Review Article - Interventional Cardiology (2012) Volume 4, Issue 5
Overview of platelet functional testing methods and their controversial role in the clopidogrel- treated patient
- Corresponding Author:
- Sandra A Weiss
Christiana Care Health Network
Newark, DE, USA
Tel: +1 302 366 1929
Fax: +1 302 366 1006
E-mail: Arina.Bingeliene@uhn.ca
Abstract
Keywords
antiplatelets, light transmission aggregometry, platelet reactivity testing, VerifyNow®
Platelets were first identified as distinct cellular blood components over 100 years ago, with contemporary platelet functional testing rooted in the 1960s. However, throughout most of the 20th century, platelet functional testing was unreliable, in part, because of the difficultly in simulating hemostasis in vitro and inhibiting artifactual activation with platelet manipulation. Although these problems have not been eliminated in modern practice, contemporary functional assays have become more adept at dealing with them.
There are numerous assays that measure platelet number and functionality. Of these, the appropriate test varies depending on the clinical situation and question at hand, such as the need to diagnose certain bleeding disorders, assessment of platelet-derived hemostasis and for monitoring response to antiplatelet medication. Regarding the latter, it is important to recognize that with the prevalent use of percutaneous interventions for coronary disease and the resulting necessary use of aspirin and P2Y12 inhibitors (e.g., clopidogrel, prasugrel and ticagrelor), appropriate response to these drugs proves critical in prevention of adverse outcomes. Highlighting this point, clopidogrel, the most widely used P2Y12 inhibitor, and clopidogrel response variability has garnered recent scientific attention, emerging as a remarkably prevalent and controversially treatable disorder associated with increased risk of ischemic cardiovascular events. It has been estimated that the prevalence of clopidogrel hyporesponse or nonresponse ranges between 15 and 40%, and varies over the time course of therapy with dual antiplatelet therapy [1]. Numerous studies have linked high levels of platelet aggregation while on treatment with clopidogrel with a significant increase in the risk of fatal and nonfatal ischemic events in timeframes ranging from 30 days to 2 years [2–15]. In addition, suboptimal platelet response to clopidogrel has been linked to stent thrombosis events, especially early in the post-percutaneous coronary intervention (PCI) period [10,16–19]. Recent data have emerged that more potent antiplatelet regimens, including prasugrel and ticagrelor, have proven more predictable and effective in achieving the desired antiplatelet effects [20,21]. However, the role of these agents in patients found to be poorly responsive or nonresponsive to clopidogrel has not been fully investigated and determined.
In light of the above discussion, the aim of this paper is to:
▪ Describe the most widely used assays used to determine response to antiplatelet medications;
▪ Compare the utility of those assays;
▪ Discuss the growing and controversial role these assays play on determining response to antiplatelet, and specifically clopidogrel, therapy.
Platelet functional analysis: assays testing antiplatelet inhibition
In broad strokes, the functional tests used to determine response to antiplatelet therapy include measurements of flow cytometry, activation- dependent signaling and platelet aggregation.
▪ Flow-cytometry/activationdependent signaling
Platelets are identified and counted using samples of platelets incubated with a fluorescent monoclonal antibody directed against an antigen expressed on the platelet surface. Furthermore, the detection of activation-dependent receptors that are extruded onto the platelet surface when stimulated can be quantified by targeting receptors such as P-selectin and activated GPIIb/IIIa. Platelet receptor reactivity can also be analyzed by detecting the end-product of intracellular signaling, such as VASP. However, despite these techniques being specific for activation status of the P2Y12 receptor, this method says little about the activation status of the platelet overall, which is affected by several biological factors. Therefore, although useful in many clinical scenarios, information about receptor expression and clinical outcomes are lacking. Furthermore, this technique is hampered by its complexity, requirement for highly specialized staff and prohibitively high cost.
▪ Platelet aggregation
Light transmission aggregometry (LTA) was developed in the 1960s, and has since become the gold standard of platelet functional testing. Blood is centrifuged to obtain platelet-rich plasma, which is then suspended between a light source and photocell. Platelet-rich plasma is suspended between two platinum wire electrodes that become covered with platelets, producing a baseline measure of light transmission. Agonists are used to induce platelet aggregation with a resulting increase in light transmission as recorded by the photocell. For tests of clopidogrelinduced platelet inhibition, ADP is used as an agonist (typically ranging from 5–20 μmol/l), although thromboxane, thrombin and collagen are also employed in test-appropriate scenarios. The response depends on the baseline function of the platelet and the concentration of agonist, which change the response curve accordingly. Despite its accuracy, reproducibility and widespread acceptance as the gold standard, LTA has proven to be time and labor intensive, costly and requires significant technical expertise for performance and interpretation.
Recently, simplified point-of-care antiplatelet assays based on the principles of platelet aggregation have become available. They are widely based on the idea of whole-blood analysis, which was developed as an alternative to the plateletrich plasma systems described previously. One such assay, VerifyNow® (Accumentrics, CA, USA), employs a turbidimetric-based optical detection system which quantifies platelet coagglutination with agonist-coated styrene microbeads [22]. The VerifyNow assay contains a preparation of human fibrinogen-coated microbeads mixed with agonist (arachidonic acid for the aspirin assay and ADP [20 μmol/l] for the P2Y12 assay). Fixed aliquots of whole-blood sample are automatically drawn into sample wells from the collection tube where platelets come in contact with an agonist. Aggregation is measured as functions of platelet-microbead coagglutination and light transmittance through sample using a proprietary algorithm, reporting values as aspirin reaction units or P2Y12 reaction units (PRU). Higher aspirin reaction units and PRU values reflect greater reactivity, and thus, platelet aggregation. For the P2Y12 assay, a second channel containing a fixed concentration of the potent platelet agonist iso-TRAP, serves as a measure of maximal platelet aggregation, eclipsing the antiplatelet activity of clopidogrel and thereby obviating the need for a drug-naive sample to serve as a reference to the on-treatment value.
