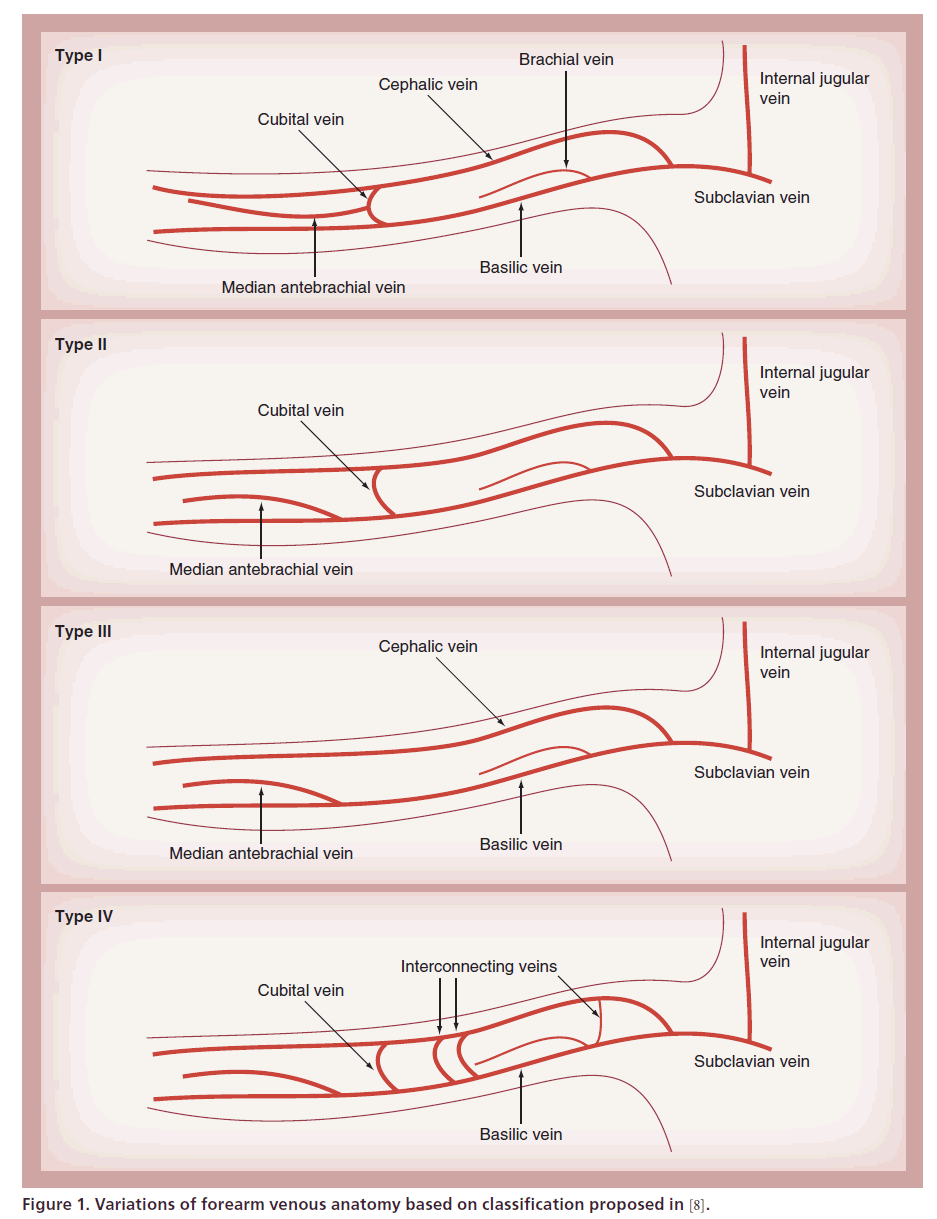
Unlike the arterial system, the venous system is ripe with redundancy. There are frequently cross channels between the different upper extremity venous systems, which can often be transversed by catheters. Several different anatomical schemes have attempted to classify and bring order to the various patterns seen at the antecubital fossa. A recent analysis of 128 cadaveric arms [8] proposed a classification into four types, each with two subtypes. In this classification, the median cubital vein that connects the cephalic system with the basilic at the antecubital fossa is used for reference, as seen in Figure 1. Type I systems are defined as one with a median antebrachial vein originating in the forearm and terminating into the cubital vein. Type II has the median antebrachial vein combining with the basilic system before the junction with the cubital vein. Type III systems have no cubital vein so the cephalic and basilic systems exist in isolation, while Type IV systems have multiple superficial veins interconnecting the cephalic and basilic systems. Overall, approximately 50% of arms will be Type I, 30% Type II, and 10% both Type III and Type IV.
