Research Article - Journal of Medicinal and Organic Chemistry (2025) Volume 8, Issue 1
Medicinal Perspective of Quinazolinone Derivatives as an Anticancer Agent
Anushri Deulkar*
Department of Medicinal Chemistry, Arni University, Kangra, India
- *Corresponding Author:
- Anushri Deulkar
Department of Medicinal Chemistry, Arni University, Kangra, Indi
E-mail: anushrideulkar24@gmail.com
Received: 01-Jan-2024, Manuscript No. JMOC-24-124220; Editor assigned: 03-Jan-2024, PreQC No.JMOC-24-124220 (PQ); Reviewed: 17-Jan-2024, QC No.JMOC-24-124220; Revised: 13-Feb-2025, Manuscript No.JMOC-24-124220 (R); Published: 20-Feb-2025, DOI: 10.37532/ jmoc.2025.8(1).282-290281
Abstract
Cancer is a disease in which abnormal cells divide uncontrollably and destroy body tissue. Cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020 or nearly one in six deaths. Although there are many anticancer medications available to treat cancer, their therapeutic efficacy is limited for a variety of reasons. Therefore, the discovery of novel cancer treatments is required. Quinazoline, Quinoxaline, Cinnoline, and Phthalazine are the four isomers of quinazoline that comprise up the class of bicyclic aromatic ring structures that have a benzene ring bonded to a two-nitrogen aromatic ring. A broad range of compounds known as quinolines have a variety of pharmacological actions. They have a variety of additional uses, including anti- histaminic, anti-cancer, anti-convulsant, anti-tubercular and anti-viral properties. Many FDA-approved anticancer drugs have their principal chemical structure as 4-aminoarylquinazoline. The purpose of this review is to present several aspects of medicinal chemistry, including drug design, the structure activity relationship and the mode of action of several distinct anticancer quinazoline derivatives. This review provides medicinal chemists with a comprehensive viewpoint to assist in the development of novel quinazolines as targeted chemotherapeutic agents.
Keywords
Quinazoline • Anti-cancer • 4-aminoarylquinazoline • Development
Introduction
Introduction to Quinazoline and it’s derivatives
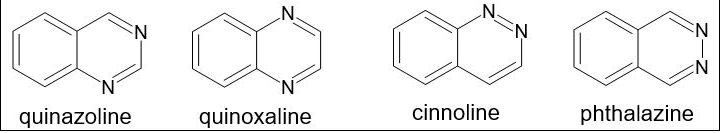
Weddige was the researcher who originally gave the Quinazoline its name since he saw that it is isomeric with two different substances, cinnoline and quinoxaline [1]. There are four known isomers of quinazoline, which have a place with the class of bicyclic fragrant ring structures that incorporate a benzene ring connected to a sweet-smelling ring that contains two nitrogen molecules. These are pyridazine, pyrimidine, and pyrazine. Their primary equations are represented in Figure 1. This gathering of isomers, otherwise called diazanaphthalenes, can be recognized by where the nitrogen particle is situated inside the heterocyclic ring. Two melded six-membered basic sweet-smelling rings, a pyrimidine ring and a benzene ring, make up the substance known as quinazoline. Phthalazine has a benzene ring and a pyridazine ring and is otherwise called benzo-orthodiazine or benzopyridazine. A benzene ring in addition to a pyrazine ring make up quinoxaline, ordinarily known as a benzopyrazine. Cinnoline is a substance with a heterocyclic twofold ring structure that comprises of a pyridazine ring and a benzene ring [2].
Figure 1. Quinazoline isomer.