A second point-of-care assay based on whole-blood sampling and electrical impedance described with some frequency in the literature is multi-electrode platelet aggregometry (MEA; Dynabyte, Munich, Germany). The adhesion and aggregation of platelets on the device surface increases the electrical impedance between two sensor electrodes. The increase of impedance due to the increasing platelet attachment is detected and the electrical signal is transformed to an arbitrary aggregation unit (AU) that is plotted against time (AU × min). This device quantifies the aggregation measurements as the area under curve of AU × min. Whole blood is used with various target specific agonists: arachidonic acid and collagen for aspirin, thrombin receptor activating peptide for IIb/IIIa antagonists and ADP for P2Y12 inhibitors.
Finally, Plateletworks® (Helena Laboratories, TX, USA) is yet another point-of-care assay which determines degree of platelet aggregation. Whole-blood aliquots are added to both baseline and agonist reagent tubes (collagen, ADP and/or arachidonic acid). The samples are then run on a standard impedance cell counter. Within several minutes, percentage aggregation can be determined based on platelet baseline and agonist counts. However, one drawback to this assay is that it must be performed within 10 min of blood withdrawal to prevent spontaneous platelet activation.
▪ Comparison of assays measuring platelet inhibition
Many studies have looked at the correlation between assays evaluating platelet inhibition and, to a lesser extent, the comparison of said assays to predict outcomes. Especially given their ease of use and rapid output, recent attention has been paid to point-of-care assays, comparing them to the more traditional, validated laboratory- based methods LTA and VASP.
Regarding this, the widest body of evidence exists for VerifyNow, with varied results. The earliest studies suggested a poor correlation between VerifyNow and LTA (the current gold standard tool for evaluating platelet inhibition), with correlation coefficients as low as 0.36 [23,24]. However, these same studies were performed during the earliest iterations of the VerifyNow assay. More recent literature has demonstrated a robust correlation between VerifyNow and LTA, with correlation coefficients as high as 0.7–0.88 [25–27]. Similar trends have been noted for VerifyNow and VASP [25], which in some situations tended to correlate more strongly and with less variability than with LTA [28]. Interestingly however, the most contemporary study comparing VerifyNow with VASP determined that definite stent thrombosis events were associated with PRU values of more than 222, while VASP was found to have no association, calling its predictive capability into question [29].
MEA and its correlation with laboratory-based techniques, as well as with VerifyNow, have also been recently evaluated, again with somewhat disparate results. One study suggested a correlation with LTA as high as 0.71, with similar correlation to VerifyNow (r = 0.62) [30]. However, others have not been able to substantiate this finding in as far as it was compared with VerifyNow, demonstrating more modest correlation between the two assays ranging from 0.39–0.47, and even less strongly to VASP [31,32]. Despite this, one recent study did demonstrate that low response to clopidogrel as assessed by MEA was significantly associated with an increased risk of stent thrombosis in patients undergoing PCI [33].
Plateletworks was noted in a recent study to similarly predict outcomes alongside LTA and VerifyNow [34], but little is known about how well its findings correlate with other standard assays. Although Plateletworks has demonstrated promise in being able to accurately determine levels of platelet inhibition, further study is warranted to determine its reliability.
Importantly, the above discussion highlights that VerifyNow has tended to outperform other point-of-care assays in demonstrating platelet inhibition, has comparable capabilities to the laboratory-based standard LTA and in some studies has even outperformed proven laboratory- based techniques such as VASP [29,35]. It is based on these findings that several studies looking at tailored therapy described below have utilized VerifyNow as part of their study design.
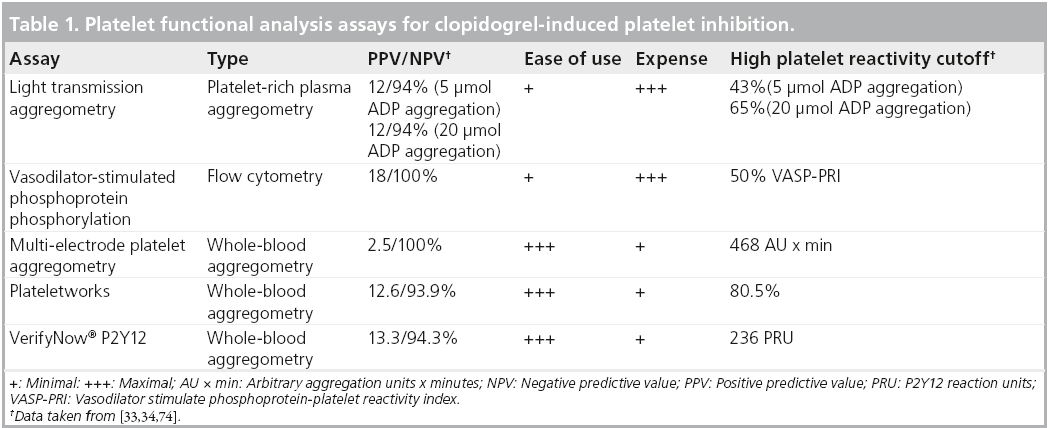
However, to complete a full evaluation of test performance, one should also look at the reproducibility of these assays. One study looked at the repeatability of LTA, MEA and VerifyNow when induced with arachidonic acid, and found that coefficients of variation for duplicate measurements at baseline and during treatment with aspirin varied widely across assays (0.4–12 and 3–46%, respectively). The day-to-day variability was also quite marked (3–37%). VerifyNow varied the least (0.4–3%), while MEA varied the most (8–46%) between baseline and ontreatment measurements [36]. Similarly, LTA was shown to have reasonable short term reproducibility, but the long-term reproducibility was quite poor [37]. A review of the various assays specific to clopidogrel-induced platelet inhibition is presented in Table 1.
In summary, if one is interested in testing platelet inhibition, no test is perfect. Either one accepts time and expense for laboratory-based standards, or accepts the potential limitations of reliability and reproducibility in all methodologies, but especially point-of-care measures. Despite their imperfections, these tools are what we have available and play an important role in the growing field of patient-centered care. It is with this in mind that we turn our discussion to the arena of platelet reactivity testing in patients treated with antiplatelet medications.
