Research Article - Clinical Investigation (2023) Volume 13, Issue 1
Preventing migraine by reducing nasal surface contaminants & restoring nasal mucosa integrity: Clinical efficacy of a new generation of polymeric osmotic treatment- MIG SPRAY
- Corresponding Author:
- Rémi Shrivastava
Research Scientist in Neurology, PhD, University of Clermont Auvergne, Department of Neurology-Neuro-Dol, BP 10448, F-63000, Clermont-Fd, France
E-mail: remi.s@naturveda.fr
Abstract
leading cause of years lived with disability worldwide. This is a multifactorial disease with complex pathology involving multiple external and internal triggering factors, nasal mucosa, brain meninges, vascular changes, and abundant secretion of vasoactive peptides and cytokines in the blood and on the nasal surface. A high concentration of GCRP neuropeptide and inflammatory cytokines such as IL-6, IL-12, TNF-α, and TGF-β is found on the nasal surface, generating strong nasal mucosa inflammation and damage.
Objective: Nasal mucosa is one of the key organs playing a role in releasing and accumulating migraine triggering factors, allowing their easy diffusion into the circulation, and generating migraine triggering threshold circulating concentrations. None of the currently available treatments are multi-target, efficient, and free of adverse effects. We conceived a medical device which not only removes continuously these factors from the nasal surface but also cleans and restores nasal mucosa integrity to keep circulating concentrations of these factors below migraine triggering threshold levels.
Methods: This trial included 125 patients having episodic migraine and diagnosed for more than a year for migraines and meeting the migraine criteria as defined in the International Classification of Headache Disorder (ICHD-3 :1.1). Baseline data were collected for 28 days before the start of the three-month treatment period. Patients were randomized in a 1:1 ratio to receive either MIG SPRAY or placebo for a period of 12 consecutive weeks. The primary end point was the mean change in the average number of migraine days per month, comparing the baseline 28-day pre-intervention period with 9 week to 12 week after the first dose of the trial regimen. Secondary endpoints were the percentage of patients with a reduction of at least 50% in the average number of migraine days per month and days of use of any acute headache medication per month. The HIT-6 and MIDAS scores were also evaluated vs. baseline between the two groups.
Results: Compared to baseline, a statistically significant mean reduction in MIG SPRAY vs placebo was observed for the number of migraine days per month, HIT-6 and MIDAS mean scores, without any adverse effect of change in systemic parameters.
Conclusions: MIG SPRAY is a new generation of highly effective polymeric migraine prevention treatment with the advantage of being multi-target, natural, mechanically acting, and totally safe.
Keywords
MIG SPRAY • Clinical trial • New generation • Polymeric treatment • Migraine prevention • CGRP • Nasal mucosaAbbreviations
NS: Nasal Mucosa, TG: Trigeminal Ganglion, CGRP: Calcitonin Gene Related Peptide, ICH: International Headache Society, CTRI: Clinical Trial Registry India, ICHD: International Classification of Headache Disorders, HIT: Headache Impact Test.Introduction
Migraine is a major global health issue that affects over 10% of the population (≈ 1 billion people globally) and is the second leading cause of years lived with disability worldwide [1]. Migraine symptoms interfere with normal day to day life, including family, education, work, and contributes to the development of comorbidities such as cardiovascular disease, depression and anxiety [2, 3].
Migraine attacks typically present several prominent symptoms, the most significant are recurrent headaches lasting 4 h to 72 h with moderate to severe pulsating pain, nausea and/or vomiting, and sensitivity to light or sound (photophobia and phonophobia, respectively) [4]. The past several decades have seen significant progress in understanding the pathophysiology of migraine, and it is now widely accepted that activation of the tri gemino vascular system and release of Calcitonin-Gene Related Peptide (CGRP), cytokines, interleukins and other pro inflammatory substances are critical elements [3, 5].
The trigemino vascular system is an essential element in the physiopathology of migraine. The trigeminal nerves are the fifth pair of cranial nerves that innervate the sensitivity of the face, the Nasal Mucosa (NM), the meninges (the envelope of the brain) and its blood vessels [6]. Its activation during a migraine attack leads to an important inflammatory response with the activation of immune system cells (macrophages, lymphocytes, mast cells, etc.), releasing multiple proinflammatory proteins (cytokines, interleukins, CGRP, etc.) [3]. These substances are massively released in the meninges and NM which is richly vascularized.
The nasal cavity in migraine plays an important role. It acts as a barrier between the body and the external environment. In the migraine patient, it is the first defense line against incoming environmental chemical molecules, allergens, heavy metals, pollution or other substances that may induce the onset of a migraine. Inflammation of the NM is a common condition that falls within the diagnostic criteria for migraine [7]. 76% of patients report suffering from nasal symptoms (congestion, irritation, pain) associated with airway obstruction [3, 8]. This inflammation activates the trigemino vascular system, leads to the release of pro inflammatory substances (CGRP, interleukins, cytokines, etc.) within the NM and participates in triggering the migraine attack [4]. Several studies have shown a high level of these pro inflammatory substances in the NM of migraine patients during and between migraine attacks [9-11]. Thus, in a chronic way, the NM of patients is inflamed, weakened and porous to the factors triggering the attacks.
