Review Article - Interventional Cardiology (2014) Volume 6, Issue 6
Mitral valve repair with the MitraClip
- Corresponding Author:
- Vinod H
Thourani
Joseph B Whitehead Department of Surgery
Division of Cardiothoracic Surgery
Emory Structural Heart & Valve Center
Emory University School of Medicine, Atlanta, GA 30308, USA
Tel: +1 404 686 2513
Fax: +1 404 686 4959
E-mail: vthoura@emory.edu
Abstract
Mitral valve regurgitation is a common disease, traditionally treated with valve repair or replacement using open cardiac surgery. The most common etiologies of mitral regurgitation (MR) include degenerative and functional pathologies. MitraClip® (Abbott Vascular, CA, USA), a new device for transcatheter mitral valve repair, which gained CE mark approval for use in Europe in 2008, is now approved in the USA for use in patients with symptomatic degenerative mitral valve regurgitation who are deemed to be prohibitive risk surgical candidates. For these patients, MitraClip offers a safe and effective treatment option. Currently, trials are underway to assess its efficacy in high surgical risk patients with functional MR. The data from these trials will hopefully provide guidance to physicians to determine which patients would benefit from the device and which patients would benefit from the standard surgical approach. There is evidence that the MitraClip will continue to have an important role in treating both high-risk and nonsurgical patients with degenerative and functional MR now and in the future.
Mitral valve regurgitation is a common disease, traditionally treated with valve repair or replacement using open cardiac surgery. The most common etiologies of mitral regurgitation (MR) include degenerative and functional pathologies. MitraClip® (Abbott Vascular, CA, USA), a new device for transcatheter mitral valve repair, which gained CE mark approval for use in Europe in 2008, is now approved in the USA for use in patients with symptomatic degenerative mitral valve regurgitation who are deemed to be prohibitive risk surgical candidates. For these patients, MitraClip offers a safe and effective treatment option. Currently, trials are underway to assess its efficacy in high surgical risk patients with functional MR. The data from these trials will hopefully provide guidance to physicians to determine which patients would benefit from the device and which patients would benefit from the standard surgical approach. There is evidence that the MitraClip will continue to have an important role in treating both high-risk and nonsurgical patients with degenerative and functional MR now and in the future.
Keywords
edge-to-edge repair, minimally invasive, MitraClip®, mitral regurgitation, mitral valve repair, percutaneous, surgery, trans-septal
Mitral regurgitation (MR), either from primary valve disease (degenerative) or secondary (functional) to left ventricular dysfunction, affects more than 4 million Americans, or almost one in ten people over age 75 years. When moderately severe or severe, MR progresses and results in the deterioration of left ventricular function resulting in congestive heart failure, increased mortality and a significant decrease in quality of life (QoL). Chronic MR also increases the risk of atrial fibrillation and stroke, both of which can have a debilitating impact on patients [1]. Definitive treatment of this problem traditionally requires surgical intervention, either with repair or replacement of the diseased valve through a median sternotomy or a lateral thoracotomy [2]. Minimally invasive open-heart techniques such as endoscopic repair through the right chest or robotic mitral valve repair are available but are less frequently used; all surgical approaches require cardiopulmonary bypass [3,4].
Given that patients with severe, symptomatic MR often have increased surgical risk due to impaired cardiac function, advanced age and associated comorbidities, newer approaches that do not utilize invasive techniques requiring cardiopulmonary bypass would be welcome. Transcatheter mitral valve repair with MitraClip® (Abbott Vascular, CA, USA) is one such technology that uses femoral venous access to repair the mitral valve without requiring cardiac arrest and is the primary subject of this review.
Percutaneous valve interventions
The standard of care for the treatment of MR has been and continues to be surgical repair or replacement of the valve [5]. Conversely, other valvular diseases have been successfully treated by transcatheter interventions. Mitral valve stenosis was first treated with balloon commissurotomy 30 years ago [6]. In 2000, the first pulmonic valve was implanted via a transcatheter delivery system [7]. This was followed shortly after by successful transcatheter aortic valve implantation in 2002 for severe aortic stenosis [8].
Aortic valve stenosis, a common disease in the elderly, has seen a remarkable increase in successful treatment due to the expanding availability and excellent results of the transcatheter aortic valve replacement technology. The PARTNER trial demonstrated superior results compared with medical therapy alone and similar outcomes to open-heart aortic valve surgery in high-risk patients [9]. To expand the application of the therapy, trials evaluating intermediate-risk patients are ongoing. Given the current success, several transcatheter mitral valve technologies are under development to parallel this approach and are starting to be tested in humans. However, the success of these therapies has been more challenging secondary to technical and anatomic considerations specific to the mitral valve.
Traditional mitral valve surgery: successes & pitfalls
Mitral valve surgery is used to treat both primary and secondary MR. Surgery, whether mitral valve repair or replacement, has been the standard of care in treating patients with primary (degenerative) MR. Patients with secondary MR are often treated medically, but surgery is also often employed, especially if the patient is already undergoing another cardiac operation such as a coronary artery bypass. Repair has recently been favored over mitral valve replacement in order to preserve as much of the native valve and subvalvular apparatus as possible. Additionally, the most common mitral valve pathologies includes flail or billowing leaflets that are amenable to resection or repair using artificial chords. These techniques, when performed correctly, result in a durable repair that is associated with reduced mortality, reduce risk of endocarditis and obviate the need for life-long anticoagulation [10–14].