The current controversy over platelet reactivity testing
Platelet activation occurs independently through binding of circulating and locally released ADP to the ADP P2Y12 platelet surface receptor. This receptor modulates phosphorylation of intracellular VASP via a G-protein coupled mechanism, with resultant conformational activation of the platelet glycoprotein IIb/IIIa receptor. Clopidogrel, the most commonly prescribed P2Y12 inhibitor, irreversibly binds the P2Y12 receptor, disabling this activation pathway for the life of the platelet [38]. Whereas large-scale clinical trials conducted over the past several decades have demonstrated the utility of various antiplatelet regimens in the primary and secondary prevention of atherothrombotic cardiovascular events, many treated patients continue to accrue such events. In the landmark CURE trial, clopidogrel in combination with aspirin – that is, dual antiplatelet therapy, was demonstrated to reduce the risk of cardiovascular end points by 20% compared with aspirin alone in the overall study population [39]. However, 10% of patients continued to have events on dual antiplatelet therapy [38], suggesting that the protective effects of this therapy were perhaps incomplete. More recent mechanistic studies support this contention and have revealed the existence of significant interpatient clopidogrel response variability. It has been estimated that the prevalence of clopidogrel hyporesponse or nonresponse ranges from 15 to 40% [40]. In patients who remain adherent to clopidogrel therapy, clinical factors such as diabetes mellitus, increased BMI, impaired intestinal absorption and competitive antagonism of liver CYP isozymes by conjunctive pharmacotherapies may contribute to clopidogrel response variability. Cellular factors including increased ADP exposure, upregulation of P2Y12 receptors and accelerated platelet turnover add to overall resistance, and polymorphisms in the genes encoding the various CYP isozymes also appear to significantly contribute to clopidogrel response variability. CPY2C19 in particular appears to play a particularly prominent role in both the biotransformation of clopidogrel, as well as clopidogrel resistance.
It follows that the utilization of the tools previously described that measure degree of ontreatment platelet reactivity would be of clinical value. In fact, these tools have been increasingly utilized in studies correlating high platelet reactivity to clinical outcomes [14,15]. For example, the ARMYDA-PRO study showed that the highest quartile platelet reactivity measured by VerifyNow was associated with higher incidence of major adverse cardiovascular events compared with the lowest quartile reactivity [15], an outcome that was mostly driven by incidence of periprocedural myocardial infarction. Similarly, Marcucci et al. noted that platelet reactivity greater than or equal to 240 PRU in patients undergoing PCI was an independent predictor of 12-month cardiovascular death and nonfatal myocardial infarction [14].
Although platelet reactivity testing is attractive in theory, widespread support for routine use has been hindered by several scientific and practical issues. First, there has been a lack of definitional standardization for clopidogrel ‘nonresponsiveness’ or ‘resistance.’ Some studies have looked at absolute difference between baseline (pretreatment) platelet aggregation and posttreatment aggregation [41], while others have looked at the baseline-referenced percentage decrease or inhibition of platelet aggregation [19]. Neither methodology, however, adheres to a uniform cutoff value for nonresponse. A second important impediment has been the lack of a widely-accepted standard methodology for assessment of on-treatment platelet reactivity/ aggregation and the question of nonuniform agreement among tests, as described earlier. Finally, and probably most importantly, there is only a modest ability for these tests to accurately predict clinical outcomes. This was evaluated in the POPULAR study, in which on-treatment platelet reactivity was evaluated with several different assays in over 1000 patients on clopidogrel following elective stent implantation [34]. Although the incidence of cardiovascular events at one year in POPULAR was significantly higher in patients with high on-treatment platelet reactivity compared with those with normal platelet reactivity, consistent with the finding of Marcucci et al. and Patti et al. (on average 12 vs 6%) [42,43], the ability for the three most robust tests (LTA, VerifyNow and Plateletworks) to discriminate those having and not having an event was at best modest, with sensitivities of 55–63% and specificities of 59–64%. This was further supported by the recent evaluation of VerifyNow in patients with definite and probable stent thrombosis in two studies, the largest of which was the ADAPT-DES trial. The study demonstrated that although high on-treatment PRU was associated with events in the overall population of over 11,000 patients (three-fold increased risk of 30-day stent thrombosis), there existed considerable overlap in on-treatment PRU levels in patient with and without stent thrombosis [29,44]. The limited sensitivity and specificity made the test questionably helpful in the individual patient. This was thought to be in part due to the low stent thrombosis rate in the overall population (0.46%), and more pronounced in stable patients without acute coronary syndrome (ACS; stent thrombosis rate 0.22%).
A great deal of attention has been paid to the VerifyNow assay, which is demonstrated by the above discussion. It is impossible to avoid such discussion as a significantly larger body of evidence simply exists for VerifyNow compared with other assays, and especially other point-of-care assays. This is, in part, because of its ease of use and proven reliability compared with standard techniques. However, ADAPT-DES was the first large-scale analysis to call the utility of the VerifyNow assay into question and perhaps giving pause to its growing popularity.
▪ Drug metabolite measurements
Although the previous discussion focused on assays used to measure response to antiplatelet therapy, a brief word about drug metabolite measurements, specifically the products of clopidogrel metabolism, is warranted. Not a great deal of research has been devoted to this area and limited information exists regarding comparisons of drug metabolite measurements to standard measurements of platelet reactivity. However, the limited data that do exist suggest the main product of clopidogrel metabolism, carboxylic acid, is significantly variable from patient to patient, consistent with the variability noted by assays aimed at platelet reactivity [45]. However, beyond potentially having a role in determining patient compliance with clopidogrel by the presence of quantifiable drug metabolite, its scope in detecting degree of platelet inhibition is limited to the research arena.
▪ Can high on-treatment platelet reactivity be overcome and should platelet reactivity testing play a part?