Maintaining the integrity of the NM is a key factor in limiting the onset of migraine. For this, it is necessary to have a multifactorial action to create a favorable environment for the regeneration of the mucosal cells, to eliminate pro inflammatory substances such as CGRP, cytokines, interleukins, TNF-alpha and others and to protect the mucosa from external triggering factors.
Migraine management is based either on nonpharmacological approaches, such as suppressing triggering factors (if they are known), or pharmacological approaches, such as abortive (acute) treatment and/or preventive (prophylactic) treatment [12]. The following classes of medications are used for migraine prevention: antiepileptic drugs, antidepressants, beta blockers, calcium channel antagonists, serotonin antagonists, botulinum neurotoxins and NSAIDs [12]. A drug is chosen based on its efficacy, its safety profile and the presence of any comorbid conditions. Recently, antibodies directed against CGRP or its receptors have been developed [13]. The efficacy results are promising but because of the potential heavy side effects, this treatments are reserved for a very small minority of patients (<1%) who are refractory to all conventional treatments [4, 14].
All these therapies are symptomatic, have often insufficient efficacy and may have variable side effects [15]. Today, surprisingly, no treatment focuses on the nasal cavity, which is the gateway to many migraine triggers. There is also no multifactorial treatment capable of targeting simultaneously all the pro inflammatory proteins released on NM.
This multifactorial disease should be treated with a “multitarget” therapy which may eliminate or at least suppress most migraine triggering and maintenance factors simultaneously. Unfortunately, there is no such treatment yet discovered nor envisaged in the future, because no chemical or biological molecule can possess these diverse properties simultaneously. This is the reason why migraine treatment still represents a therapeutic challenge due to poor response and long-term side effects [3, 16].
The best hypothetical approach for a multifactorial treatment consists in protecting the NM against migraine triggering factors and simultaneously reducing the concentration of CGRP and other proinflammatory cytokines from the NM surface to minimize NM inflammation and to provide ideal conditions for NM repair [17, 18]. An intact NM should reduce systemic entry of these proteins into the circulation and probably help keep the circulating concentration of inflammatory cytokines below threshold level which triggers migraine attack. Migraine, being a chronic disease, such a treatment should act topically only on the surface of NM, without systemic absorption or cellular interactions to avoid any side-effects during regular long-term use. Such a multi-target and mechanical approach should help reduce intensity, frequency, duration of migraine attacks, and in turn the need for chemical treatments to improve quality of life of patients. As no single chemical entity can fulfill these multiple yet basic requirements, we conceived an osmotic polymeric liquid (MIG SPRAY) which forms a protective, resistant, non-irritant, osmotic, and absorbent film over the NM surface. MIG SPRAY was supplied as 15 ml nasal spray and its efficacy in migraine prevention was compared against a glycerol +water containing placebo.
Material and Methods
Clinical trial oversight
The trial was performed as a double-blind, randomized, placebo-controlled study by Mudra ClinCare, located at Awaskar Building 402107 Mumbai, India, certified to conduct clinical investigations on human subjects (N° UQ-2022122821 following ISO-14155 guideline) by Dr. S. Sadgune as overall clinical trial coordinator at Dnyaneshwari Clinic & Hospital, Department of Medicine, Mumbai, MS, India. The trial was registered under the number CTRI/2021/07/034627 in July 2021 and was approved by relevant ethics committees (Altezza Institutional Ethics Committee) and institutional review boards. The authors vouch for the conduct of the trial, adherence to the protocol, the accuracy and completeness of the data and analyses, and the reporting of adverse events. The trial complied with the International Conference on Harmonization Guidelines for Good Clinical Practice, the principles of the Declaration of Helsinki, and relevant national and local regulations. At the time of screening, participants signed consent forms. The trial sponsor, VITROBIO France; ISO 13485 certified), provided the trial medications, safety profile studies, instructions for use and storage.
Trial participants
The first patient was recruited in July 2021 and the last patient followed was in December 2021.
Inclusion criteria
The patients eligible to enter this study were males or females, aged between 18 years and 55 years. All patients had been diagnosed for more than a year for migraines and met the definition of the International Classification of Headache Disorder version 3 (ICHD-3:1.1). Twenty-eight days of observation before the start of the three-month treatment period was used to establish the baseline. Patients with less than five days of migraine or migraine attacks lasting less than four hours were not included in the study. Participants were asked to keep a migraine diary to evaluate migraines, study parameters and medication use. Patients, who used a new migraine treatment or were diagnosed with medication overuse headaches less than six months prior to the study, were not retained. The crisis treatment was authorized to the patient during the study period if they were already using it for more than six months. All other migraine treatments were prohibited during the study.
Exclusion criteria
The main non-inclusion criteria were allergies to salicylates and camphor or hypersensitivity to study medication, drug abuse or dependency, chronic psychiatric or systemic diseases, pregnant and breastfeeding women, and subjects taking antidepressants, neuroleptics, anxiolytics, or prophylactic treatment for migraine within three months before the start of the study. The exclusion criteria were based on safety concerns and to avoid biased results.