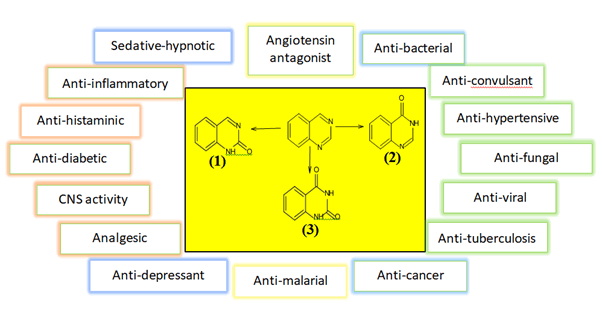
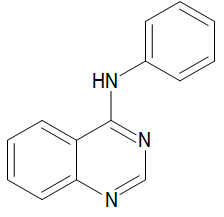
The quinazoline framework might comprise of either quinazoline-2(1H)-one (2-quinazolinone) 1 (Figure 2) or quinazoline-4(3H)-one (4-quinazolinone) 2, where the oxo bunch (=O) structures the carbonyl gathering (C=O) [3-7]. If not, quinazoline-2,4-dione 3 (quinazolinedione) might be shaped by the presence of two oxo bunches at C-2 and C-4. A different arrangement of mixtures known as quinolines have various pharmacological activities [8-11]. Various more purposes incorporate pain relieving, hostile to hypertensive, calming, against bacterial, against diabetic, narcotic entrancing, hostile to histaminic, hostile to malignant growth, against convulsant, against tubercular, and against viral specialists [12-16].
Figure 2. The quinazolines and some of their pharmacological activities.
Literature Review
Introduction to cancer
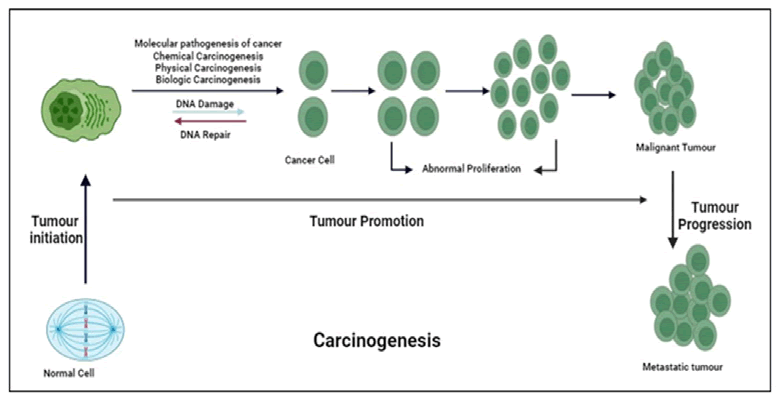
Carcinogenesis is the process of changing a healthy, normal cell into a tumor cell. It requires several genes and genetic alterations. Origination, promotion and progression are all parts of the multifaceted process known as carcinogenesis [17]. The first stage begins with the formation of a cell that has undergone permanent alteration and is typically connected to a mutation and several start pathways. The second stage, which is most likely a benign lesion, sees the original changed cells develop into a visible mass of cells. During the promotion stage, epigenetic factors that affect the starting cells’ ability to proliferate are undoubtedly present. Mostly benign or noncancerous cells or occasionally pre-cancerous cells, are the end result of promotion. These benign cells undergo a few more genetic alterations before they develop into cancerous cells. The third and last stages of carcinogenesis are distinct from the first two stages due to involvement of the development of malignant tumors from benign non-cancerous tumors [18]. Due to a range of physical, chemical or biological stimuli, including viruses, stem cells play a critical role in the initiation of carcinogenesis. Preneoplastic cells must undergo consecutive processes in order to change malignantly since once began, these cells would be exposed to a stimulus that would speed up the production of fully neoplastic cells (Figure 3).
Figure 3. The quinazolines and some of their pharmacological activities.
Why cancer?
In 2020, cancer will be the primary cause of approximately 10 million deaths or one in every six deaths. Breast (2.26 million cases), lung (2.21 million cases), colon and rectum (1.93 million cases), prostate (1.41 million cases), skin (nonmelanoma) (1.20 million cases) and stomach (1.09 million cases) were the most prevalent types of cancer in 2020 in terms of new cases.