A variety of techniques have emerged to repair the prolapsing mitral valve: traditional quadrangular and triangular resection techniques, replacement of the ruptured chordae with artificial neochordae, placement of an edge-to-edge stitch to anchor the prolapsing leaflet cusp to the opposing stable cusp, and implantation of a mitral annuloplasty ring to improve leaflet coaptation and avoid future annular dilatation. These techniques for primary mitral valve lesions have been quite successful, demonstrated by long-term durability of these repairs.
Unlike primary MR, secondary or functional MR remains a surgical challenge. It is often treated with implantation of an undersized annuloplasty ring or valve replacement. The durability of these repairs is suboptimal, with recurrence rates of 15–60% reported in the literature [15,16]. In the latter-half of the last decade, significant efforts have focused on improving results for surgical mitral annuloplasty with the development of adjustable annuloplasty rings whose size and shape can be adjusted postimplantation on a beating heart (e.g., MitraSolutions®, St Jude Medical [MN, USA], DynaTek [MO, USA] and ValTech [Yehuda, Israel], among others). These approaches have championed technological advancement in mitral valve repair and laid a strong foundation for substantial innovative efforts toward the development of percutaneous valve therapies. However, they are not yet part of clinical practice and remain investigational devices to date.
Percutaneous treatment of MR
Early development in percutaneous mitral valve repair focused on indirect annuloplasty by deploying a device under tension into the coronary sinus to reduce the size of the annulus. This technique utilizes the anatomical proximity of the coronary sinus to the mitral annulus, and seeks to reduce the mitral annular area via annular cinching. Clinical experience with various coronary sinus annuloplasty approaches on reduction of MR has been disconcerting due to several anatomical constraints and mismatch of the coronary sinus in relation to mitral annulus in dilated ventricles [17]. Another complication is compression of a coronary artery (usually the left circumflex) which can lead to myocardial ischemia [18]. One such indirect annuloplasty device, the Carillon mitral contour system (Cardiac Dimensions, Inc., WA, USA) has European CE mark approval and is currently undergoing trials in Europe.
Alternative direct annuloplasty approaches have since emerged. A direct suture annuloplasty system is under development from MitrAlign® (MA, USA). This device uses a suture-pledget system to cinch the mitral annulus by placing two pairs of pledgets into the opposing sides of the mitral annulus. They are then cinched together to reduce the mitral orifice area. This device is not commercially available as of yet, but early results are encouraging [19]. The Accucinch® (CA, USA), which has a similar conceptual form but uses multiple anchors along the entire posterior annulus, is under early development [20]. Finally, the CardioBand® system (Valtech Cardio) uses a trans-septal approach to deliver a flexible ring to the annulus via an automated suture technique. An animal model has demonstrated short-term success, and human studies are underway [21].
Finally, the number of transcatheter mitral valve replacement technologies has grown given the success of transcatheter aortic valve replacement. Commercially available transcatheter aortic valves have been successfully implanted in the mitral position inside of previously implanted mitral valves (valve-in-valve). Much less success has been seen with implantation of these devices directly into the native mitral valve [22]. Limited success has been seen in the mitral position for a variety of reasons. For one, percutaneous access to the mitral valve is more challenging than the aortic valve. Additionally, the mitral annulus is large, irregularly shaped, and is intimately involved in left ventricle (LV) geometry.
Development of the MitraClip
The most common mitral valve pathology resulting in severe symptomatic MR is myxomatous degeneration of the posterior leaflet (primary MR) [23]. As aforementioned, this pathology is most often managed with mitral valve repair. The various techniques for repair have evolved greatly over the last half century. Carpentier described a partial resection of the posterior leaflet of the mitral valve [24], a technique that is used frequently today with excellent long-term durability [25,26]. This technique is best tailored for posterior leaflet prolapse; other pathologies such as anterior prolapse or Barlow’s disease often require more complex repair [27,28]. In an effort to simplify the repair of complex mitral valve pathology, a new technique was developed by Alfieri et al. [29].
The Alfieri repair, originally described in 1991, treats MR by fixing the cusps of the anterior and posterior leaflets together using a double stitch placement at the point of maximal regurgitation. This ‘edge-toedge’ technique creates a double orifice mitral valve, with two smaller inflow orifices to the LV. Long-term results have been good and this technique represents yet another technique for surgeons to repair the valve [30]. To improve the durability of results, the Alfieri repair is typically combined with implantation of a partial band or complete annuloplasty ring, except in cases with a severely calcified mitral annulus [31]. This concept by Alfieri has served as a proof-of-concept for the development of the MitraClip.
Description of the MitraClip
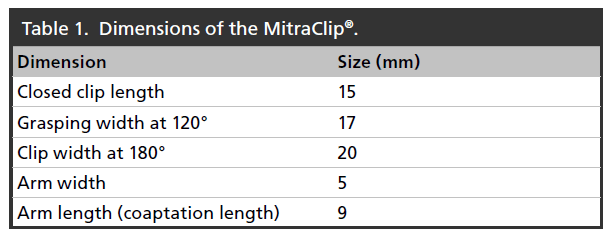
The MitraClip is a single-size clip device that has been used to treat patients with functional, mixed and degenerative MR. The Clip has a dual arm structure, with grippers above the arms to assist with capture of the mitral valve leaflets and their approximation while the heart is beating. Table 1 highlights key MitraClip dimensions.