Trial end points
The primary end point was the mean change in the average number of migraine days per month between 9 week to 12 week, compared to the 28 day pre-intervention baseline period. Secondary end points were the average number of migraine days per month, between 1 week to 4 week and 5 week to 8 week, compared to baseline 28 day pre intervention period. Another key endpoint was the percentage of patients with a reduction of at least 50% average number of migraine days per month, and the mean change from the baseline in the average number of days during 9 week to 12 week when any acute migraine medication was used. A migraine day was defined as any day on which the patient had a migraine or probable migraine, when pain lasted for at least 4 consecutive hours and met criteria for migraine or probable migraine (subtype in which only one migraine criterion is absent), or a day when an acute migraine specific medication was used to treat a headache of any duration. Other secondary end points included the mean change in the score on the six-item Headache Impact Test (HIT-6). HIT-6 test was designed to provide a global measure of adverse headache impact. HIT-6: scores range between 36 to 78, where higher scores indicate a greater degree of headacherelated disability. Safety and side-effect profiles were evaluated according to reported adverse events, change in vital signs (systolic and diastolic blood pressure, pulse, body temperature, and respiratory rate), physical condition, cardiovascular parameters, respiratory, gastrointestinal, musculoskeletal and nervous system parameters.
Study design
This randomized, double-blind, placebo-controlled trial consisted of a screening visit, a 28 day preintervention period, and a 12 week intervention period, with a final evaluation week 12. Patients satisfying all the ICHD-3 inclusion criteria and none of the exclusion criteria were enrolled and and randomly allocated to a 1:1 ratio as per randomization schedule to receive MIG SPRAY or placebo. Randomization was performed by using SAS Version 9.1.3. The randomization schedule was generated with block randomization methodology. Patients were seen at five scheduled visits to protocol-specified evaluations: at screening, baseline, 4 week, 8 week, and 12 week, or at the time of early withdrawal from the trial. Patients who withdrew prematurely had final protocol-specified evaluations performed as soon as possible after withdrawal. Headache data (e.g., occurrence, duration, and pain severity; occurrence of photophobia, phonophobia, nausea, or vomiting; and any use of migraine medication) were captured daily through an individual headache diary.
Conception of an osmotic and stable nasal spray
The MIG SPRAY nasal contained glycerol as an osmotic solution which was rendered filmogen by incorporating glycerol binding polymers as described by Shrivastava et al [19, 20]. The technology used to conceive MIG SPRAY osmotic, contaminant trapping, stable, non-irritant polymeric film has already been described by Shrivastava et al [21, 22]. When applied on the NM, this polymeric film attracts hypotonic liquid from the NM tissue, thereby detaching and draining all NM surface contaminants towards the absorbent film where they can be trapped. The aim was to protect the NM from environmental migraine triggering factors and to create favourable conditions for NM reconstitution.
Test and comparator products
The Test Product (TP), designated as MIG SPRAY, commercialized in Europe as a medical device, was supplied by Vitrobio Pharma in France (ISO13485 certified) in a 15 ml plastic container fitted with a spray for nasal application ( ± 125 sprays: 120µl/ spray). The solution contained glycerol which was rendered filmogen by adding small quantities of Migcyanidin polymeric premix derived from Salix alba, Tanacetum parthenium, Curcuma longa, and Vitis vinifera; Acacia and Xanthan gums as thickeners; and traces of Eucalyptus oil, potassium sorbate, sodium benzoate, and citric acid, as stabilizers. The placebo spray was presented identical to MIG SPRAY with the exception that it contained only glycerol with a mixture of Acacia and Xanthan gums at the same concentrations as MIG SPRAY. The presentations of the two test products were blinded with a sticker and the sprays were used as 2 sprays to 3 sprays per nostril, 2 times to 3 times a day, during the entire study period.
Statistical
Estimations based on the observational trial of MIG SPRAY in episodic migraine (Shrivastava 2021) and the Cochrane systemic review to evaluate the efficacy of Tanacetum parthenium as a preventive treatment for migraine (Wider, Pittler, et Ernst 2015) predicted that a sample of 80 patients who had completed the trial and could be evaluated would provide 90% power to detect a means (± SD) difference of 1.4 ± 0.5 in the average number of migraine days per month. With an anticipated rate of discontinuation of 30%, 125 participants were planned for randomization in this trial. Analyses were conducted in the modified intention- to-treat population, which included all randomly assigned patients who received at least one dose of a trial product and had at least 10 days of post baseline efficacy assessments regarding the primary end point. Safety analyses included all randomly assigned patients who received at least one dose of a trial product. Demographic and baseline characteristics were summarized descriptively with a Student’s test for comparison between the two groups and Fisher’s exact test for analysis of contingencies. The primary efficacy outcome was analysed with two-way repeated measure ANOVA followed by the post hoc Bonferroni’s test. The mean change from the baseline with standard errors (± SD) is presented for each treatment group, and the difference versus placebo with 95% Confidence Interval (CI). For management of missing data in the primary analysis, the average number of headache days per month during the 12 week period was prorated to a 28 day equivalent with the use of all post baseline observations. The same analyses were used for relevant secondary end points. For the percentage of patients with a reduction of at least 50% and 30% in the average number of headache days per month, the Cochran Mantel Haenszel test was used. P<0.05 was considered statistically significant. Adverse events data are collected during the double-blind, placebo-controlled intervention period. The safety population included all the patients who underwent randomization and received at least one dose of a trial product. Statistical analysis was performed by Chi-square test for comparison of adverse events between the two groups. The analyses were carried out with the software GraphPad Prism version 8.4.2, (La Jolla, USA).