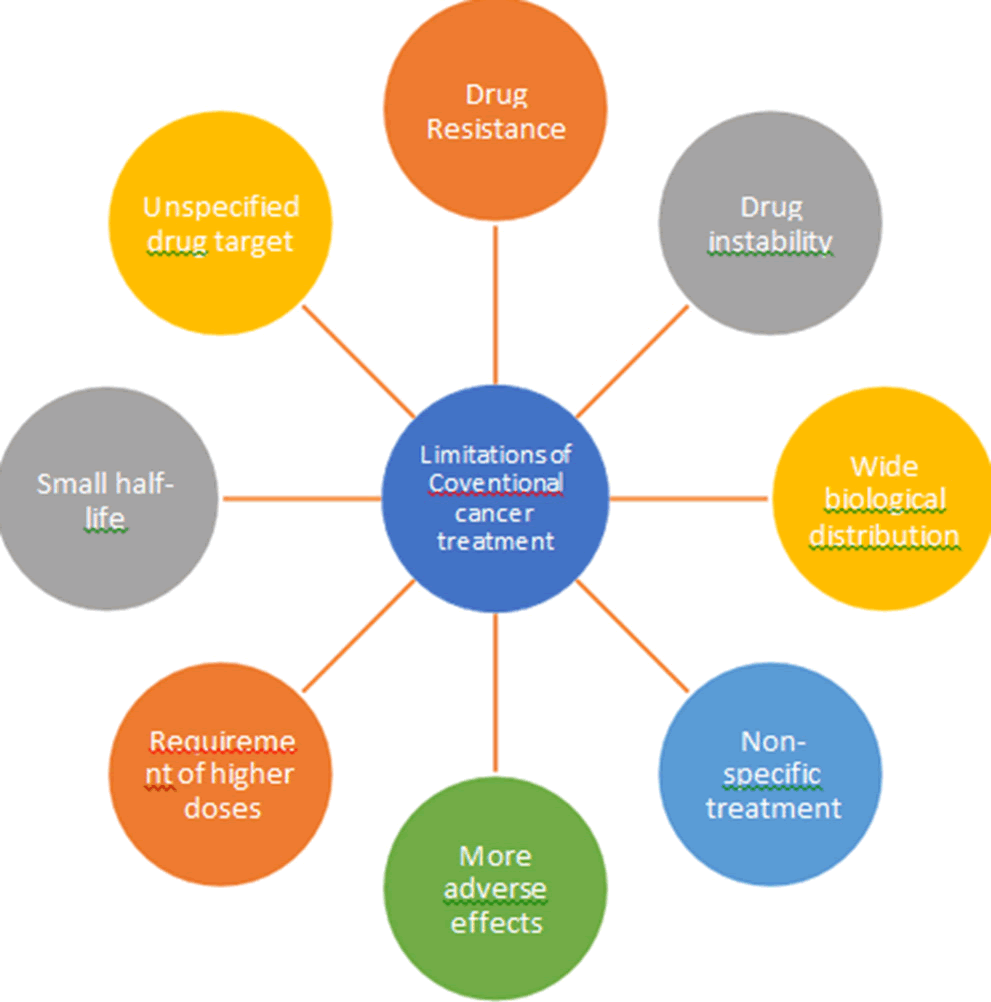
Lung (1.80 million deaths), colon and rectum (916,000 deaths), liver (830,000 deaths), stomach (769,000 deaths) and breast (685,000 deaths) were the most prevalent cancer mortality causes in 2020. 400,000 kids are diagnosed with cancer in children every year. The most prevalent malignancies differ between nations. In 23 nations, cervical cancer is the most prevalent type. There are many anticancer medications available to treat cancer, but their therapeutic efficacy is limited for a number of reasons, including nonspecific treatment, greater adverse effects, higher dosage requirements, short half-lives, widespread biological distribution, drug resistance, drug instability and lack of drug targeting.
A major barrier to effective cancer treatment is the development of resistance by cancerous cells to anticancer drugs. This resistance develops due to a variety of mechanisms, including drug efflux, epigenetics, DNA mutation, cell death inhibition, drug degradation, drug inactivation and drug target alteration. Higher anticancer medication doses are necessary, which increases non-cancerous cell toxicity and multidrug resistance. Anticancer medication instability and poor water solubility are further challenges in the treatment of cancer.
Limitations of conventional cancer treatment given in Figure 4.
Figure 4. Limitations of conventional cancer treatment.