Cephalic vein
Further up the arm from the antecubital fossa, the cephalic vein may have some cross connections with the basilic system in Type IV arrangements, but otherwise usually runs up to its termination in the axillary vein. Variations intermination do exist and instead of the axillary vein, rarely the cephalic will end in the external jugular, internal jugular or subclavian vein [9]. These variants should still allow flexible catheter passage from the forearm, but could interfere with other uses of the proximal cephalic vein such as for permanent pacemakers or result in unusual curves if passing stiffer equipment to the central venous system.
Basilic vein
The basilic vein runs along the medial to the biceps and combines with the deeper venous lateral and medial brachial branches. Only rarely is the basilic vein absent [10], in which case a more dominant cephalic system may exist or passage may be through the deeper brachial veins. The brachial veins run deeper than the superficial basilic vein, but with the advent of ultrasound techniques to obtain access, may become more common sites of entry into the venous system. Like all the upper arm vasculature, variations exist in the deep brachial system [11], although the significance for central venous access is unknown.
Acquired forearm venous anatomy
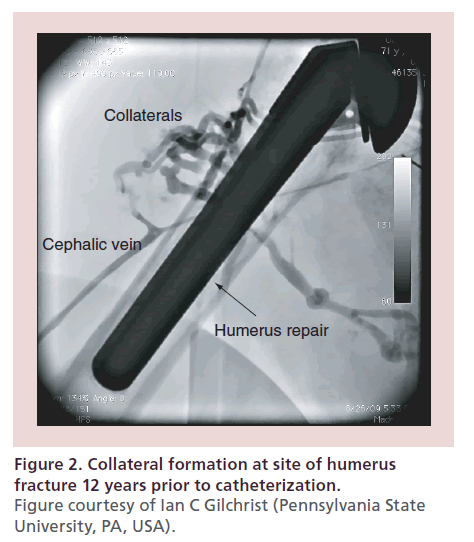
Beyond the innate patterns of venous drainage, variations in drainage can develop from acquired conditions. If there has been trauma or other forms of damage to the venous system in the past, rich collateral systems may have evolved, as shown in Figure 2. These may help or otherwise hinder passage to the central venous system, depending on the circumstances. In the cardiovascular patient group, electrophysiologic devices placed via the subclavian or jugular systems are perhaps the most common cause for changes in the venous flow. The device leads may cause thrombosis and stimulate collateral formations that subsequently result in alternative routes into the central system.
Practical approach to invasive peripheral venous access
Equipment
The technique for invasive peripheral venous access essentially uses the same tools already in use by physicians accessing the radial artery and does not require new investments in inventory. The only consideration is based on the entry point for venous access. The distance from the distal forearm near the wrist is longer than the typical distance from the groin to the pulmonary wedge position. Since industry to date has not been willing to manufacture central venous catheter devices longer than approximately 110 cm, access in patients with longer arms is relegated to the mid-forearm or antecubital region. If longer catheters become available (125 cm), this concern will no longer remain and any vein will be usable down to the wrist.