Results
Demographics
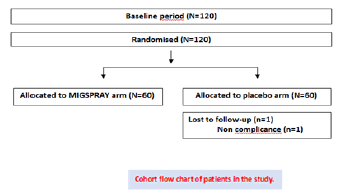
125 patients were included, after analysis of the migraine diary and eligibility, for the 28-day observation period. Patients were randomly assigned to either the MIG SPRAY group (n=60) or the placebo group (n=60). Only one patient was discontinued from the treatment and dropped-out from the study. Overall, 119 of 125 patients (95%) remained in the study until week 12. The demographic data shown in Cohort chart, indicated no significant difference between groups (Figure 1). A fisher’s exact test was performed for the malefemale proportions between groups. The population included all the patients who underwent randomization and received at least one dose of a trial regimen (n=125).
Mean change in average number of migraine days
The baseline characteristic of patients in both groups is shown in Table 1 A and 1B. Statistical analysis was performed by paired two-tailed. Mean migraine days at baseline (during the 28 day screening period) was 6.2 ± 1.7 in MIG SPRAY group and 5.8 ± 1.8 in the placebo group. During 9 week to 12 week, mean of migraine days was 4.8 ± 2.0 and 5.4 ± 2.1, respectively. MIG SPRAY demonstrated statistically significant reduction from the baseline in the frequency of migraine days during 9 week to 12 week compared to placebo: -1.1 ± 0.3 [95% Confidence Interval (CI) -1.6 to -0.5], p<0.001 vs placebo) (Table 2). Mean results of primary and secondary parameters ± SD for the MIG SPRAY and Placebo groups. Statistical analysis was performed by paired two-tailed Student’s test for comparisons with baseline within the same group (¤p<0.05; ¤¤p<0.01; ¤¤¤p<0.001). A two-way ANOVA followed by the post hoc Bonferroni’s test for the difference versus placebo (*p<0.05; **p<0.01; ***p<0.001). Student’s test to compare the means at the baseline between the two groups.
Table 1 A: Baseline characteristics of the patients, according to trial group.
| Characteristic | MIGSPRAY (n=60) | Placebo (n=60) | P-value |
|---|---|---|---|
| Male, n (%) | 35 (29.2) | 34 (28.3) | 0.92 |
| Female, n (%) | 25 (20.8) | 26 (21.7) | 0.93 |
| Mean age, years | 33.5 ± 7.1 | 36.0 ± 6.2 | 0.47 |
| Mean weight, kg | 51.63 ± 7.9 | 54.15 ± 6.6 | 0.84 |
| Mean height, cm | 159.89 ± 7.6 | 162 ± 7.3 | 0.77 |
| Mean no. of migraine days at baseline | 6.2 ± 1.7 | 5.8 ± 1.8 | 0.21 |
| >Mean days of use of any acute headache medication per month at baseline | 4.8 ± 2.1 | 5.1 ± 1.1 | 0.52 |
Table 1 B: Quantitative parameters are presented as mean ± SD.
| Mean HIT-6 score at baseline | 65.1 ± 6.9 | 67.5 ± 6.3 | 0.09 |
|---|---|---|---|
| Mean MIDAS score at baseline (days; grade) | 16.8 ± 5.0 (III) | 17.7 ± 4.5 (III) | 0.44 |
Table 2: Primary and secondary endpoints
| MIG SPRAY (n=60) | Placebo (n=60) | |
|---|---|---|
| Primary end point | ||
| Average no. of migraine days per month | ||
| Mean value, 9 week to 12 week | 4.8 ± 2.0 | 5.4 ± 2.1 |
| Mean change from baseline, 9 week to 12 week | -1.4 ± 0.4 ¤¤¤ | -0.4 ± 0.4 |
| Difference vs. placebo | -1.1 ± 0.3 [-1.6 to -0.5] *** | |
| Secondary end points | ||
| Average no. of migraine days per month | ||
| Mean value, 1 week to 4 week | 5.7 ± 2.0 | 5.5 ± 2.1 |
| Mean change from baseline, 1 week to 4 week | -0.5 ± 0.3 | -0.3 ± 0.4 |
| Difference vs. placebo | -0.2 ± 0.2 [-0.7 to -0.2] | |
| Mean value, 5 week to 8 week | 4.9 ± 1.8 | 5.4 ± 2.1 |
| Mean change from baseline, 5 week to 8 week | -1.3 ± 0.3 ¤¤¤ | -0.5 ± 0.4 |
| Difference vs. placebo | -0.8 ± 0.6 [-1.5 to -0.2] ** | |
| ≥ 50% Reduction in average no. of headache days per month at 9 week to 12 week - no. of patients (%) | 14 (22.2) | 5 (8.1) |
| Days of use of any acute headache medication per month | ||
| Mean change from baseline, 9 week to 12 week | -2.4 ± 0.2 ¤¤ | -0.9 ± 0.3 |
| Difference vs. placebo | -1.5 ± 0.3 *** | |
| HIT-6 score | ||
| Mean value, 9 week to 12 week | 56.8 ± 5.8 | 65.7 ± 7.1 |
| Mean change from baseline, 9 week to 12 week | -8.3 ± 1.2 ¤¤¤ | -1.8 ± 1.2 |
| Difference vs. placebo | -5.7 ± 1.1 [-7.8 to -3.5] *** | |
| MIDAS score | ||
| Mean value, 9 week to 12 week | 10.15 ± 3.6 | 15.5 ± 4.7 |
| Mean change from baseline, 9 week to 12 week | -6.6 ± 0.8 ¤¤¤ | -2.1 ± 0.8 ¤ |
| Difference vs. placebo | -5.4 ± 0.8 [-6.7 to -2.4] *** | |
Secondary end points
The results of secondary endpoints as shown in Table 2 indicate that the 1st 4 four weeks of both treatments do not show any significant difference in number of migraine days between the MIG SPRAY and the placebo group. However, during the 5 week to 8 week, MIG SPRAY demonstrated statistically significant reduction from baseline in the frequency of migraine days compared to placebo -0.8 ± 0.6 [-1.5 to -0.2] , p=0.0058 vs placebo), similarly to 9 week to 12 week. These findings show that both treatments need a certain delay to exert effects, the changes are progressive with time, and that MIG SPRAY is much more active compared to the placebo treatment. The analysis of secondary endpoints showed that 22.2% of patients in the MIG SPRAY group experienced a reduction of at least 50% in the number of migraine days per month during 9 week to 12 week, compared to 8.1% in the placebo group (difference from placebo [95%CI] of 14.1% [-9.9% to 22.1%; p<0.001]).