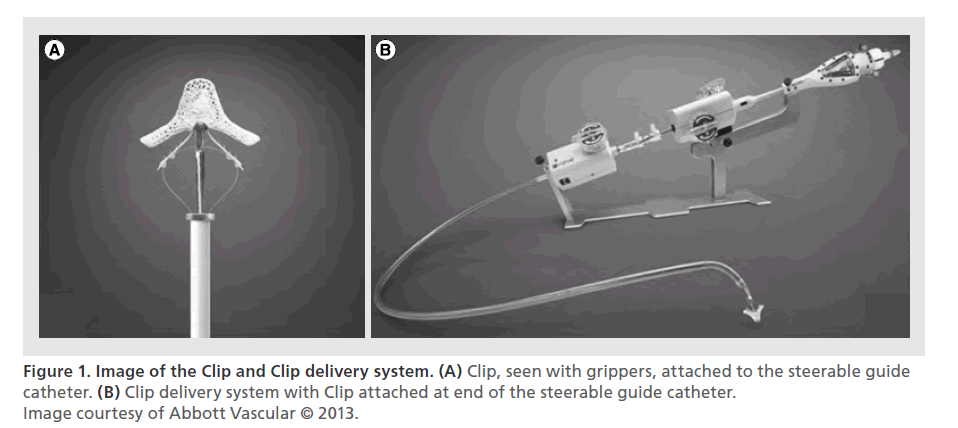
The system is introduced via femoral venous access and uses trans-septal puncture to enter the left atrium. The system includes the Steerable Guide Catheter (SGC) through which the Clip Delivery System (CDS) is introduced. The SGC has a diameter of 24 Fr at the skin and 22 Fr at the interatrial septal puncture site. The Clip itself, which is at the end of CDS, is made of cobalt–chromium and is covered with polypropylene fabric to promote tissue in-growth, and it is certified for use in a MRI up to a magnetic field of 3 T [20]. These materials are commonly used in other cardiovascular implants. Figure 1 shows the Clip and delivery system.
The procedure
The MitraClip is implanted in a percutaneous procedure that is performed using both fluoroscopic and echocardiographic guidance. First, femoral venous access is obtained via standard percutaneous techniques. Then, trans-septal puncture of the interatrial septum is ideally performed at the fossa ovalis to gain access to the left atrium. The patient should then be heparinized to an activated clotting time greater than 250 s, where it should remain for the duration of the procedure. The septal puncture must be appropriately positioned relative to the mitral valve, so that the device can be oriented perpendicular to the mitral valve. All maneuvers are done under echocardiographic visualization including real-time 3D transesophageal echocardiographic guidance (3D TEE). Therefore, an experienced and dedicated echocardiographer facile in not only in 2D but also 3D echocardiography is critical to the performance of these procedures. The device is positioned directly above the regurgitant jet and advanced across the mitral valve into the LV, with the two arms of the Clip perpendicular to the valve leaflets.
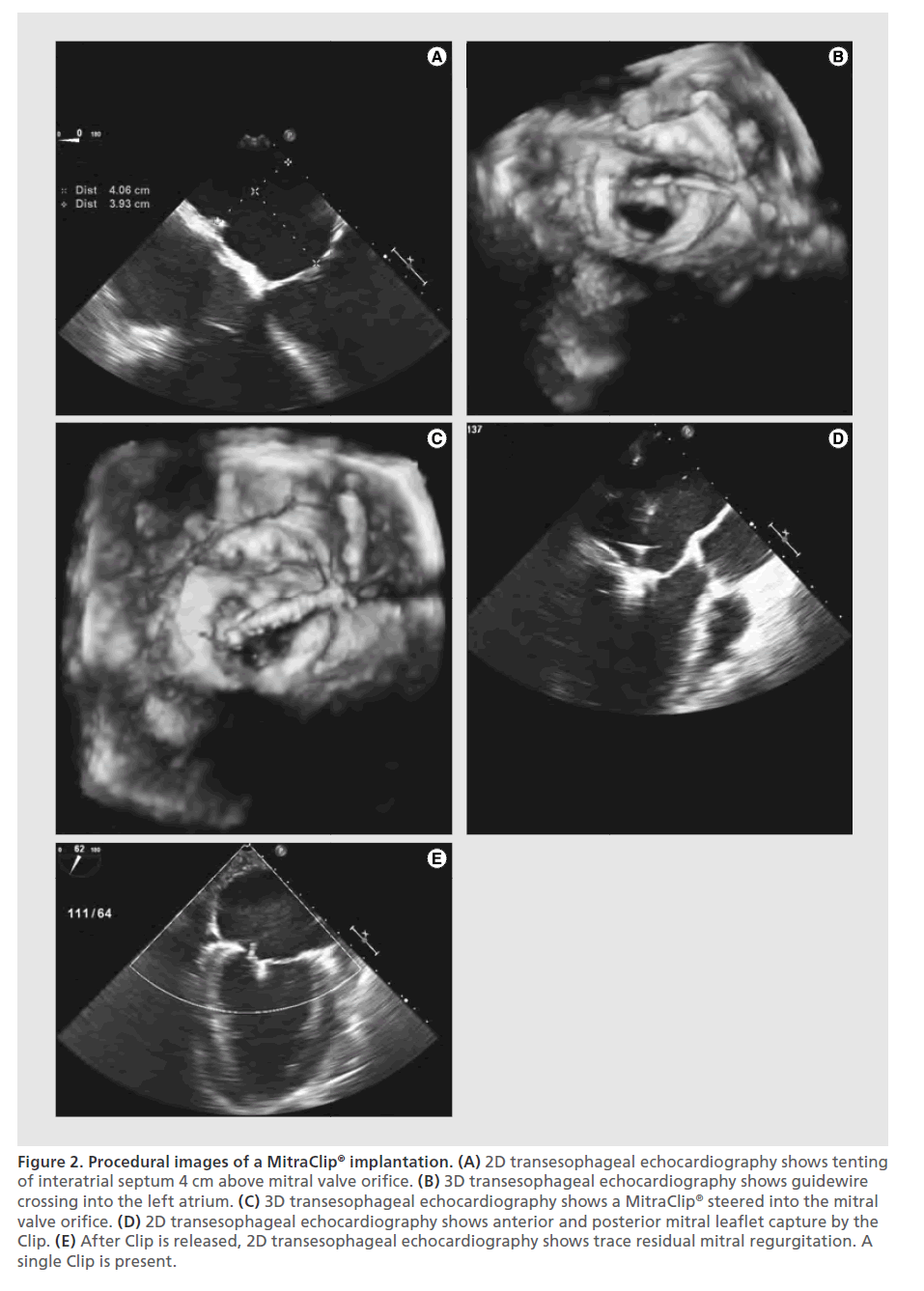
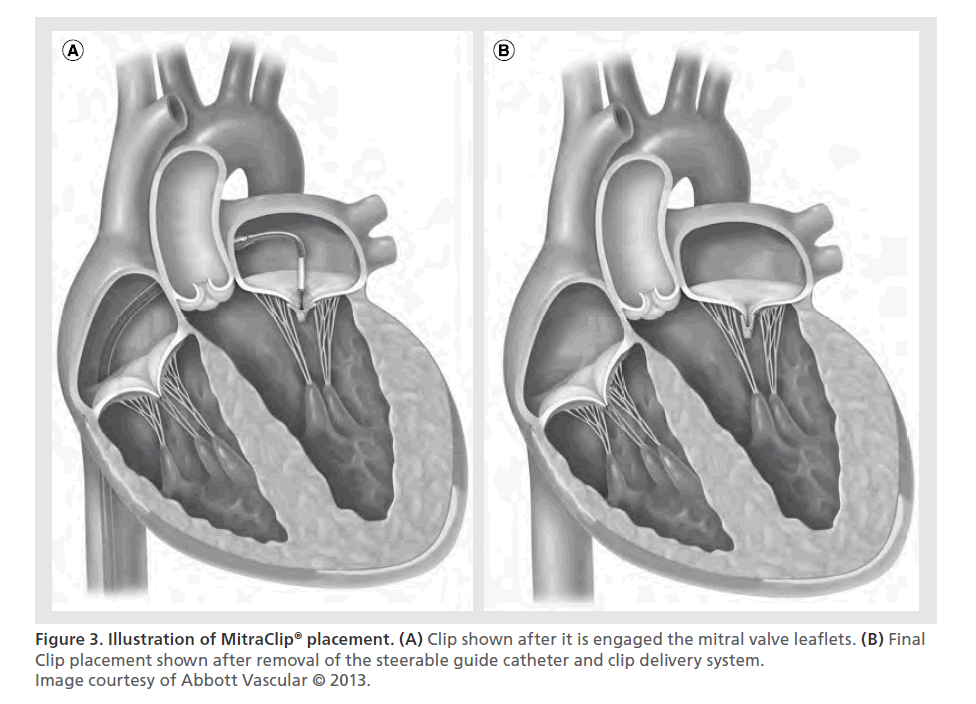
The Clip is then retracted toward the mitral valve leaflets, so it can engage the appropriate segments of the mitral valve. The arms and grippers of the Clip are then closed and if the leaflet insertion is judged acceptable by TEE, the degree of residual MR is assessed. If reduction is inadequate, the Clip can be released and repositioned. When the reduction