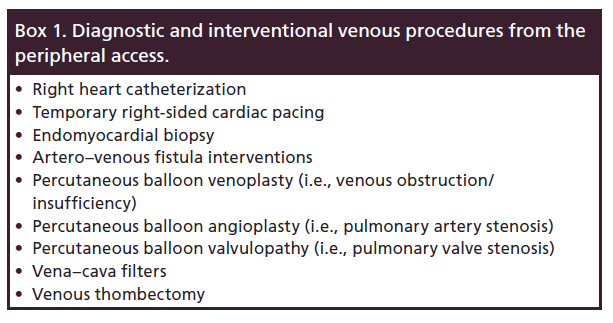
Balloon-tipped, right heart catheters are commercially available in the USA at 4 Fr and larger, while thermodilution devices start at 5 Fr. The diameter of the catheter and inner lumen will dictate to some extent the diameter of any wire one might need to place within the catheter. Likewise, pacing catheters are available with balloon tips starting at 4 Fr and work well from a peripheral entry site. Endomyocardial biopsy equipment that otherwise can reach from the femoral vein will also work from the forearm. A variety of venous procedures are listed in Box 1. Feasibility for just about every procedure is more a function of available working catheter length rather than diameter of device.
Initial access technique
While arterial access is classically obtained in the catheterization laboratory itself, the initial venous access is most efficiently obtained if performed by preprocedure staff, prior to entry into the cath-eterization laboratory. Ambient temperatures in the catheterization laboratory are typically cool as the staff is wearing lead. Combined with patient anxiety, the catheterization laboratory is conducive for veno-constriction. Preprocedural areas are typically much warmer and friendlier environments. Patients can be positioned more easily to optimize successful venipuncture. In general, any vein that can accept a 20-gauge angiocath will usually be sufficient to allow passage of a 4- or 7-Fr vascular access sheath and catheter. The vein should appear healthy and not sclerotic or spiral in nature like a collateral. Once an angiocath is in place, it can be capped off as a heparin-well for later use in the catheterization laboratory.
The staff obtaining venous entry site needs to understand that this site will be used for a cardiac catheterization and proper technique should be observed in the process. Likewise, the operator should not be enticed into using an old, chronically dwelling intravenous line for convenience. These sites will be chronically colonized and impossible to adequately clean in the catheterization laboratory. Pathogens will exist not only on the catheter’s external parts, but also in the track leading to the vein where antiseptic fluid is unlikely to reach.
If venous access is not possible outside of the catheterization laboratory, it is almost always possible in the catheterization laboratory itself. Warmth and adequate sedation may reduce vascular tone [12]. If routine venipuncture is not possible due to nonvisible or usable superficial vein, ultrasound techniques will locate a deeper vein and make arm access almost universally possible. Tourniquet techniques [7], adding warmth to the room or surface of the skin can augment success. Likewise, arterial angiography of the forearm with levophase filling can also be used to localize deeper veins [13].
Placement of venous vascular sheath
Venous sheath placement should be carried out before arterial access to minimize total arterial time and radial artery occlusion. Once venous entry is confirmed, the typical radial access kit can be used to first pass the kit’s wire through the angiocath’s heparin- well cap, or venous needle, and then exchanged out for the vascular sheath that is appropriate in size for the central venous device or catheter needed. If the initial venous entry was obtained outside of the catheterization laboratory, it will most likely be clean but not sterile. Attention to adequate site preparation with alcohol-based preparation solution or other fluid capable of denaturing proteins on the external venous catheter components is important. During the exchange, any part of the intravenous catheter placed prior to arrival in the catheterization laboratory should only be contacted directly with gauze or gloves that will be removed or changed so as to not contaminant the sterile field. To date, no reports of hospital-acquired infection have been made using this approach and it is compliant with the CDC’s ‘2011 guidelines for the prevention of intravascular catheter-related infections’ [14].