Indeed, while several studies have demonstrated that increasing the dose of clopidogrel load and maintenance can reduce platelet hyporesponse [46–49], trials evaluating dose adjustment in response to high platelet reactivity and clinical outcomes have been equivocal. One study demonstrated that VASP-guided repeat loading with clopidogrel (up to three additional doses) in patients undergoing PCI with a more than 50% VASP index (high platelet reactivity) to achieve adequate inhibition resulted in significantly fewer major adverse cardiovascular events at 1 month compared with standard treatment [50]. Similarly, two prospective studies demonstrated tailored loading of clopidogrel effectively reduced high on-treatment platelet reactivity and decreased 30-day cardiovascular events [51,52]. However, the GRAVITAS trial, the largest prospective randomized study evaluated over 2200 subjects with high platelet reactivity on clopidogrel as measured by VerifyNow following PCI with a drug-eluting stent [53]. The groups were randomly assigned to standard-dose clopidogrel (no load, 75 mg/day) or high-dose clopidogrel (600 mg load, 150 mg/day thereafter) for 60 days. The primary end point (6-month incidence of death from cardiovascular causes, nonfatal myocardial infarction or stent thrombosis) was nearly identical between groups, a remarkable low 2.3%. However, the study’s ability to obtain reduced platelet reactivity in patient in the high maintenance-dose clopidogrel arm was hampered as 40% of that population did not achieve this goal. Therefore, as a means to either support or refute the findings in GRAVITAS, the ARCTIC study (NCT00827411) proposed that dose adjustment of clopidogrel, based on biological monitoring by VerifyNow, would reduce the rate of severe cardiovascular complications compared with a conventional strategy in patients scheduled for drug-eluting stent implantation and followed up for 1 year [54,101]. The study has completed enrollment, but the results have not yet been reported.
A second potential avenue for overcoming poor response to clopidogrel may lie in the new P2Y12 agents prasugrel and ticagrelor. They are of clinical interest in that they have been shown to consistently reduce platelet reactivity as they are not affected by the cytochrome alleles that reduce clopidogrel effectiveness [55,56]. However, whether choosing one of these alternatives a priori in patients with high on-treatment platelet reactivity with clopidgrel results in improved clinical outcomes, has not been shown. For example, the TRIGGER-PCI trial, which randomized patients with high on-treatment platelet reactivity, as measured by VerifyNow, to either prasugrel or continued clopidogrel, was terminated prematurely due to futility [57].
In accordance with the limitations noted above, several societies, including the 2008 American College of Chest Physicians clinical practice guidelines, a 2010 report from the American College of Cardiology Foundation and the American Heart Association, a 2010 report endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons and a 2010 ‘white paper’ from the Journal of the American College of Cardiology do not endorse routine testing of platelet reactivity or changing therapy based on results of this testing [58–60].
▪ The role of genetic testing
One final related consideration is that of testing for reduced-function CYP alleles. Although it does not speak to the functionality of platelets in patients treated with antiplatelet medications, it is becoming increasingly difficult to separate functionality from the genetic make-up that creates said functionality in patients treated with clopidogrel. This is highlighted by the black box warning for clopidogrel (which does not point to functional testing, but genetic testing) and the growing number of studies that are aimed at both functional assessment, as well as genetic determination. Therefore, the authors will briefly focus on the role for genetic testing.
Individuals with the wild-type CYP2C19 variant account for approximately 35% of the general population, and are termed extensive metabolizers. This variant results in 40–60% platelet inhibition on average by LTA. Individuals with the gain-of-function allele account for another 30% of the general population, and are termed ultra-rapid metabolizers. However, several lossof- function alleles have been identified and confer a 45–55% relative reduction in platelet inhibition compared with extensive metabolizers. As such, carriers of these loss-of-function allele combinations have been termed poor metabolizers. However, poor metabolizers are relatively rare, comprising approximately 2% of the Caucasian population [61–63].
Recent studies have found that ACS patients with reduced-function CYP2C19 alleles are significantly more likely to develop cardiovascular events and acute stent thromboses compared with noncarriers in a timeframe ranging from 15 months to 8 years after initiation of clopidogrel therapy [64–68]. In a substudy of the TRITON-TIMI 38 trial, clopidogrel-treated carriers of a reduced-function CYP2C19 allele had a 53% relative increase in cardiovascular death, myocardial infarction and stoke compared with noncarriers. It was additionally noted that carriers of the reduced-function CYP2C19 allele had a significant increase in platelet aggregation as seen by LTA compared with noncarriers as they were exposed to 32.4% less active drug [66]. This further held true in a population mostly comprised of post-PCI patients (91%), where 1.6-fold increased risk of death, myocardial infarction or stroke was demonstrated among carriers of one loss-offunction allele and 1.8-fold increased risk was demonstrated if the patient carried two such alleles [61]. However, not all evidence supports these findings. For instance, retrospective analyses from the CURE and the PLATO trials, which included both PCI and medically treated patients, failed to demonstrate that loss-offunction alleles were associated with increased cardiovascular risk [62,63]. Furthermore, a pooled analysis of observational cohorts only showed a weak 1.12-fold increased risk of cardiovascular events associated with carriage of a single loss-of-function allele and a 1.22-fold risk in those who carry two [61]. The utility of genomic testing is further called into question when loss-of-function carriers are common, but the rate of events, and specifically the rate of stent thrombosis, which is intertwined with clopidogrel nonresponse, is rather infrequent by comparison.