of MR is judged to be adequate, the Clip is released from the delivery system. If after releasing the Clip, MR of greater than mild is apparent and the mean gradient is less than 5 mmHg, another Clip can be implanted. More than one Clip is implanted in about 50% of patients and implantation of five Clips has been described in the literature [32]. The addition of Clips is limited by, among other factors, development of a transmitral valve diastolic gradient, which is a surrogate measure for potential development of mitral stenosis. After the Clip(s) are deployed, the delivery catheter is retracted and removed from the patient. Protamine is often administered to reverse the heparinization. Manual compression, use of a temporary subcutaneous suture, or placement of a percutaneous closure device may be used to close the femoral vein access site. Figure 2 shows echocardiographic still images of the key steps in implantation of the MitraClip. Figure 3 shows an illustration of MitraClip placement. An illustrated animation of the procedure is available on Abbott’s website [33].
Figure 2. Procedural images of a MitraClip® implantation. (A) 2D transesophageal echocardiography shows tenting of interatrial septum 4 cm above mitral valve orifice. (B) 3D transesophageal echocardiography shows guidewire crossing into the left atrium. (C) 3D transesophageal echocardiography shows a MitraClip® steered into the mitral valve orifice. (D) 2D transesophageal echocardiography shows anterior and posterior mitral leaflet capture by the Clip. (E) After Clip is released, 2D transesophageal echocardiography shows trace residual mitral regurgitation. A single Clip is present.
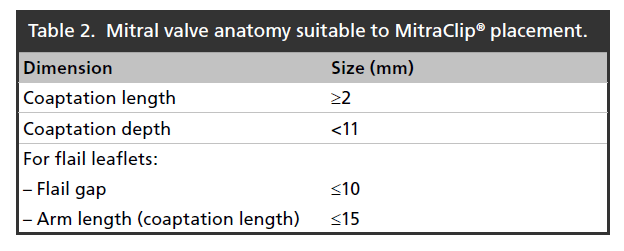
Patients must have suitable anatomy for the Clip to be able to properly grasp and attach to the valve leaflets and reduce MR. The EVEREST I trial [34] defined suggested mitral valve echocardiographic measurements, summarized in Table 2. In addition to these and other anatomic considerations, other important contraindications to the procedure include pre-existing mitral stenosis, active endocarditis, inability to tolerate procedural anticoagulation or post-procedure antiplatelet therapy and clinical frailty of the patient [35].