Once the vascular sheath is in place, it can be flushed prior to use. Attempting to draw back blood with a syringe, similar to what is done after placing an arterial sheath, often results in no fluid as the vein collapses on the end of the sheath. This is not of concern if sheath placement was otherwise uneventful and it freely flushes. No special flush solution or cocktail is needed in the venous sheath. Unlike the arterial system, spasm is not usually a problem. Veins are not responsive to calcium channel blockers [15]. Nitrates do induce venodilation and can be used either via the sheath or topically if spasm presents itself. As maximal vasodilation occurs at 42°C [16], local application of heat can also be used for spasm. Likewise, cold flush can induce spasm and should be avoided.
Passage of catheters to right heart & central venous system
Routine procedure
The intravenous vascular sheath now provides a gateway to the central system. To pass a right heart catheter, be it a thermodilution or monitoring catheter, is usually very simple. Catheter passage should be smooth and without resistance. Balloon tips should not be fully inflated until the device enters the larger portion of the venous system such as the subclavian and certainly never in the cephalic system. Catheters passing up the basilic system can at times be brought all of the way to the pulmonary wedge position just under hemodynamic guidance. In general, positioning the x-ray system over the shoulder should be done to localize whether the catheter is passing up the basilic or cephalic vein. Those catheters in the cephalic may need some manipulation at the T-junction with the axillary in order to be positioned in the correct direction for successful central venous entry. An appropriately sized hydrophilic or otherwise similar soft-tipped wire passed up the central lumen of the catheter, or asking the patient to take a deep breath, may at times be necessary to facilitate the catheter’s passage through a particularly awkward junction.
Troubleshooting
If passage from the peripheral entry site to the central vein is problematic, re-evaluation is in order. Force should never be applied, as the venous walls are easy to damage. Rather, the situation is often best assessed with a limited venogram to define the anatomy. Venography may at times demonstrate a normal vein with only a normal venous valve obstructing passage. This is easily remedied with either a bolus of fluid to open the valve, using a soft wire passed through the catheter, or minimally adding air to the catheter’s balloon to redirect the catheter towards the lumen center and through the valve.
Other times, venography demonstrates complex collaterals from prior trauma that was not recognized in the precatheterization history. These challenges may be met by either using an alternative vein (other arm), or by attempting to navigate the collaterals and around thrombus. Enthusiasm for such adventures needs to be tempered with the understanding that veins are relatively thin walled and excessive pressure will cause perforation. Unlike arterial perforation in the radial artery that can be managed with wire passage through the lumen and tamponade from within the artery, venous perforation usually appears as a tear and salvage of the vein for continued procedural use is difficult. Unless a wire is already in place beyond the perforation, these cases are best dealt with by finding an alternative venous site and placing a pressure dressing at the site of perforation.
Catheters without end holes such as temporary pacing catheters can also be passed up peripheral veins to central system [17]. Without a central lumen, one is unable to take venograms from the tip and it is not possible to pass a wire through the lumen. On the other hand, balloon tips common on these catheters can be slightly inflated and therefore allow relatively atraumatic passage analogous to balloon-assisted tracking [18]. Another option to guide passage is to instill a small volume of contrast into the side arm of the vascular entry sheath and this will outline the course of the vein. The catheter can then trace this route under fluoroscopy to the central system.
Finishing the procedure & hemostasis
At the conclusion of the procedure, venous sheath removal is similar to any large intravenous removal in the peripheral system. Prior to removal of balloontipped catheters, the balloon needs to be deflated so as to not traumatize the vein on disengagement. The vascular sheath can then be removed and a pressure dressing appropriate for venous closure applied. There is no need for special hemostatic devices as used in the radial artery closures.
There has been limited experience with allowing peripheral lines to the central system to remain for further therapy after the cardiac catheterization. Devices such as peripherally inserted central catheter lines have been purposely left dwelling in the peripheral veins while providing central access for extended periods of time. This experience must be balanced against the known hazard of venous thrombosis from a relatively large catheter in small veins. While removal is probably preferable, lines can remain if needed on an as short as possible basis if the risk/benefit appears clinically reasonable. For example, one might consider maintaining a temporary pacemaker placed for high-grade heart block during an acute inferior wall myocardial infarction that is resolving, but the operators would feel better knowing that back-up pacing was available, at least for a few more hours.