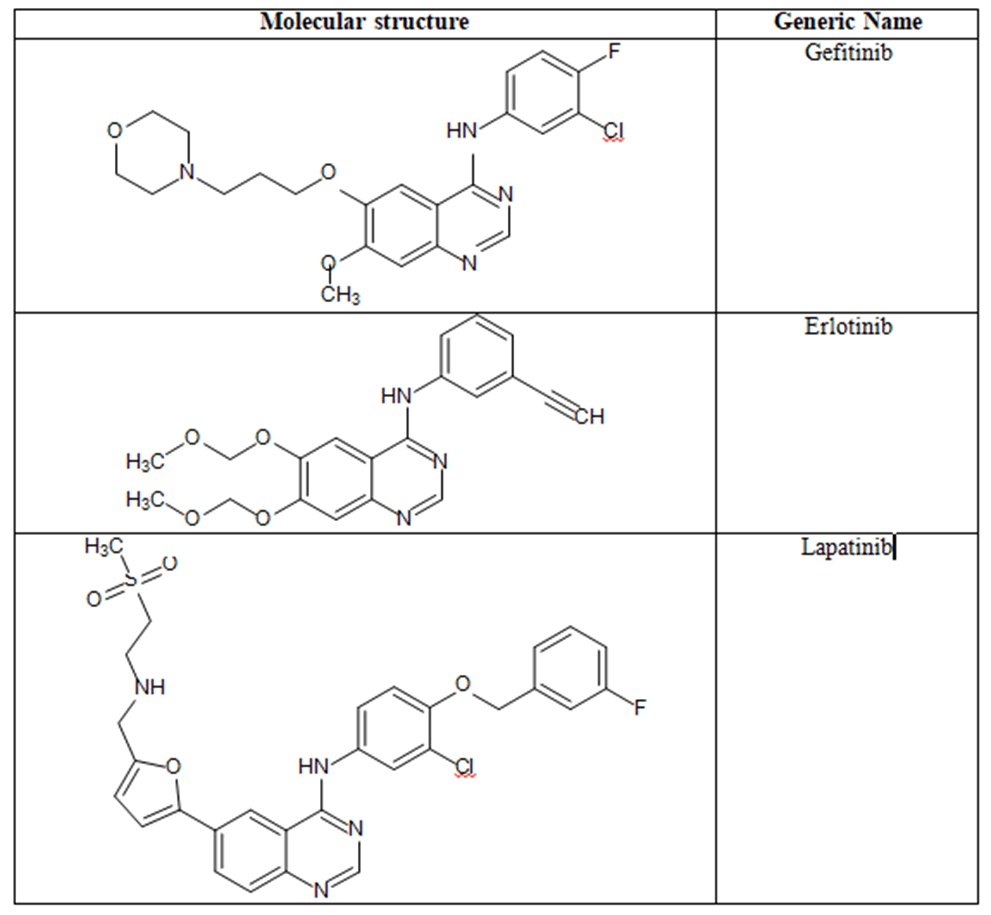
Some quinazoline based FDA-approved anticancer agents: Some FDA-approved anti-cancer agents which are illustrated in Figure 5.
Figure 5. Molecular structures and generic names of some marketed anticancer quinazolines.
Physicochemical characters of the center primary element of anticancer quinazolines: The 4-aminoarylquinazoline is the center construction of numerous FDA-endorsed anticancer specialists (Figure 6). The physicochemical characters of 4-aminoarylquinazolineare displayed in the Table 1.
Figure 6. 4-aminoarylquinazoline.
| Character | 4-aminoarylquinazoline |
| Molecular formula | C8H9N3 |
| Molecular weight | 147.18 g/mol |
| Number of heavy atoms | 11 |
| Number of aromatic heavy atoms | 6 |
| Fraction Csp3 | 0.12 |
| Number of rotatable bonds | 0 |
| Number of H-bond acceptors | 2 |
| Number of H-bond donors | 2 |
| Molar refractivity | 51.25 |
| Tropological polar surface area | 50.41 A2 |
| Lipophilicity | 0.66 |
| Water solubility | Soluble |
| GI absorption | High |
| BBB permeation | No |
| Bioavailability score | 0.55 |
| Lipinski | Yes |
| Synthetic accessibility | Easy |
Table 1. The Physicochemical characters of 4-aminoarylquinazoline.