Effect on crisis treatment
The use of crisis treatments per day was also analysed during the 9 week to 12 week and compared to placebo. It revealed a decrease of -2.4 days ± 0.2 days for the MIG SPRAY group and -0.9 days ± 0.3 days for the placebo group, with a difference between both groups of -1.5 ± 0.3 [95% (CI) -1.9 to -0.87] p<0.001.
Change in HIT-6
The HIT-6 scores indicated a decrease of -8.3 ± 1.2 points in the MIG SPRAY group and -1.8 ± 1.2 points in the placebo group compared to the baseline. The difference between both groups is -5.7 ± 1.1 points [95% (CI) -7.8 to -3.5] p<0.001 (Table 3) The % of patients in MIG SPRAY and placebo groups having any of the side effects listed in the table, during the study period. The results include all the patients who underwent randomization and received at least one dose of a trial regimen. Statistical analysis was performed by Chi-square test for comparison of adverse events between the two groups. “ns”: not significant.
Table 3: Adverse Events.
| MIGSPRAY | Placebo | p-value | |
|---|---|---|---|
| Sneezing | 3% | 0% | ns |
| Headache | 2% | 2% | ns |
| Dizziness | 2% | 3% | ns |
| Nausea | 2% | 2% | ns |
| Heartburn | 0% | 2% | ns |
| Tingling sensation in throat | 1% | 0% | ns |
| Irritation in throat | 3% | 3% | ns |
| Dryness in nose | 2% | 2% | ns |
| Drowsiness | 0% | 1% | ns |
| Runny nose | 1% | 5% | ns |
| Tingling sensation in nose | 2% | 2% | ns |
Safety and adverse events
A total of 119 patients received the entire treatment of MIGSPRAY/ Placebo, only 1 patient was discontinued from the treatment and dropped-out from the study. No serious side effects were reported in any of the groups. Among the minor side effects, there was no significant difference between the two groups. These data confirm the safety of the treatment. The mean results of secondary health parameters in MIG SPRAY and Placebo group patients who underwent randomization and received at least one dose of a trial regimen. SBP: Systolic Blood Pressure (mmHg); DBP: Diastolic Blood Pressure (mmHg); PR: Pulse Rate (beat/min); RR: Respiratory Rate (breath/ min), T°: Axillary body temperature (Celsius). Statistical analysis was performed by repeated two-way ANOVA for comparison between MIG SPRAY and placebo at Baseline (BL) and after 12 weeks of treatment.“ns”:notsignificant. Additional physical examination did not reveal any change of the vital signs or other health parameters in MIG SPRAY-treated or placebo-treated patients through out the trial period or after 12 weeks of treatment (Table 4).
Table 4: Health parameters.