Review of published data
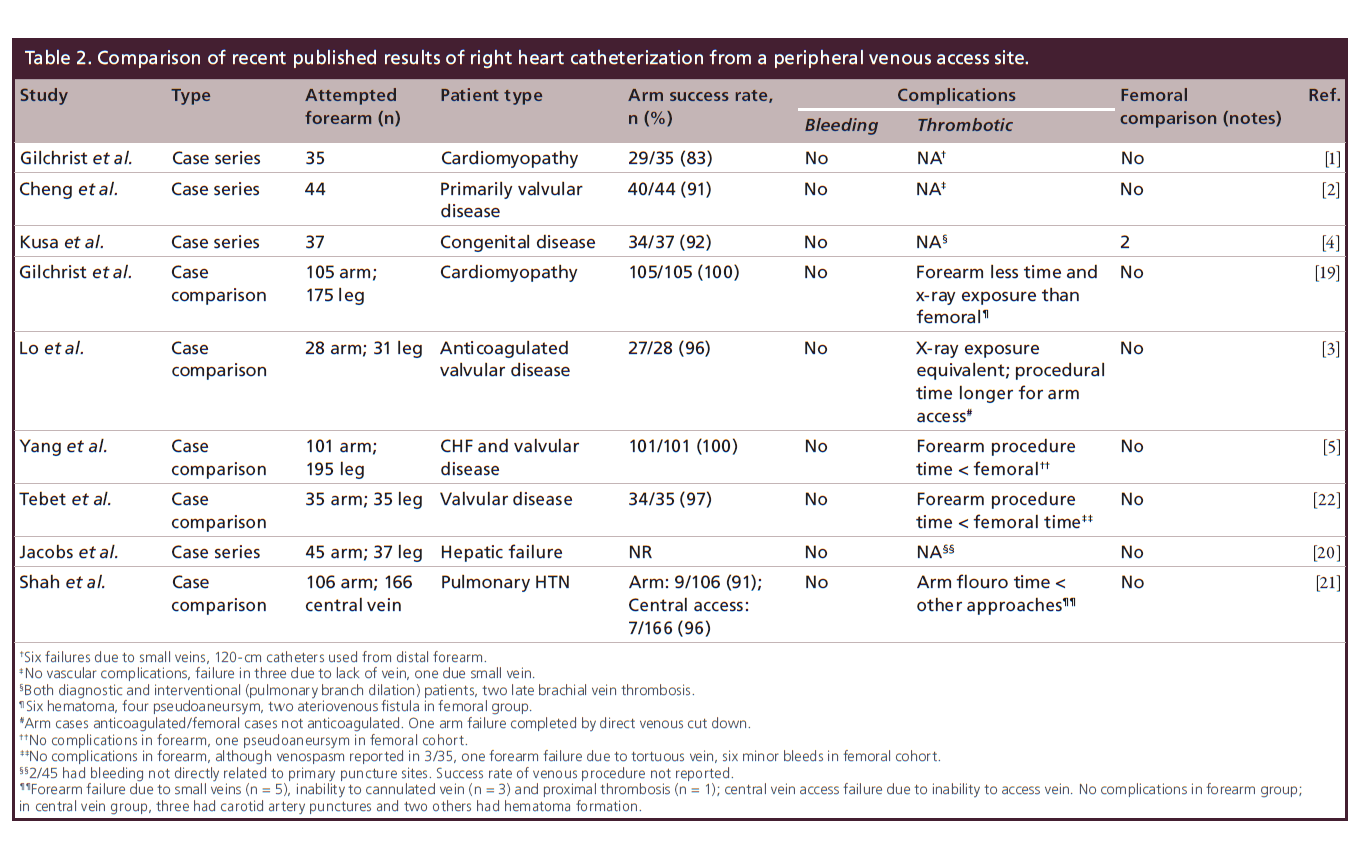
Several groups [1–5,19–22] have published their results using a forearm approach for venous access to the central venous system. Each has presented different patient populations, but the results are remarkably similar, as demonstrated in Table 2. While publication bias is clearly possible, each experience is positive for the forearm approach. Complications reported are minimal and comparisons to traditional femoral or deep neck veins are favorable. This is especially notable since many of the populations reported are not low risk patients.