The US FDA recently distributed a black box warning for clopidogrel, noting that reduced effectiveness of the drug could be seen in patients who are poor metabolizers, that tests are available to identify genetic variants that could lead to poor clopidogrel metabolism and that healthcare professionals should consider alternative agents or dosing strategies for such patients. Exactly what those alternatives are remains to be seen, but recent evidence shows treatment with high-dose maintenance clopidogrel (150– 300 mg daily) compared with standard dose fails to alter high on-treatment platelet reactivity in homozygote carriers of loss-of-function alleles, nor does it reliably overcome the influence of loss-of-function alleles in heterozygotes [69–71]. Furthermore, the often noted gastric side effects of high-dose clopidogrel make this regimen impractical. Regarding alternative agents, carriers of loss-of-function alleles in TRITON TIMI- 38 treated with prasugrel did not demonstrate an increased risk of stent thrombosis while, as previously noted, those treated with clopidogrel had a significantly increased rate of stent thrombotic events [72]. Similarly, in a genetic substudy of PLATO, ticagrelor was associated with a reduced incidence of events compared with clopidogrel regardless of genotype status [63]. In summary, prasugrel and ticagrelor may mitigate the adverse effects of loss-of-function alleles, but currently only retrospective analyses are available. Ongoing prospective PAPI-2 trial (NCT01452152), which will randomize genetically- proven poor-responders to clopidogrel or prasugrel, seeks to shed light on whether geneticguided therapy choices will result in improved outcomes [102].
Conclusion and future perspective
Moving forward, the use of guided therapy may find its niche in clinical practice but currently the field remains murky. Point-of-care platelet reactivity testing has proven itself to be a reasonably reliable and rapid method of estimating clopidogrel response compared with our laboratory- based standards. These tests, and more recently CYP2C19 genetic testing, have become increasingly available, making it much easier to make such determinations. With the multitude of variables affecting the metabolism of clopidogrel, many clinicians are left wondering how to maneuver when high levels of platelet reactivity are noted on therapy, especially considering the relatively low rate of manifest stent thrombotic events. This may also have major implications now that clopidogrel has a generic formulation and chosing between the market standard versus superior but more expensive alteranatives becomes an everyday decision. When faced with borderline platelet inhibition values in patients loaded and maintained on clopidogrel and given the current disparity of clinical trial outcomes, it is unclear whether the practice of additional clopidogrel loading or a change to a different agent is of value, a statement that succinctly highlights the opinion of the authors in this very unsettled arena. ARCTIC will attempt to support or refute the knowledge garnered from GRAVITAS in demonstrating that dose adjustment of clopidogrel will yield improved cardiovascular outcomes. Further, the P2Y12 substudy of TRILOGY-ACS (NCT00699998) aims to compare prasugrel with clopidogrel among medically-managed non-ST elevation ACS patients [73,103]. It’s goal will be to provide the largest prospective data integration of functional analysis via VerifyNow and pharmacogenomics in patients with ACS treated with clopidogrel versus prasugrel. It has the potential to extend the scope of both functional and genomic testing to a larger patient subset in that the study is uniquely testing only medically-managed patients. Finally, the pharmacogenomics piece will also be substantiated or refuted by the ongoing PAPI-2 study. In summary, however, until we prove that changing the dose or type of therapy because of the results of functional or genomic testing is beneficial, we will continue to maneuver somewhat blindly.
Executive summary
Platelet functional analysis: assays testing antiplatelet inhibition
▪ The functional tests used to determine response to antiplatelet therapy include measurements of flow cytometry, activation-dependent signaling and platelet aggregation.
▪ Light transmission aggregometry is considered the gold standard but is labor intensive and expensive.
▪ Point-of-care assays that are widely based on whole-blood aggregometry include VerifyNow® (Accumentrics, CA, USA), multi-electrode aggregometry and Plateletworks® (Helena Laboratories, TX, USA).
Comparison of assay measuring platelet inhibition
▪ VerifyNow is the point-of-care assay that has demonstrated the greatest degree of reproducibility and reliability in determining degree of platelet inhibition compared with the gold standard, light transmission aggregometry.
▪ Less is known about multi-electrode aggregometry and Plateletworks as the body of evidence is not as robust as for VerifyNow. Multi-electrode aggregometry has less data suggesting its reliability and predictive capability, but Plateletworks has demonstrated some promise.
The current controversy over platelet reactivity testing
▪ Dual antiplatelet therapy with aspirin and a thienopyridine is the mainstay of treatment for patients with manifest coronary disease, and specifically those undergoing percutaneous intervention. Several factors, however, limit the effectiveness of this therapy, with thienopyridine and, specifically, clopidogrel resistance playing a prominent and widely publicized role.
▪ Studies have been aimed at identifying patients at risk for major cardiovascular events and stent thrombosis using the assays described above, with VerifyNow prevalently utilized as the assay of choice.
▪ Although initially attractive, the use of platelet reactivity testing has been hampered by several factors:
- A lack of definitional standardization for clopidogrel ‘nonresponsiveness’ or ‘resistance’;
- The lack of a widely-accepted standard methodology for assessment of on-treatment platelet reactivity/aggregation and the question of uniform agreement among tests;
- The, at best, modest ability for these test to accurately predict clinical outcomes.
Drug metabolite measurements
▪ Clopidogrel metabolism products demonstrated wide inter-patient variability, similar to that seen with absolute antiplatelet response to clopidogrel by platelet inhibition testing.
▪ Beyond potentially having a role in determining patient compliance with clopidogrel by the presence of quantifiable drug metabolite, its scope in detecting degree of platelet inhibition is limited to the research arena.
Can high on-treatment platelet reactivity be overcome & should platelet reactivity testing play a part?
▪ Studies aimed at altering clinical outcomes by assay-guided changes in clopidogrel dosing have resulted in no statistically significant improvement to the present time.
▪ Studies aimed at altering clinical outcomes by assay-guided changes in P2Y12 inhibitor choices to newer, more potent agents, have been similarly disappointing.
▪ Ongoing studies directed at the above questions will attempt to either support or refute the previously reported findings.
▪ Guidelines and the major cardiovascular societies do not currently support the role of platelet reactivity testing to guide decisions about drug dosing and agent choices.
The role of genetic testing
▪ CYP2C19 contributes largely to the metabolism of clopidogrel. CYP2C19 alleles further make up a large component of the genetic variation leading to clopidogrel-response variability.
▪ Patients considered poor metabolizers of clopidogrel via loss-of-function alleles have been found to be at increased risk of cardiovascular events after stent placement.