Some methods of preparation of quinazolines
The first quinazoline derivative, 2-cyano-3,4- dihydro-4-oxoquinazoline, was synthesized in 1869 by Griess et al. By decarboxylating the 2-carboxy molecule, Bischler and Lang produced analogs of the quinazoline that are similar. Gabriel synthesized quinazoline in 1903 by converting o-nitrobenzylamine to o-aminobenzylamine, which then condensed with formic acid to produce dihydroquinazoline, which was then further oxidized to produce quinazolin-4-one.
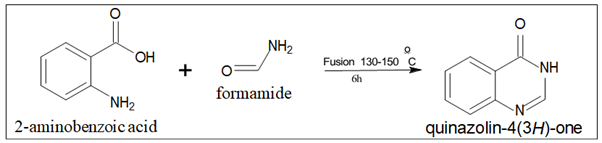
- Niemtowski technique: Quinazolin-4(3H)- one is produced with a 40% yield by fusing anthranilic acid (2-aminobenzoic acid) and formamide at 130°C–150°C for six hours (Figure 7).
Figure 7. Quinazoline isomer.
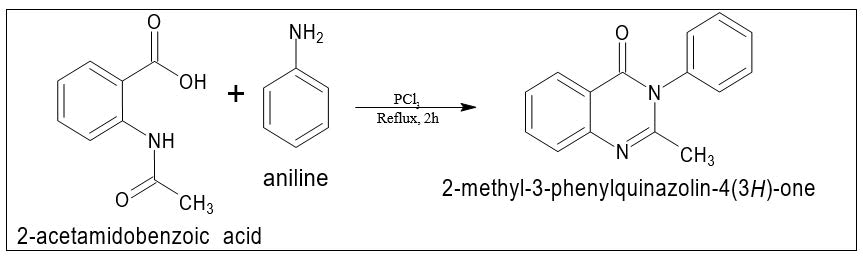
- Morgan technique: 2-methyl-3- phenylquinazolin-4(3H)-one with a 45% yield, was produced by refluxing 2-acetamidobenzoic acid and an aromatic amine in toluene for two hours while employing phosphorous trichloride (PCl3) as a catalyst (Figure 8).
Figure 8. Quinazoline isomer.
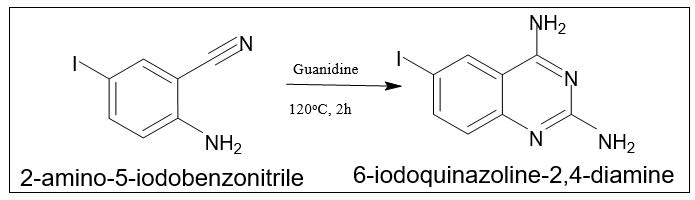
By reaction of guanidine with 2-aminobenzonitrile: III. By the reaction of guanidine with 2-aminobenzonitrile: Guanidine and 2-aminobenzonitrile can be heated at 120°C for two hours to produce 6-iodoquinazoline-2,4-diamine with a 90% yield (Figure 9).
Figure 9. Synthesis of 6-iodoquinazoline-2,4-diamine.
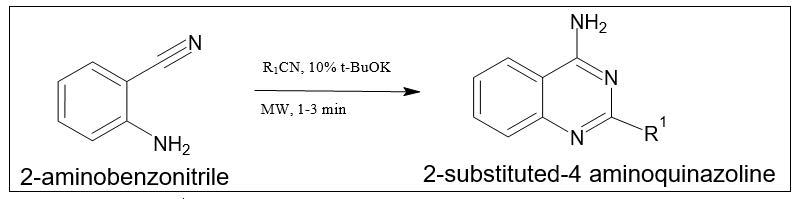
By reaction of 2-aminobenzonitrile with nitrile: By mixing 2-aminobenzonitrile with nitriles under basic conditions in the microwave for one to three minutes, 2-substituted-4-aminoquinazolines can be produced with a yield of 76 to 93 percent (Figure 10).
Figure 10. Synthesis of 2-substituted-4-methylquinazoline.