Table 2. Mitral valve anatomy suitable to MitraClip® placement.
Clinical evaluation of the MitraClip
After evaluation in a porcine model [36], the MitraClip was evaluated in a Phase I safety trial (EVEREST I) [37]. In this trial, the MitraClip was implanted in 107 patients who were candidates for mitral valve repair. After the first ten patients, it was noted that inadequate reduction in MR was often achieved with only one Clip, so the trial was amended to allow for implantation of a second Clip. Results of the trial demonstrated that the device was safe as there were relatively few complications, with ten patients (9%) having a major adverse event. These were defined as: death, myocardial infarction, nonelective cardiac surgery for adverse events, renal failure, transfusion of greater than two units of blood, reoperation for failed surgery, stroke, gastrointestinal complications requiring surgery, ventilation for greater than 48 h, deep wound infection, septicemia and new onset of permanent atrial fibrillation (determined at 12 months). Ten patients (9%) had evidence of partial Clip detachments at 30 days, but there were no Clip embolizations. The degree of reduction in MR persisted throughout the 12-month study period [34].
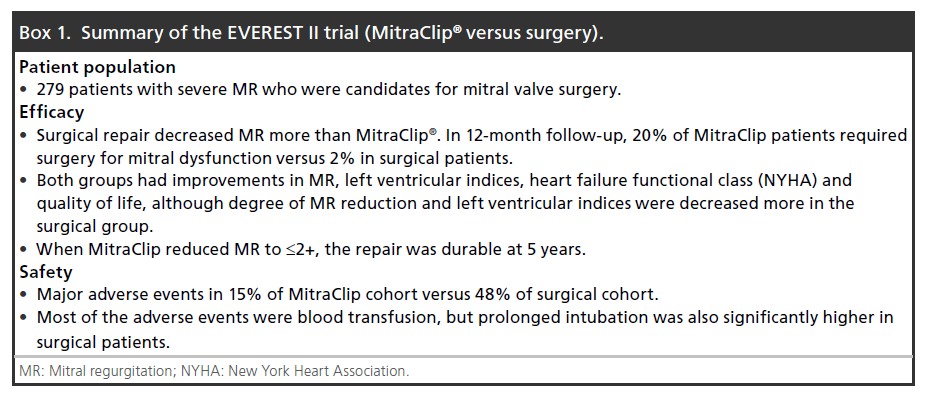
Given the success of this safety trial, the MitraClip was evaluated in a Phase II clinical trial (EVEREST II) [38] comparing the MitraClip directly to mitral valve surgery in a randomized sample of 279 patients. These included patients with both degenerative and functional MR. The primary outcome was freedom from death, surgery for mitral valve dysfunction, or ≥3+ MR, for which surgery was found to be superior. However, short-term outcomes with the Clip were excellent, with only 15% of patients experiencing a major adverse event compared with 48% of surgical patients (as defined by the study). In total, 45% of the patients in the surgical group received a transfusion of ≥2 units of blood. Prolonged mechanical ventilation was also more common in the surgical patients, although only affected 4% of patients (none in the MitraClip arm). While the need for a few units of blood may not be seen as an important complication, it is important to note that blood transfusions have been associated with worse early and late outcomes in cardiac surgery patients [39]. While the degree of MR reduction was larger in the surgical patients, those who achieved a good immediate result with MitraClip showed excellent durability of the repair (at 5 years). Nearly 80% of patients were free from 3+ or 4+ MR after Clip placement and thus avoided surgery in 12-month follow-up. Other important clinical indicators that improved in both groups included heart failure functional status (New York Heart Association), ejection fraction, LV dimensions and QoL. Among patients in the MitraClip arm, there were no incidences of device embolization or clinically significant cases of mitral stenosis. Box 1 summarizes the results of the EVEREST II trial.
Recovery from the MitraClip procedure is rapid, with patients typically only staying in the hospital for 2–3 days, significantly less than after mitral valve surgery (4–7 days). Additionally, patients are more routinely discharged to home after MitraClip implantation when compared with surgery [40]. There has not been any evidence of development of an acute low-output state after MitraClip implantation [41], a complication that has been suggested in the surgical literature of correction of MR [42]. Four-year follow-up of patients in the EVEREST II trial has shown no increase in late MR recurrence compared with surgery [43].
Device approval & current practice
As of October 2013, the MitraClip device has been implanted in over 11,000 patients. In Europe, CE mark was received in March 2008. US FDA approval was first sought in 2010, with the indications of treating surgical and inoperable patients with degenerative and function MR. This approval was denied because FDA did not feel that MitraClip demonstrated an appropriate risk–benefit ratio. Through a series of negotiations between Abbott and FDA, Abbott narrowed their indications to include high-risk and inoperable patients with degenerative MR. This, however, was again not approved. Although the device did show safety in this patient population [44,45], FDA did not feel that the device convincingly showed efficacy due in part to the heterogeneity of MR in these studies. The indications were again amended to only include inoperable patients with degenerative MR. In contrast to treatment for symptomatic functional MR, where medical therapy and other procedures like cardiac resynchronization may improve symptoms and long-term outcomes, there remains no satisfactory minimally invasive options for inoperable patients with degenerative MR. Based partly on this consideration, FDA approval of the MitraClip was granted in October 2013 for patients with severe symptomatic degenerative MR that are considered to be too high risk for conventional mitral valve surgery as determined by an evaluation made by a heart team [46].