▪ However, the results are controversial as the high prevalence of reduced-function alleles does not correlate with the much lower rate of stent thrombotic events.
▪ Studies to date have not reliably demonstrated an improvement in platelet reactivity or clinical outcomes in clopidogrel dose adjustments in patients with loss-of-function alleles. However, retrospective analyses have suggested promise in alternative P2Y12 inhibitor choices in patients with loss-of-function alleles.
▪ Studies are currently underway, which will attempt to demonstrate an improvement in clinic outcomes by changing therapy based on genetically proven clopidogrel poor response.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪ ▪ of considerable interest
- Gurbel PA, Becker RC, Mann KG, Steinhubl SR, Michelson AD. Platelet function monitoring in patients with coronary artery disease. J. Am. Coll. Cardiol. 50(19), 1822–1834 (2007).
- Angiolillo D, Bernardo E, Sabate M et al. Impact of platelet reactivity on cardiovascular outcomes in patients with Type 2 diabetes mellitus and coronary artery disease. J. Am. Coll. Cardiol. 50, 1541–1547 (2007).
- Bliden K, DiChiara J, Tantry U, Bassi A, Chaganti S, Gurbel P. Increased risk in patients with high platelet aggregation on chronic clopidogrel therapy undergoing PCI: is the current antiplatelet therapy adequate? J. Am. Coll. Cardiol. 49, 657–666 (2007).
- Bonello L, Paganelli F, Arpin-Bornet M et al. Vasodilator-stimulated phosphoprotein phosphorylation analysis prior to percutaneous coronary intervention for exclusion of postprocedural major adverse cardiovascular events. J. Thromb. Haemost. 5, 1630–1636 (2007).
- Cuisset T, Frere C, Quilici J et al. High post-treatment platelet reactivity identified low-responders to dual antiplatelet therapy at increased risk of recurrent cardiovascular events after stenting for acute coronary syndrome. J. Thromb. Haemost. 4, 542–549 (2006).
- Cuisset T, Frere C, Quilici J et al. Benefit of a 600mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation actue coronary syndrome undergoing coronary stenting. J. Am. Coll. Cardiol. 48, 1339–1345 (2006).
- Frere C, Cuisset T, Quilici J et al. ADPinduced platelet aggregation and platelet reactivity index VASP are good predictive markers for clinical outcomes in non-ST elevation actue coronary syndrome. J. Thromb. Haemost. 98, 838–843 (2007).
- Geisler T, Langer H, Wydymus M et al. Low response to clopidogrel is associated with cardiovascular outcome after coronary stent implantation. Eur. Heart J. 27, 2420–2425 (2006).
- Gurbel P, Bliden K, Guyer K et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE Post-Stenting study. J. Am. Coll. Cardiol. 46, 1820–1826 (2005).
- Gurbel P, Bliden K, Zaman K, Yoho J, Hayes K, Tantry U. Clopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS) study. Circulation 111, 1153–1159 (2005).
- Hochholzer W, Trenk D, Bestehorn H et al. Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective stent placement. J. Am. Coll. Cardiol. 48, 1742–1750 (2006).
- Lev E, Patel R, Maresh K et al. Aspirin and clopidogrel drug response in patients undergoing percutaneous coronary intervention: the role of dual drug resistance. J. Am. Coll. Cardiol. 47, 27–33 (2006).
- Matetzky S, Shenkman B, Buetta V et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 109, 3171–3175 (2004).
- Marcucci R, Gori A, Baniccia R et al. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by point-of-care assay: a 12-month follow-up. Circulation 119, 237–242 (2009).
- Patti G, Nusca A, Mangiacapra F, Gatto L, D’Ambrosio A, DiSciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention: results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Antioplasty- Platelet Reactivity Predicts Outcome) study. J. Am. Coll. Cardiol. 52, 1128–1133 (2008).
- Ajzenberg N, Aubry P, Huisse M et al. Enhanced shear-induced platelet aggregation in patients who experience subacute stent thrombosis: a case-control study. J. Am. Coll. Cardiol. 45, 1753–1756 (2005).
- Barragan P, Bouvier J, Roquebert P et al. Resistance to thienopyridines: clinical detection of coroanry stent thrombosis by monitoring of vasodilator-stimulated phosphoprotein phosphorylation. Catheter. Cardiovasc. Interv. 59, 295–302 (2003).
- Buonamici P, Marcucci R, Migliorini A et al. Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. J. Am. Coll. Cardiol. 49, 2312–2317 (2007).
- Mueller I, Best F, Schulz C, Massberg S, Schoenig A, Gawaz M. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. J. Thromb. Haemost. 89, 783–787 (2003).
- Wallentin L, Becker RC, Budaj A et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361(11), 1045–1057 (2009).
- Wiviott SD, Braunwald E, McCabe CH et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357(20), 2001–2015 (2007).
- Malinin A, Pokov A, Swaim L, Kotob M, Serebruany V. Validation of a VerifyNow P2Y12 cartridge for monitoring platelet inhibition with clopidogrel. Methods Find. Exp. Clin. Pharmacol. 28, 315–322 (2006).
- Cuisset T, Frere C, Poyet R et al. Clopidogrel response: head-to-head comparison of different platelet assays to identify clopidogrel non responder patients after coronary stenting. Arch. Cardiovasc. Dis. 103(1), 39–45 (2010).
- Lordkipanidze M, Pharand C, Nguyen TA, Schampaert E, Palisaitis DA, Diodati JG. Comparison of four tests to assess inhibition of platelet function by clopidogrel in stable coronary artery disease patients. Eur. Heart J. 29(23), 2877–2885 (2008).
- Varenhorst C, James S, Erlinge D et al. Assessment of P2Y(12) inhibition with the point-of-care device VerifyNow P2Y12 in patients treated with prasugrel or clopidogrel coadministered with aspirin. Am. Heart J. 157(3), 562 e561–569 (2009).