While randomized data are not available, the comparisons in the published series are very favorable for the peripheral approach to the central system. There are indications that forearm access to the central system is similar in procedural time to that of other central vein approaches and some reports suggest less time. Radiation exposure can also be minimized as passage to the pulmonary artery can at times be done with little or no exposure compared with that required for femoral vein approaches.
Part of the published experience represents a learning process and may not reflect the best results that can be obtained today. For instance, the experience published by Lo et al. [3] was promising for forearm access in anticoagulated patients, but showed extra procedural time in this subgroup compared with the traditional femoral vein approach. On closer inspection, venous access was obtained in the catheterization laboratory versus allowing preprocedural staff to obtain a venous access site prior to entering the laboratory. This group changed its practice after publication and the time difference resolved [Nolan J, Pers. Comm.]. Another example involves the changing use of technology. Specifically, ultrasound that was not routinely used in the early published experiences. Vascular ultrasound permits use of both superficial and deep veins in the forearm. With ultrasound, the ability to establish access is markedly improved to the point that even right ventricular biopsies can be reliably performed using large basilic and brachial veins.
Peripheral access of the central venous system has expanded the use for right heart procedures. No longer is the level of anticoagulation a deterrent or delay for right heart catheterization. Patients on complex live support devices and therapies with conflicting noninvasive findings can have their central hemodynamics checked easily by a peripheral passage of a catheter rather than risk the hazards of central puncture. Passage of the catheters down from the upper venous system into a failing heart with insufficient valves and elevated filling pressures is often much easier than manipulating a similar catheter from the femoral vein.
Given the relatively low priority for studying the science of right heart venous access, the extent of formal publications of further series comparing transfemoral/ deep neck veins versus forearm access is probably limited. Advances in access techniques will need to be implied from advances to the technique as described in case reports and series. The risk of death from right heart catheterization has been estimated at 1–2:10,000 [23,24], powering a trial to show mortality advantage from the peripheral access site will not be feasible. Nevertheless, the anatomical unlikelihood of a retroperitoneal hemorrhage, pneumothorax or carotid puncture, to note but a few complications from direct central venous access, continues to offer an obvious advantage for peripheral venous access for central cardiac catheterization when such a procedure is indicated.
Conclusion
Invasive peripheral vein access to the central venous system is an old technique that has been reinvigorated by technological improvements in catheter technology and the demand for safer invasive procedures. The anatomy of the arm provides a route to the central venous system without the potential access comorbidity associated with the direct puncture techniques. Access and passage of right heart catheters is straightforward and easier than transradial arterial procedures. A variety of other technologies can also be applied using similar principles, although each device may have nuances and limitations that need to be considered before attempting use from a peripheral arm vein.
Experience to date has been very promising, without reports of thrombosis or loss of long-term venous access analogous to the issues raised with radial artery occlusion, nor have there been reports of infection. There has not been a truly randomized study against other vascular approaches and an adequately powered comparison is realistically unlikely. Nevertheless, there are logical advantages to avoidance of the anatomical hazards of direct central vein access approaches. As hemorrhagic complications are not expected, the peripheral approach to central access potentially extends the patient subsets that can safely undergo invasive access to the central venous system. Whether these peripherally placed central lines can be used for longer-term monitoring or therapy such as temporary pacing remains a question to be answered.