| MIG SPRAY | Placebo | p-value | ||
|---|---|---|---|---|
| SBP | BL | 121.2 ± 3.1 | 121.2 ± 2.4 | ns |
| After 12 weeks | 121.5 ± 2.4 | 121.5 ± 2.7 | ns | |
| DBP | BL | 81.1 ± 3.2 | 81.2 ± 3.2 | ns |
| After 12 weeks | 81.5 ± 2.4 | 81.3 ± 2.2 | ns | |
| PR | BL | 76.2 ± 5.2 | 76.8 ± 5.5 | ns |
| After 12 weeks | 77.2 ± 6.0 | 78.7 ± 4.9 | ns | |
| RR | BL | 15.4 ± 2.3 | 15.7 ± 2.1 | ns |
| After 12 weeks | 15.5 ± 2.2 | 15.9 ± 1.9 | ns | |
| TC | BL | 36.8 ± 0.2 | 36.9 ± 0.2 | ns |
| After 12 weeks | 36.9 ± 0.2 | 36.9 ± 0.3 | ns | |
Discussion
Migraine is one of the oldest diseases in the world affecting above 11% of the world population with enormous socioeconomic impact and burden on healthcare systems, representing the 6th cause of disability worldwide [23]. Treatment for migraine has advanced over the years and our arsenal of migraine therapy has never been broader with the development of new Gepants or monoclonal antibodies, along with new and improved deliveries of current therapies, with or without non-drug options. Still after the availability of profusion of therapies, hardly one third of migraine patients are satisfied with current therapeutic options, proving that there is no effective treatment to cure or even to prevent this common disease [24]. Now it is well established that migraine can be triggered by various natural or environmental conditions which cause hypoxia or vasodilation in the nasal cavity, such as nasal congestion, exposure to nitric oxide (called sinus hypoxic nitric oxide theory of migraine), to volatile vasodilator gases, pollution particles, sinusitis, weather changes, and low barometric pressure [25, 26]. These observations reflect that the NM could be the primary site that initiates migraine through repetitive or intermittent activation of trigeminal sensory nerves and blood vessels in the NM. Even if migraine trigger is systemic or central, the activation of the trigemino vascular system produces CGRP and other pro inflammatory cytokines which are then released in the blood circulation and leak on the NM through damaged NM and vessels. The nasal events lead to massive accumulation of these peptides and cytokines on the nasal surface which follows severe NM inflammation, further cellular damage, formation of gaps on the surface of the NM, increased vascular permeability, and free exchange of migraine triggering factors between NM and body circulation. The major role played by CGRP in migraine and its accumulation on the NM surface has diverted current pharmaceutical research to directly target nasal cavity for drug delivery, either to block CGRP or CGRP release, to reduce circulating concentration of CGRP and consequently, migraine trigger or intensity [27]. The NM of migraine patients is chronically inflamed and damaged, as frequent migraine attacks do not allow sufficient time for its repair. Thus, exposure to any new migraine triggering factors nearly instantly favors a new attack. Although CGRP plays a central role in migraine, one should not forget that CGRP is accompanied by multiple other pro inflammatory cytokines such as IL-6, IL-12, TNF-α, and TGF-β, responsible for chronic NM inflammation and damage [28]. Regrettably, none of the current or future treatments are directed to suppress at least the nasal pathological events implicated in migraine and they have multiple undesired effects [29]. This is quite comprehensible because an effective treatment should not only remove nasal surface CGRP continuously but should simultaneously protect the NM from new migraine triggering environmental factors, clean other pro inflammatory cytokines, stop NM inflammation to restore its integrity, while being totally safe.
To fulfil these multi faced yet essential requirements directed to minimize at least the frequency, intensity, and duration of migraine attacks to improve the quality of life of patients, we envisaged conceiving a stable, non-irritant, and osmotic nasal barrier (MIG SPRAY) capable of keeping the NM always clean and healthy, through its exclusively mechanical mode of action.
In this study, MIG SPRAY showed a significant success in preventing migraine compared to the placebo across the primary clinical outcome. The beneficial effects of MIG SPRAY on average number of migraine days reduction starts appearing between 1 week to 4 week but the reduction was not significant compared to 5 week to 8 week and up to 9 week to 12 week, where a significant decrease vs baseline was observed (-1.4 days vs baseline, p<0.001). These findings correspond with significantly more patients getting at least 50% reduction in the average number of migraine days per month in this group vs placebo (MIG SPRAY 22.2% and placebo 8.1%; p<0.001). The degree of headache-related disability decreased between baseline and 9 weeks-12 weeks, with a reduction in HIT-6 score with MIG SPRAY (by 8.93 points ± 1.2 points) than with placebo (by 1.58 points ± 1.2 points, p ≤ 0.001 for comparison with placebo). Minimally Important Change (MIC) and Minimally Important Difference (MID) for HIT-6 are defined at -2.5 points to -6 points for MIC and -1.5 points for MID [30]. Although no MIC has been established for MIDAS, a preliminary analysis based on 25% change in monthly headache days estimated that an increase or decrease of 5 days of migraine-related disability per 3 months, represents a significant change [31]. In this study, MIDAS score reduction was -6.6 days ± 0.8 days for MIG SPRAY and -2.1 days ± 0.8 days in placebo group (p<0.001 for comparison with placebo).
Currently, propranolol is the most common preventive treatment for migraine [32]. Studies have shown that it effectively reduces the number of attacks per month by 1.3 (-2.0 to -0.62) days after 12 weeks of treatment versus placebo while topiramate, another common preventive treatment, reduces the amount of migraine days versus placebo per month by 0.9 (-1.3 to -0.39) [33]. The results with MIG SPRAY show a mean reduction of 1.1 (-1.6 to -0.5) days at 9 week to 12 week which is on the same trend compared to the efficacy of propranolol and topiramates. However, propranolols is a beta-blocker and topiramate a Ca++ channel blocker, used for cardiac conditions and are not migraine specific. Recently, antibodies directed against CGRP or its receptor have shown better results in migraine treatment and prevention compared to propranolol and topiramates, but their long-term side effects are not yet established. CGRP blocking drugs are very effective to prevent or to treat migraine but we should not forget that CGRP and its receptor are abundantly present in both the vasculature, in the peripheral and central nervous system, and are involved in several physiological processes. Being strong vasodilators, they are essential in oxygenation and cellular survival in accidental hypoxic conditions. Hence, blocking CGRP may pose a risk in subjects with comorbidities such as cerebral and cardiovasculardiseases. Therefore, ICH authorize the use of all the CGRP monoclonal antibodies only for chronic migraine patients and only for those patients who are refractory to all conventional treatments [34, 35]. Being highly expensive drugs with unknown long-term side-effects, the use of CGRP antagonists for migraine prevention seems compromised.