- Jakubowski JA, Li YG, Small DS et al. A comparison of the VerifyNow P2Y12 point-of-care device and light transmission aggregometry to monitor platelet function with prasugrel and clopidogrel: an integrated analysis. J. Cardiovasc. Pharmacol. 56(1), 29–37 (2010).
- Kim IS, Jeong YH, Kang MK et al. Correlation of high post-treatment platelet reactivity assessed by light transmittance aggregometry and the VerifyNow P2Y12 assay. J. Thromb. Thrombolysis 30(4), 486–495 (2010).
- Bidet A, Jais C, Puymirat E et al. VerifyNow and VASP phosphorylation assays give similar results for patients receiving clopidogrel, but they do not always correlate with platelet aggregation. Platelets 21(2), 94–100 (2010).
- Varenhorst C, Koul S, Erlinge D et al. Relationship between clopidogrel-induced platelet P2Y12 inhibition and stent thrombosis or myocardial infarction after percutaneous coronary intervention-a case-control study. Am. Heart J. 162(2), 363–371 (2011).
- Paniccia R, Antonucci E, Maggini N et al. Comparison of methods for monitoring residual platelet reactivity after clopidogrel by point-of-care tests on whole blood in high-risk patients. J. Thromb. Haemost. 104(2), 287–292 (2010).
- Ko YG, Suh JW, Kim BH et al. Comparison of 2 point-of-care platelet function tests, VerifyNow assay and multiple electrode platelet aggregometry, for predicting early clinical outcomes in patients undergoing percutaneous coronary intervention. Am. Heart J. 161(2), 383–390 (2011).
- von Beckerath N, Sibbing D, Jawansky S et al. Assessment of platelet response to clopidogrel with multiple electrode aggregometry, the VerifyNow P2Y12 analyzer and platelet vasodilator-stimulated phosphoprotein flow cytometry. Blood Coagul. Fibrinolysis 21(1), 46–52 (2010).
- Sibbing D, Braun S, Morath T et al. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J. Am. Coll. Cardiol. 53(10), 849–856 (2009).
- Breet NJ, van Werkum JW, Bouman HJ et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 303(8), 754–762 (2010).
- Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. Comparison of methods to evaluate clopidogrel-mediated platelet inhibition after percutaneous intervention with stent implantation. J. Thromb. Haemost. 101(2), 333–339 (2009).
- Grove EL, Hvas AM, Johnsen HL et al. A comparison of platelet function tests and thromboxane metabolites to evaluate aspirin response in healthy individuals and patients with coronary artery disease. J. Thromb. Haemost. 103(6), 1245–1253 (2010).
- Sevcikova H, Vojacek J, Bis J et al. Good short-term but not long-term reproducibility of the antiplatelet efficacy laboratory assessment. Clin. Appl. Thromb. Hemost. 18(2), 174–180 (2012).
- Angiolillo D. Variability in responsiveness to oral antiplatelet therapy. Am. J. Cardiol. 103(Suppl. 3), 27A–34A (2009).
- Yusuf S, Zhao F, Mehta S, Chrolavicius S, Tognoni G, Fox K. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation: for the Clopidogrel in Ustable Angina to Prevent Recurrent Events Trial Investigators. N. Eng. J. Med. 345, 494–502 (2001).
- Gurbel P, Becker R, Mann K, Steinhubl S, Michelson A. Platelet function monitoring in patients with coronary artery disease. J. Am. Coll. Cardiol. 50, 1822–1834 (2007).
- Gurbel P, Bliden K, Hiatt B, O’Connor C. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 107, 2908–2913 (2003).
- Marcucci R, Gori AM, Paniccia R et al. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation 119(2), 237–242 (2009).
- Patti G, Nusca A, Mangiacapra F, Gatto L, D’Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty- Platelet Reactivity Predicts Outcome) study. J. Am. Coll. Cardiol. 52(14), 1128–1133 (2008).
- Stone GW. Assessment of dual antiplatelet therapy with drug-eluting stents: a large scale, prospective, multicenter registry examining the relationship between platelet responsiveness and stent thrombosis after DES implantation. Presented at: Transcatheter Cardiovascular Therapeutics 23rd Annual Scientific Symposium. San Francisco, CA, USA 7–11 November 2011.
- Mani H, Toennes SW, Linnemann B et al. Determination of clopidogrel main metabolite in plasma: a useful tool for monitoring therapy? Ther. Drug Monit. 30(1), 84–89 (2008).
- Gladding P, Webster M, Zeng I et al. The antiplatelet effect of higher loading and maintenance dose regimens of clopidogrel: the PRINC (Plavix Response in Coronary Intervention) trial. JACC Cardiovasc. Interv. 1(6), 612–619 (2008).
- L’Allier PL, Ducrocq G, Pranno N et al. Clopidogrel 600-mg double loading dose achieves stronger platelet inhibition than conventional regimens: results from the PREPAIR randomized study. J. Am. Coll. Cardiol. 51(11), 1066–1072 (2008).
- Oestreich JH, Holt J, Dunn SP et al. Considerable variability in platelet activity among patients with coronary artery disease in response to an increased maintenance dose of clopidogrel. Coron. Artery Dis. 20(3), 207–213 (2009).
- Price MJ, Coleman JL, Steinhubl SR, Wong GB, Cannon CP, Teirstein PS. Onset and offset of platelet inhibition after high-dose clopidogrel loading and standard daily therapy measured by a point-of-care assay in healthy volunteers. Am. J. Cardiol. 98(5), 681–684 (2006).
- Bonello L, Camoin L, Arques S et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J. Am. Coll. Cardiol. 51, 1404 (2008).
- Bonello L, Camoin-Jau L, Armero S et al. Tailored clopidogrel loading dose according to platelet reactivity monitoring to prevent acute and subacute stent thrombosis. Am. J. Cardiol. 103(1), 5–10 (2009).
- Bonello L, Camoin-Jau L, Arques S et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J. Am. Coll. Cardiol. 51(14), 1404–1411 (2008).