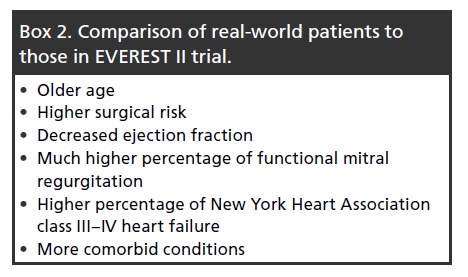
Most of the commercial experience to date is from Europe, since CE mark was granted in 2008 and FDA approval was obtained only recently in late 2013. The majority of postmarket data has been ascertained through various national registries. ACCESS-EU is a postmarket registry of MitraClip patients. A retrospective evaluation of 567 patients in this registry was performed by Maisano et al. [47], where several key differences in real-world application of the MitraClip compared with those in the EVEREST II trial were found. Box 2 summarizes the differences.
In general, these patients are more elderly and higher surgical risk candidates than those evaluated in the EVEREST II trial. More patients had functional MR, which may be related to these patients’ high surgical risk. The anatomic characteristics of the mitral valve in 70–80% of these patients were outside the inclusion criteria stated in the EVEREST II trial [48]. Therefore, in the ‘real-world’ setting it does not appear that many ‘straightforward’ candidates for mitral valve surgery are being referred for MitraClip. Nonetheless, even in this very sick group of patients, clinical outcomes with the Clip were excellent as demonstrated by improvements in degree of MR reduction and improvements in New York Heart Association heart failure classification, quality of living and 6-min-walk test results.
In subgroup analysis of the EVEREST II trial, MitraClip was equivalent to surgery in older patients (≥70 years) and those with functional MR [38]. As seen in the ACCESS-EU data, this is currently the group of patients most commonly receiving MitraClip repair, at least in Europe. This may be replicated in the US experience, although the device does not currently have an indication for functional MR. Time will tell how therapy will evolve going forward, especially as patient selection improves, new and competing technologies become available, and optimization of the procedure continues.
Future perspective
For patients with functional MR, EVEREST II and ACCESS-EU data suggest improvements in heart failure functional class, MR and QoL. However, there have been no clinical studies to date directly comparing MitraClip to medical therapy in patients with heart failure due to functional MR. Currently two such trials are underway, one in the USA (COAPT) [49] and one in Europe (RESHAPE-HF) [50] that are attempting to address this issue.
The COAPT study is a prospective, randomized trial that will compare heart failure patients treated with the MitraClip one-to-one with standard medical therapy. The primary outcome will be recurrent heart failure admissions. Similarly, the RESHAPE-HF study will compare heart-failure patients treated with the MitraClip to standard medical therapy and the primary outcome will be both heart-failure admissions as well as all-cause mortality. The results of these studies will clearly be of great importance. Should there be no differences between groups, it may temper the current practice of implanting the device in patients with functional MR. In surgical patients, there is remarkably little data to support that mitral valve surgery for functional MR improves symptoms over time or life expectancy [5]. Conversely, a positive study might lead to an FDA indication for functional MR. The results may somewhat address the ongoing question of whether functional MR itself can be improved with valve therapy of any kind; regardless of whether it is a percutaneous or surgical intervention. It is known that the mitral annulus will continue to dilate as heart failure worsens over time, so these studies will offer insight into whether this process can be reversed or held at bay with valve therapy. This will also be important in surgical patients; a positive result may support mitral valve repair or replacement for functional MR as a concomitant procedure when undergoing another open-heart procedure.
Improvements in surgical mitral valve therapies over the past decades have resulted in excellent outcomes in low- and medium-risk patients. With the recent advent of percutaneous mitral valve therapies, there is evidence of a parallel refinement in techniques and improvement in patient selection. Primarily high surgical risk patients are being referred today for consideration as candidates for MitraClip therapy. The heart team, consisting of the interventional cardiologist, cardiac surgeon, heart-failure specialist, echocardiographer and others are critical in the determining the appropriate patients with severe MR who should undergo the MitraClip procedure. Even with the success of the therapy there are opportunities to improve the current design of the device. Potential improvements could include easier steering of the device, smaller access sheath and perhaps improved Clip design to prevent single leaflet detachment and increased reduction in MR. As technology and experience progresses, it is possible that percutaneous clipping of the mitral valve will be combined with a percutaneous annuloplasty device. This would more closely resemble the Alfieri approach in surgical practice. This would provide both repair of the valve itself and address pathologies of the valvular apparatus.
For patients with coronary artery disease, surgical volumes decreased as percutaneous stenting gained acceptance. To date, it does not appear that patients who are good candidates for surgical mitral valve repair or replacement are being referred for Mitra- Clip therapy, but there is clearly a patient preference toward minimally invasive approaches, and time will tell whether healthier patients are eventually referred for MitraClip as technology and techniques advance.
Disclaimer
In addition to the peer-review process, with the authors’ consent, the manufacturer of the product discussed in this article was given the opportunity to review the manuscript for factual accuracy. Changes were made at the discretion of the authors and based on scientific or editorial merit only.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Mitral regurgitation
• Common disease of elderly, with degenerative mitral regurgitation (MR) being most common etiology.
• Surgical repair is the current gold standard.
Percutaneous approaches
• Percutaneous valve interventions have shown great success in the treatment of mitral stenosis, as well as for aortic and pulmonary valve replacements (e.g., SAPIEN [Edwards, CA, USA], CoreValve [Medtronic, MN, USA], Melody [Medtronic]).
• Percutaneous mitral annuloplasty devices, both indirect and direct approaches, are under development, but early results are mixed.
• The MitraClip® is a device that simulates the Alfieri repair (edge-to-edge) to treat functional and degenerative MR via a percutaneous approach (without associated annuloplasty).