In fact, migraine patients will be more than happy if they can find an easily applicable, and safe preventive treatment which can help to reduce migraine days, frequency &/or duration, which may allow them to live a better and fearless life. MIG SPRAY represents a yet unmet need in the field of migraine prevention [36]. The mode of action of MIG SPRAY polymeric osmotic film is mechanical and multi-target. Being filmogen, MIG SPRAY film remains on the NM for a period of 4 h to 6 h after each application. Firstly, the film forms a protective barrier against migraine triggering factors entering the body during respiration thereby reducing the chances of a new migraine attack. Secondly, it cleans the nasal surface by generating a continuous osmotic flow of hypotonic liquid from inside towards outside the NM, thus detaching and draining mechanically all the free-floating CGRP and other inflammatory cytokines toward the film, where they are blocked. This mechanical cleaning of CGRP, minimizes its reabsorption into the systemic circulation and consequently, its migraine triggering threshold circulating concentration. Thirdly, constant removal of newly released inflammatory cytokines on the NM should help reduce NM inflammation. Lastly, in the absence of contaminants and inflammation, broken NM cells can grow, restore normal defensive functions, and minimize entry of undesired molecules into the systemic circulation. In addition, MIG SPRAY, due to its totally topical mode of action on the nasal surface without any pharmacological, receptor, immunological, or metabolic effects and without being absorbed in the body represents no risk of side-effects even after long term use.
Conclusion
Migraine is the second most invalidating pathology in the world and there is still no specific prophylactic treatment for migraine. The absence of treatment relates to the complexity of the disease pathology involving multiple factors and impossibility to find a multi-target drug acting on multiple disease factors simultaneously. Recent research proves that CGRP and inflammatory cytokines have a key role in the disease process to trigger and to maintain the disease. Large quantities of these factors are released on the NM which is the most fragile, vascularized, and permeable organ in the body. We conceived MIG SPRAY as a safe device to collect and remove these factors on the NM thereby indirectly reducing their circulating concentrations and preventing the disease trigger.
The clinical results presented in this article show excellent efficacy and total safety of MIG SPRAY in preventing migraine and presents a great hope for migraine patients to live a better life. The product is now registered as a medical device in France
Acknowledgements
The design and conduct of the trial and data analysis were supported by a grant from VITROBIO & NATURVEDA SAS (ZAC de Lavaur 63500 Issoire, France), the inventor of MIG SPRAY. We thank the patients who participated in this trial and their families; all investigators, site personnel and coordinating investigators. We also strongly thank Dr. Xavier MOISSET (CHU Clermont-Ferrand, France) for his precious help in writing the article and Dr. Christelle GREMEAU-RICHARD (Neuro-dol INSERM 1107, France) for her corrections to the protocol.References
- James SL, Degu Abate, Kalkidan HA, et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. The Lancet. 392(10159):1789‑858(2018). [Google Scholar] [Crossref]
- Ducros, A. Migraine. EMC Neurol. 29(1):1‑15(2006). [Google Scholar] [Crossref]
- Goadsby PJ, Holland PR, Martins OM, et al. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev. 97(2):553-622(2017). [Google Scholar] [Crossref]
- Ashina M. Migraine. N Engl J Med. 383(19):1866‑76(2020). [Google Scholar] [Crossref]
- Han D. Association of Serum Levels of Calcitonin Gene-Related Peptide and Cytokines during Migraine Attacks. Ann Indian Acad Neurol. 22(3):277(2019). [Google Scholar] [Crossref]
- Dallel R. Advances in the Understanding and Treatment of Pain and Headache. J Neural Transm. 127(4):389‑92(2020). [Google Scholar] [Crossref]
- Barbanti P, G Fabbrini, M Pesare, et al. Unilateral Cranial Autonomic Symptoms in Migraine. Cephalalgia: Int J Headache. 22(4):256‑59(2002). [Google Scholar] [Crossref]
- Arslan H, Hüseyin ET, Üzeyir Y, et al. Evaluation of the changes in the nasal cavity during the migraine attack. J craniofacial surg. 25(5):446‑49(2014). [Google Scholar] [Crossref]
- Aydın M, Caner FD, Adalet A, et al. Plasma Cytokine Levels in Migraineurs During and Outside of Attacks. Eur J Gen Med. 12(4):307-12(2015). [Google Scholar] [Crossref]
- Cernuda ME, D LC. Ramon J. et al. Interictal Increase of CGRP Levels in Peripheral Blood as a Biomarker for Chronic Migraine. Neurology. 81(14):1191‑96(2013). [Google Scholar] [Crossref]