- Price MJ, Berger PB, Angiolillo DJ et al. Evaluation of individualized clopidogrel therapy after drug-eluting stent implantation in patients with high residual platelet reactivity: design and rationale of the GRAVITAS trial. Am. Heart J. 157(5), 818–824, 824 e811 (2009).
- Collet JP, Cayla G, Cuisset T et al. Randomized comparison of platelet function monitoring to adjust antiplatelet therapy versus standard of care: rationale and design of the assessment with a double randomization of (1) a fixed dose versus a monitoring-guided dose of aspirin and clopidogrel after DES implantation, and (2) treatment interruption versus continuation, 1 year after stenting (ARCTIC) study. Am. Heart J. 161(1), 5–12 e15 (2011).
- Gurbel PA, Bliden KP, Butler K et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 120(25), 2577–2585 (2009).
- Varenhorst C, James S, Erlinge D et al. Genetic variation of CYP2C19 affects both pharmacokinetic and pharmacodynamic responses to clopidogrel but not prasugrel in aspirin-treated patients with coronary artery disease. Eur. Heart J. 30(14), 1744–1752 (2009).
- Trenk D, Stone GW, Gawaz M et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J. Am. Coll. Cardiol. 59(24), 2159–2164 (2012).
- Bonello L, Tantry US, Marcucci R et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 56(12), 919–933 (2010).
- Holmes DR Jr, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA ‘boxed warning’: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 56(4), 321–341 (2010).
- Holmes DR Jr, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA Clopidogrel clinical alert: approaches to the FDA ‘boxed warning’: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation 122(5), 537–557 (2010).
- Mega JL, Simon T, Collet JP et al. Reducedfunction CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 304(16), 1821–1830 (2010).
- Pare G, Mehta SR, Yusuf S et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N. Engl. J. Med. 363(18), 1704–1714 (2010).
- Wallentin L, James S, Storey RF et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet 376(9749), 1320–1328 (2010).
- Collet JP, Hulot JS, Pena A et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 373(9660), 309–317 (2009).
- Giusti B, Gori AM, Marcucci R et al. Relation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosis. Am. J. Cardiol. 103(6), 806–811 (2009).
- Mega JL, Close SL, Wiviott SD et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 360(4), 354–362 (2009).
- Sibbing D, Stegherr J, Latz W et al. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur. Heart J. 30(8), 916–922 (2009).
- Simon T, Verstuyft C, Mary-Krause M et al. Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 360(4), 363–375 (2009).
- Jeong YH, Kim IS, Park Y et al. Carriage of cytochrome 2C19 polymorphism is associated with risk of high post-treatment platelet reactivity on high maintenance-dose clopidogrel of 150 mg/day: results of the ACCEL-DOUBLE (Accelerated Platelet Inhibition by a Double Dose of Clopidogrel According to Gene Polymorphism) study. JACC Cardiovasc. Interv. 3(7), 731–741 (2010).
- Price MJ, Murray S, Angiolillo DJ et al. Influence of genetic polymorphisms on the effect of high- and standard-dose clopidogrel after percutaneous coronary intervention: the GIFT (Genotype Information and Functional Testing) study. J. Am. Coll. Cardiol. 29, 1928–1937 (2012).
- Mega JL, Hochholzer W, Frelinger AL 3rd et al. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA 306(20), 2221–2228 (2011).
- Mega JL, Close SL, Wiviott SD et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation 119(19), 2553–2560 (2009).
- Chin CT, Roe MT, Fox KA et al. Study design and rationale of a comparison of prasugrel and clopidogrel in medically managed patients with unstable angina/ non-ST-segment elevation myocardial infarction: the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Am. Heart J. 160(1), 16–22 e11 (2010).
- Bonello L, Paganelli F, Arpin-Bornet M et al. Vasodilator-stimulated phosphoprotein phosphorylation analysis prior to percutaneous coronary intervention for exclusion of postprocedural major adverse cardiovascular events. J. Thromb. Haemost. 5(8), 1630–1636 (2007).
▪ Demonstrates that VerifyNow® (Accumentrics, CA, USA) and Plateletworks® (Helena Laboratories, TX, USA) were found to similarly predict cardiovascular outcomes in patients with high on-treatment platelet reactivity compared with the laboratory-based standard, light transmission aggregometry. Other assays on the market did not prove reliable.
▪▪ Demonstrates that high on-treatment platelet reactivity as measured by VerifyNow could predict clinical events in the population at large. However, the considerable overlap in on-treatment measures in patients with and without stent thrombosis calls the utility of the test for the individual patient into question.
▪▪ Large scale, prospective study to demonstrate that tailored dosing of clopidogrel in patients with high on-treatment platelet reactivity as measured by VerifyNow does not alter clinical outcomes.
▪▪ First large-scale study to demonstrate that a priori changes to more potent P2Y12 inhibitors (i.e., prasugrel) because of functional evidence of high on-treatment platelet reactivity does not alter event rates.
▪ Demonstrates that patients found to be carriers of loss-of-function alleles to clopidogrel metabolism, more potent P2Y12 inhibitors (in this case ticagrelor) may confer benefit.
▪ Demonstrates that escalated doses of clopidogrel in genetically-proven clopidogrel poor responders do not mediate the effects of the loss-of-function alleles.
▪▪ Demonstrates that patients found to be carriers of loss-of-function alleles to clopidogrel metabolism, more potent P2Y12 inhibitors (in this case prasugrel) may confer benefit.
▪ Websites
- Double Randomization of a Monitoring Adjusted Antiplatelet Treatment Versus a Common Antiplatelet Treatment for DES Implantation, and Interruption Versus Continuation of Double Antiplatelet Therapy (ARCTIC). http://clinicaltrials.gov/ct2/show/NCT00827411
- Pharmacogenomics of Anti-platelet Intervention-2 (PAPI-2) Study. http://clinicaltrials.gov/ct2/show/ NCT01452152
- A Comparison of Prasugrel and Clopidogrel in Acute Coronary Syndrome Subjects (TRILOGY ACS).