Future perspective
The societal pressures for safer and better procedures will continue to push evolution in cardiovascular medicine. Migration to either safer, alternative, noninvasive technologies or safer, invasive procedures will continue in the future. With further refinement of established techniques such as coronary stenting with small equipment and catheters, the peripheral access of the arm will continue to grow and become the default entry site. As new percutaneous procedures such as valve implants and repair are developed, they will need the large bore access that the femoral vasculature can provide. Even when femoral access is used, the forearm may provide adjunctive access to assist in closure of the femoral sites to facilitate femoral vascular closure, provide back-up pacing capacity, or provide a rapid method to monitor central pressures. Some of the barriers for using the forearm for both invasive arterial and venous procedures has been the lack of enthusiasm by industry to invest in products with shaft lengths long enough to operate from the peripheral locations. Many countries across Europe and Asia have seen their forearm cardiac procedures surpass numerically their femoral procedures. Now that there is a rapid penetration in the US market, capitalism will most likely drive industry to follow the lead of the innovative vascular physicians and develop products for use from the peripheral venous system into the central venous system. These products will need to include both diagnosis catheters and interventional tools for right-sided valve disease, structural heart disease, congenital heart disease and thromboembolic disease.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Historical perspective
• Development of transradial arterial access.
• Advent of invasive peripheral venous access for right heart catheterization.
• Is right heart catheterization still needed?
Anatomy of invasive peripheral venous access for right heart catheterization
• Venous forearm anatomy defined by a lateral cephalic and medial basilic system.
• Antecubital region allows variable cross communication between venous systems.
• Cephalic vein drains up the lateral aspects of the arm.
• Basilic vein drains up medial aspects of the arm joining deeper brachial veins.
• Acquired forearm venous anatomy may exist from prior trauma or thrombosis.
Practical approach to invasive peripheral venous access
• Equipment is similar to that needed for arterial access.
• Initial access technique is most efficient if started before entering catheterization laboratory.
• Placement of venous vascular sheath uses standard vascular techniques.
Passage of catheters to right heart & central venous system
• Routine procedure should be free of resistance and uneventful.
• Troubleshooting if deviation from routine should occur early to avoid trauma.
• Finishing the procedure and hemostasis.
Review of published data
• Diverse set of case series and comparisons.
• High success rate; few complications reported.
• No randomized data against legacy approaches.
• Need for equipment modifications to optimize potential of technique.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- Gilchrist IC, Kharabsheh S, Nickolaus MJ, Reddy R. Radial approach to right heart catheterization: early experience with a promising technique. Cathet. Cardiovasc. Interv. 55, 20–22 (2002).
- Cheng NJ, Ho WC, Ko YH et al. Percutaneous cardiac catheterization combining direct venipuncture of superficial forearm veins and transradial arterial approach – a feasible approach. Acta Cardiol. Sin. 19, 159–164 (2003).
- Lo TSN, Buch AN, Hall IR, Hildick-Smith DJ, Nolan J. Percutaneous left and right heart catheterization in fully anticoagulated patients utilizing the radial artery and forearm vein: a two-center experience. J. Intervent. Cardiol. 19, 258–263 (2006).
- Kusa J, Fernández RB, Mejía SM et al. [Brachial percutaneous venous access. Its usefulness in the diagnostic and interventionist catheterism of complex cardiopathies]. Arch. Cardiol. Mex. 74(4), 271–275 (2004).
- Yang C-H, Guo B-F, Yip H-K et al. Bilateral cardiac catheterization: the safety and feasibility of a superficial forearm venous and transradial arterial approach. Int. Heart J. 47, 21–27 (2006).
- Patel MR, Bailey SR, Bonow RO et al. ACCF/SCAI/AATS/ AHA/ASE/ASNC/HFSA/HRS/SCCM/SCCT/SCMR/STS. 2012 Appropriate use criteria for diagnostic catheterization. J. Am. Coll. Cardiol. 59(22), 1–33 (2012).
- Chun HJ, Byun JY, Yoo S-S et al. Tourniquet application to facilitate axillary venous access in percutaneous central venous catheterization. Radiology 226, 918–920 (2003).