- Yan J, Ohannes KM, Theodore JP, et al. Sensitization of Dural Afferents Underlies Migraine-Related Behavior Following Meningeal Application of Interleukin-6 (IL-6). MOL PAIN. 8(6)1-9(2012). [Google Scholar] [Crossref]
- Lanteri MM, D Valade, G. Géraud, et al. Prise en charge diagnostique et thérapeutique de la migraine chez l’adulte et chez l’enfant. Rev Neurol. 169(1):14‑29(2013). [Google Scholar] [Crossref]
- Diener HC, Stefanie F, Charly G, et al. Prevention of Migraine with Monoclonal Antibodies against CGRP or the CGRP Receptor: Addition to the S1 Guideline: Therapy of Migraine Attacks and Prevention of Migraine. Recommendations of the Germany Society of Neurology and the German Migraine and Headache Society. Neurol Res Pract. 2(1):11(2020). [Google Scholar] [Crossref]
- Ferrari Michel D, Hans CD, Xiaoping N, et al. Fremanezumab versus Placebo for Migraine Prevention in Patients with Documented Failure to up to Four Migraine Preventive Medication Classes (FOCUS): A Randomised, Double-Blind, Placebo-Controlled, Phase 3b Trial. The Lancet. 394(10203):1030‑40(2019). [Google Scholar] [Crossref]
- Géraud G. Les Céphalées en 30 leçons. Elsevier Masson. (2015) [Google Scholar] [Crossref]
- Becker WJ. Acute Migraine Treatment in Adults. Headache. 55(6):778‑93(2015). [Google Scholar] [Crossref]
- Martin V, John H, Sheena K, et al. Nasal Delivery of Acute Medications for Migraine: The Upper Versus Lower Nasal Space. J Clin Med. 10(11):2468(2021). [Google Scholar] [Crossref]
- Behairy EA, Tarek ElH, Khaled HA, et al. Evaluation of Nasal and Paranasal Findings in Cases of Migraine. Menoufia Med J. 32(4):1423(2019). [Google Scholar] [Crossref]
- R Shrivastava. Non-solid composition for local application. Int. publ. (2000). [Google Scholar] [Crossref]
- Shrivastava RM and Shrivastava R. A filmogen glycerol for topical application. Int pat. (2014). [Google Scholar] [Crossref]
- Shrivastava L, Schütte H, Malik P, et al. A New Class of Polymeric Anti-Allergen Nasal Barrier Film Solution for the Treatment of Allergic Rhinitis. J Allergy Ther. 8(3): 1000263(2017). [Google Scholar] [Crossref]
- Shrivastava R, Shrivastava R, Johansen B, et al. Allain T. Anti-Inflammatory and Antiviral Osmotic Polymeric Film to Treat Covid-19 Early-Stage Infection. J Inflamm Res 14:1195-206(2021). [Google Scholar] [Crossref]
- Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants. J Neurol Sci. 372:307-15(2017). [Google Scholar] [Crossref]
- Olesen A, Schytz HW, Ostrowski SR, et al. Low adherence to the guideline for the acute treatment of migraine. Sci Rep. 12:8487(2022). [Google Scholar] [Crossref]
- Rathnasiri Bandara SM. Paranasal sinus nitric oxide and migraine: a new hypothesis on the sino rhinogenic theory. Med Hypotheses. 80(4):329-40(2013). [Google Scholar] [Crossref]
- Marmura MJ. Triggers, Protectors, and Predictors in Episodic Migraine.Curr Pain Headache Rep. 22(12):81(2018). [Google Scholar] [Crossref]
- Abo El-Enin HA, Mostafa RE, Ahmed MF, et al. Assessment of Nasal-Brain-Targeting Efficiency of New Developed Mucoadhesive Emulsomes Encapsulating an Anti-Migraine Drug for Effective Treatment of One of the Major Psychiatric Disorders Symptoms. Pharmaceutics. 14(2):410(2022). [Google Scholar] [Crossref]
- Kawamura N, Tamura H, Obana S, et al. Differential effects of neuropeptides on cytokine production by mouse helper T cell subsets. Neuroimmunomodulation. 5(1-2):9-15(1998). [Google Scholar] [Crossref]
- Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 71(22):1821-28(2008). [Google Scholar] [Crossref]
- Smelt, Antonia FH, Caroline BT, et al. What Is a Clinically Relevant Change on the HIT-6 Questionnaire? An Estimation in a Primary-Care Population of Migraine Patients. Cephalalgia. 34(1):29‑36(2014). [Google Scholar] [Crossref]
- Lipton R, P Desai, S Sapra, et al.How much change in Headache-Related Disability is clinically meaningful? Estimating minimally important difference or change in MIDAS using data from rhe AMPP study. Boston Am Headache Soc Annu Meet. (2017) [Google Scholar] [Crossref]
- Silberstein, Stephen D. Preventive Treatment of Headaches. Curr Opin Neurol. 18(3):289‑92(2005). [Google Scholar] [Crossref]
- Jackson JL, Elizabeth C, Rafael SD, et al. A Comparative Effectiveness Meta-Analysis of Drugs for the Prophylaxis of Migraine Headache. PloS One. 10(7):e0130733(2015). [Google Scholar] [Crossref]
- Altamura C, Brunelli N, Marcosano M, et al. Gepants - A long way to cure: a narrative review. Neurol Sci. 43(9):5697-708(2022). [Google Scholar] [Crossref]
- Sandor PS, Andreas RG, Heiko Pohl, et al. Neurologie: Anticorps monoclonaux anti-CGRP: des «game changers» dans la prophylaxie de la migraine? Forum Méd Suisse. 19(0102):24‑25(2019). [Google Scholar] [Crossref]
- Ailani J, Burch RC, Robbins MS, et al. The American Headache Society Consensus Statement: Update on integrating new migraine treatments into clinical practice. Headache. 61(7):1021-39(2021). [Google Scholar] [Crossref]