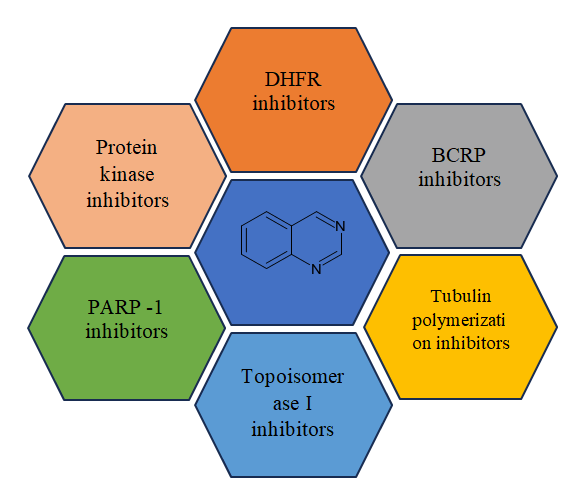
Modes of action anti-cancer quinazolines
Modes of action anti-cancer quinazolines shows in Figure 11.
Figure 11. Modes of action anticancer Quinazolines.
Discussion
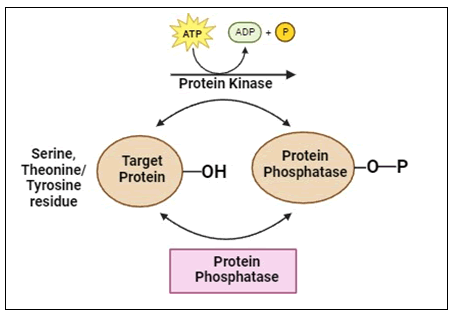
Protein kinase inhibitors: Protein kinases are intracellular enzymes that control the division and development of cells. Protein kinases are phosphate-binding enzymes that phosphorylate serine, threonine or tyrosine residues in cells by attaching phosphate to their side chains. Protein kinases provide ATP to a serine, threonine or tyrosine residue in a protein (Figure 12).
Figure 12. Phosphorylation by protein kinase.
A “molecular shift” caused by phosphorylation makes it possible to directly activate or deactivate proteins. On the other hand, protein phosphatases catalyze the removal of the phosphate from the target protein, which suppresses kinase activity and reverses the effects of phosphorylation. Over one third of all protein phosphorylation events (O-phosphorylation) occur on serine (Ser or S), threonine (Thr or T) and tyrosine (Y or Y) residues. Both the 568 protein kinases and the 156 protein phosphatases found in the human genome regulate phosphorylation events and as a result, they are essential for biological processes such cell division, proliferation and apoptosis.
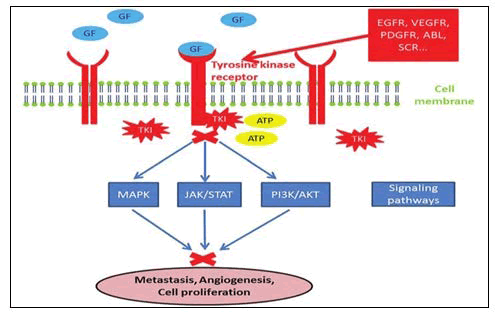
Tyrosine kinase inhibitors: Tyrosine Kinases (TK) are an essential part of the oncoproteins connected to the different kinds of malignancies. They were chosen as a promising biological target for anticancer drugs. Transmembrane receptor related and non-Receptor Tyrosine Kinase (nRTK) are the two types of protein tyrosine kinases. RTKs come in more than 20 different varieties, according to research. One of these extracellular tyrosine kinase receptors (RTK) becomes dimerized when it binds the ligand. Various cell signaling pathways, including Phosphoinositide 3-Kinases (PI3Ks), Mitogen-Activated Protein Kinase (MAPK) and signal transducer activator of transcription 3 (STAT3), are launched as a result of this process, which causes the cytoplasmic tyrosine kinase to phosphorylate various types of tyrosine residues (Figure 13). The non-receptor tyrosine kinase (nRTK), on the other hand, are enzymes that play a significant part in controlling cell proliferation, differentiation, migration, adhesion and death.
Figure 13. Phosphorylation by protein kinase.