Implanting the MitraClip
• Standard trans-septal access is obtained. Fluoroscopic and echocardiographic guidance are used during the procedure.
• Position the device perpendicular to the mitral valve and advance into the left ventricle.
• Retract device toward mitral valve and capture leaflets.
• Assess reduction in MR immediately with transesophageal echocardiography, while the heart remains beating.
• Clip can be removed and repositioned to improve reduction in MR.
• Additional Clips can be implanted if necessary.
• Surgical options are still preserved.
Clinical evaluation
• EVEREST I trial demonstrated clinical safety.
• EVEREST II trial showed good success, although not as effective as surgery. Procedure was very safe, and reduced the need for blood transfusion and mechanical ventilation.
• In total, 20% of patients required surgery for persistent MR in 12-month follow-up.
• When initially success in reducing MR, the Clip showed durability over 5 years.
• Few device-related complications, most commonly single leaflet detachment.
Device approval & current practice
• CE mark granted in 2008 and FDA approved in 2013. Majority of commercial practice to date has been in Europe.
• Functional MR has been treated more often than degenerative (as opposed to EVEREST II trial) in the commercial experience in Europe.
• Most patients selected for MitraClip are high-risk surgical candidates.
Future perspective
• COAPT and RESHAPE-HF trials underway to demonstrate the benefit of the therapy in high-risk patients with heart failure due to functional mitral regurgitation.
• These will compare MitraClip directly to medical therapy. Goal is to reduce mortality and heart-failure admissions.
• Future advances could include smaller delivery system and improved design to prevent partial leaflet detachment.
References
Papers of special note have been highlighted as: • of interest
- Nkomo VT, Gardin JM, Skelton TN et al. Burden of valvular heart diseases: a population-based study. Lancet 368, 1005–1011 (2006).
- Glower DD. Surgical approaches to mitral regurgitation. J. Am. Coll. Cardiol. 60, 1315–1322 (2012).
- Casselman FP, Van Slycke S, Wellens F et al. Mitral valve surgery can now routinely be performed endoscopically. Circulation 108, II-48–54 (2003).
- Murphy DA, Miller JS, Langford DA et al. Endoscopic robotic mitral valve surgery. J. Thorac. Cardiovasc. Surg. 132, 776–781 (2006).
- Nishimura RA, Otto CM, Bonow RO et al. 2014 AHA/ ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 129(23), e521–e643 (2014).
- Inoue K, Owaki T, Nakamura T et al. Clinical application of transvenous commissurotomy by a new balloon catheter. J. Thorac. Cardiovasc. Surg. 87, 394–402 (1984).
- Zahn EM, Hellenbrand WE, Lock JE, McElhinney DB. Implantation of the melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit. Early results from the US clinical trial. J. Am. Coll. Cardiol. 54, 1722–1729 (2009).
- Cribier A, Eltchaninoff H, Bash A et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 106, 3006–3008 (2002).
- Smith CR, Leon MB, Mack MJ et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 364, 2187–2198 (2011).
- Vassileva CM, Mishkel G, McNeely C et al. Long-term survival of patients undergoing mitral valve repair and replacement: a longitudinal analysis of medicare fee-forservice beneficiaries. Circulation 127, 1870–1876 (2013).
- Gillinov MA, Faber C, Houghtaling PL et al. Repair versus replacement for degenerative mitral valve disease with coexisting ischemic heart disease. J. Thorac. Cardiovasc. Surg. 125, 1350–1362 (2003).
- Thourani VH, Weintraub WS, Guyton RA et al. Outcomes and long-term survival for patients undergoing mitral valve repair versus replacement. Effect of age and concomitant coronary artery bypass grafting. Circulation 108, 298–304 (2003).
- Feringa HH, Shaw LJ, Poldermans D et al. Mitral valve repair and replacement in endocarditis: a systematic review of literature. Ann. Thorac. Surg. 83, 564–571 (2007).
- Enriquez-Sarano M, Schaff HV, Orszulak TA et al. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation 91, 1022–1028 (1995).
- McGee EC, Gillinov AM, Blackstone EH et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J. Thorac. Cardiovasc. Surg. 128, 916–924 (2004).
- Kuwahara E, Otsuji Y, Iguro Y et al. Mechanism of recurrent/persistent ischemic/functional mitral regurgitation in the chronic phase after surgical annuloplasty: importance of augmented posterior leaflet tethering. Circulation 114, I529–I534 (2006).
- Webb JG, Harnek J, Munt BI et al. Percutaneous transvenous mitral annuloplasty: initial human experience with device implantation in the coronary sinus. Circulation 113, 851–855 (2006).
- Masson J, Webb JG. Percutaneous treatment of mitral regurgitation. Circ. Cardiovasc. Interv. 2, 140–146 (2009).
- Mandinov L, Büllesfeld L, Kuck K-H, Grube E. Early insight into mitralign direct annuloplasty for treatment of functional mitral regurgitation. Interv. Cardiol. Rev. 6, 170–172 (2011).
- Feldman T, Young A. Percutaneous approaches to valve repair for mitral regurgitation. J. Am. Coll. Cardiol. 63, 2057–2068 (2014).
- Maisano F, Vanermen H, Seeburger J et al. Direct access transcatheter mitral annuloplasty with a sutureless and adjustable device: preclinical experience. Eur. J. Cardiothorac. Surg. 42, 524–529 (2012).
- Wilbring M, Alexiou K, Malte Tugtekin S et al. Pushing the limits—further evolutions of transcatheter valve procedures in the mitral position, including valve-in-valve, valve-in-ring, and valve-in-native-ring. J. Thorac. Cardiovasc. Surg. 147, 210–219 (2014).