- Mikuni Y, Chiba S, Tonosaki Y. Topographical anatomy of superficial veins, cutaneous nerves, and arteries at venipuncture sites in the cubital fossa. Anat. Sci. Int. 88, 46–57 (2013).
- Kim DI, Han SH. Venous variations in neck region: cephalic vein. IJAV 3, 208–210 (2010).
- Singh SP, Ekandem GJ, Bose S. A study of the superficial veins of the cubital fossa in Nigerian subjects. Acta. Anat. 114, 317–320 (1982).
- Yang HJ, Gil Y-C, Jin J-D, Cho H, Kim H, Lee H-Y. Novel findings of the anatomy and variations of the axillary vein and its tributaries. Clin. Anat. 25, 893–902 (2012).
- Deftereos S, Giannopoulos G, Raisakis K et al. Moderate procedural sedation and opioid analgesia during transradial coronary interventions to prevent spasm: a prospective randomized study. JACC Cardiovasc. Interv. 6(3), 267–273 (2013).
- Pancholy SB, Sweeney J. A technique to access difficult to find upper extremity veins for right heart catheterization: the levogram technique. Catheter. Cardiovasc. Interv. 78(5), 809–812 (2011).
- O’Grady NP, Alexander M, Burns LA et al. Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 52(9), e162–e193 (2011).
- Low RI, Takeda P, Mason DT, DeMaria AN. The effects of calcium channel blocking agents on cardiovascular function. Am. J. Cardiol. 49, 547–553 (1982).
- Taylor WF, Johnson JM, O’Leary DS, Park MK. Effect of high local temperature on reflex cutaneous vasodilatation. J. Appl. Physiol. 57, 191–196 (1984).
- Deftereos S, Giannopoulos G, Raisakis K et al. Feasibility of peripheral venous access for temporary right ventricular pacing. Hellenic J. Cardiol. 53, 340–342 (2012).
- Patel T, Shah S, Pancholy S. Balloon-assisted tracking of a guide catheter through difficult radial anatomy: a technical report. Catheter. Cardiovasc. Interv. 81, e215–e218 (2013).
- Gilchrist IC, Moyer CD, Gascho JA. Trans-radial right and left heart catheterization: a comparison to traditional femoral approach. Catheter. Cardiovasc. Interv. 67, 585–588 (2006).
- Jacobs E, Singh V, Damluji A et al. Safety of transradial cardiac catheterization in patients with end-stage liver disease. Catheter. Cardiovasc. Interv. 83(3), 360–366 (2013).
- Shah S, Boyd G, Pyne CT et al. Right heart catheterization using antecubital venous access: feasibility, safety and adoption rate in a tertiary center. Catheter. Cardiovasc. Interv. doi:10.1002/ccd.25249(2013) (Epub ahead ofprint).
- Tebet MA, de Andrade PB, de Andrade MVA, Gentile M, de Mattos LAP, Labrunie A. [Safety and efficacy of transradial right and left heart catheterization compared with transfemoral approach: an initial experience]. Rev. Bras. Cardiol. Invas. 16(3), 317–321 (2008).
- Hoeper MM, Lee SH, Voswinckel R et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J. Am. Coll. Cardiol. 48, 2564–2552 (2006).
- Shah KB, Rao TLK, Laughlin S, El-Etr AA. A review of pulmonary artery catheterization in 6245 patients. Anesthesiology 61, 271–275 (1984).
• Early transradial experience of central venous access using veins down to the level of the wrist.
•• High-volume experience with procedures in fully anticoagulated patients without normalizing coagulation.
•• Early experience using arm veins to do complex right-sided heart procedures percutaneously.
•• An important guideline for acceptable infection control. Understanding recommendations helps define an access protocol compliant with these guidelines.
•• Comparison between procedures performed via peripheral forearm or femoral vein showing favorable results of peripheral access for procedure times, x-ray exposure and complications.
• Experience showing the feasibility of adoption of this technique in a single center.