- Olson LJ, Subramanian R, Ackermann DM et al. Surgical pathology of the mitral valve: a study of 712 cases spanning 21 years. Mayo Clin. Proc. 62, 22–34 (1987).
- Carpentier A. Cardiac valve surgery-the ‘French correction’. J. Thorac. Cardiovasc. Surg. 86, 323–337 (1983).
- Gillinov AM, Cosgrove DM, Blackstone EH et al. Durabilty of mitral valve repair for degenerative disease. J. Thorac. Cardiovasc. Surg. 116, 734–743 (1998).
- Braunberger E, Deloche A, Berrebi A et al. Very long-term results (more than 20 years) of valve repair with Carpentier’s Techniques in nonrheumatic mitral valve insufficiency. Circulation 104, I8–I11 (2001).
- Salati M, Moriggia S, Scrofani R, Santoli C. Chordal transposition for anterior mitral prolapse: early and longterm results. Eur. J. Cardiothorac. Surg. 11, 268–273 (1997).
- Zussa C, Polesel E, Rocco F, Valfrè C. Artificial chordae in the treatment of anterior mitral leaflet pathology. Cardiovasc. Surg. 5, 125–128 (1997).
- Fucci C, Sandrelli L, Pardini A et al. Improved results with mitral valve repair using new surgical techniques. Eur. J. Cardiothorac. Surg. 9, 621–626 (1995).
- Alfieri O, Maisano F, De Bonis M et al. The doubleorifice technique in mitral valve repair: a simple solution for complex problems. J. Thorac. Cardiovasc. Surg. 122, 674–681 (2001).
- Maisano F, Viganò G, Blasio A et al. Surgical isolated edge-to-edge mitral valve repair without annuloplasty: clinical proof of the principle for an endovascular approach. EuroIntervention 2, 181–186 (2006).
- Paranskaya L, D’Ancona G, Bozdag-Turan I et al. Percutaneous vs surgical repair of mitral valve regurgitation: single institution early and midterm outcomes. Can. J. Cardiol. 29, 452–459 (2013).
- MitraClip®. Percutaneous mitral valve repair. www.abbottvascular.com/us/mitraclip.html
- Feldman T, Kar S, Rinaldi M et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J. Am. Coll. Cardiol. 54, 686–694 (2009).
- Mauri L, Garg P, Massaro JM et al. The EVEREST II trial: design and rationale for a randomized study of the evalve mitraclip system compared with mitral valve surgery for mitral regurgitation. Am. Heart J. 160, 23–29 (2010).
- St. Goar FG, Fann JI, Komtebedde J et al. Endovascular edge-to-edge mitral valve repair: short-term results in a Porcine Model. Circulation 108, 1990–1993 (2003).
- Feldman T, Wasserman HS, Herrmann. HC et al. Percutaneous mitral valve repair using the edge-to-edge technique. Six-month results of the EVEREST Phase I clinical trial. J. Am. Coll. Cardiol. 46, 2134–2140 (2005).
- Feldman T, Foster E, Glower DD et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 364, 1395–1406 (2011).
- Murphy GJ, Reeves BC, Rogers CA et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 116, 2544–2552 (2007).
- Rogers JH, Franzen O. Percutaneous edge-to-edge MitraClip® therapy in the management of mitral regurgitation. Eur. Heart J. 32, 2350–2357 (2011).
- Siegel RJ, Biner S, Rafique AM et al. The acute hemodynamic effects of MitraClip® therapy. J. Am. Coll. Cardiol. 57, 1658–1665 (2011).
- Detaint D, Sundt TM, Nkomo VT et al. Surgical correction of mitral regurgitation in the elderly. Outcomes and recent improvements. Circulation 114, 265–272 (2006).
- Mauri L, Foster E, Glower DD et al. 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J. Am. Coll. Cardiol. 62, 317–328 (2013).
- Glower D, Kar S, Lim DS et al. Percutaneous MitraClip device therapy for mitral regurgitation in 351 patients - high risk subset of the EVEREST II study. J. Am. Coll. Cardiol. 64, 172–181 (2014).
- Lim DS, Reynolds MR, Feldman T et al. Improved functional status and quality of life in prohibitive surgical risk patients with degenerative mitral regurgitation following transcatheter mitral valve repair with the MitraClip System. J. Am. Coll. Cardiol. 64, 182–192 (2014).
- MitraClip® Delivery System – P100009. www.fda.gov/MedicalDevices/ ProductsandMedicalProcedures/ DeviceApprovalsandClearances/Recently- ApprovedDevices/ucm375149.htm
- Maisano F, Franzen O, Baldus S et al. Percutaneous mitral valve interventions in the real world. Early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip® therapy in Europe. J. Am. Coll. Cardiol. 62, 1052–1061 (2013).
- Schillinger W, Athanasiou T, Weicken N et al. Impact of the learning curve on outcomes after percutaneous mitral valve repair with MitraClip® and lessons learned after the first 75 consecutive patients. Eur. J. Heart Fail. 13, 1331–1339 (2011).
- Cardiovascular Outcomes Assessment of the MitraClip® Therapy Percutaneous Therapy for High Surgical Risk Patients (COAPT). http://clinicaltrials.gov/ct2/show/NCT01626079
- A Randomized Study of the MitraClip® Device in Heart Failure Patients With Clinically Significant Functional Mitral Regurgitation (REHAPE-HF). http://clinicaltrials.gov/ct2/show/NCT01772108
• Current valvular heart disease guidelines.
• Description and results of surgical edge-to-edge repair.
• Landmark trial comparing MitraClip® directly to surgery
• Commercial usage of the MitraClip® device in Europe.