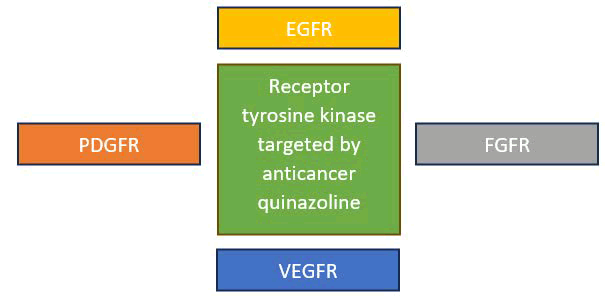
Quinazoline molecules target the (RTKs) which involve the following receptors:
- Epidermal Growth Factor Receptor (EGFR)
- Platelet Derived Growth Factor Receptor (PDGFR)
- Vascular Endothelial Growth Factor Receptor (VEGFR)
- Fibroblast Growth Factor Receptor (FGFR)
These RTKs have been discovered to be overexpressed in a variety of cancers, including ovarian, colon, breast, lung, stomach and prostate cancer. These PTKs, including EGFR, VEGFR-2, PDGFR and FGFR, were shown to be inhibited by 4-anilinquinazoline derivatives.
As a result, many anticancer medicines against various types of malignancies were investigated using these compounds (Figure 14).
Figure 14. Receptor tyrosine kinases which are targeted by the anticancer quinazoline.
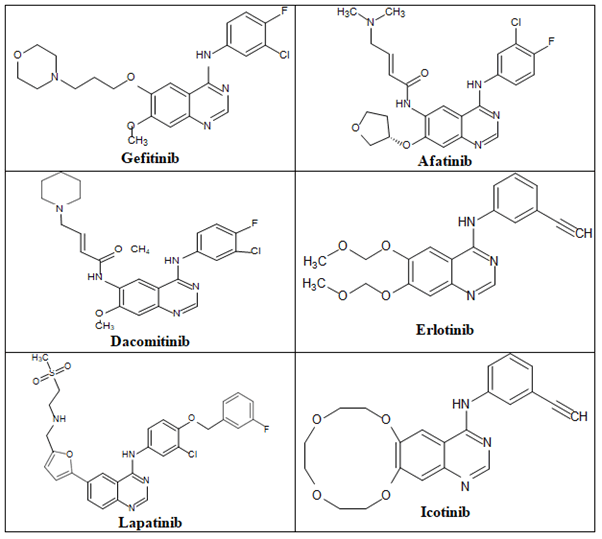
Epidermal Growth Factor Receptor (EGFR): Epidermal Growth Factor Receptor (EGFR) inhibitors are a class of medications that are used to treat various common malignancies, including breast, colon, lung and pancreatic cancer. The four members of the EGFR family share structural similarities and include the EGFR (HER1/ErbB1), ErbB2 (HER2/neu), ErbB3 (HER3) and ErbB4 (HER4). In the cell signaling pathways affecting cell proliferation, apoptosis, angiogenesis and metastatic dissemination, EGFR plays a significant mediating role. There are now two kinds of anti-EGFR medications available. These include monoclonal Antibodies (mAbs) against the extracellular domain that can prevent the dimerization of the receptor and small-molecule Tyrosine Kinase Inhibitors (TKIs) that can bind to the EGFR’s intracellular catalytic site. Gefitinib, afatinib, dacomitinib and erlotinib were the first EGFR-TKIs to be approved by the FDA for the treatment of advanced non-small-cell lung cancer. As first-line treatments for advanced NSCLC and pancreatic cancer, erlotinib, lapatinib and icotinib (Figure 15) bind to the EGFR in a reversible manner.
Figure 15. The anticancer quinazolines.
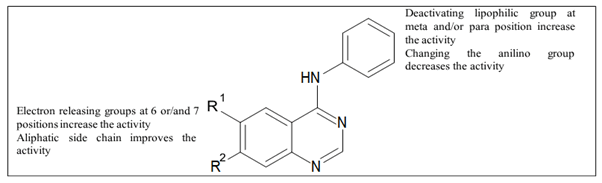
Figure 16 shows SAR of EGFR inhibitors.
Figure 16. SAR of quinazolines as EGFR inhibitors.
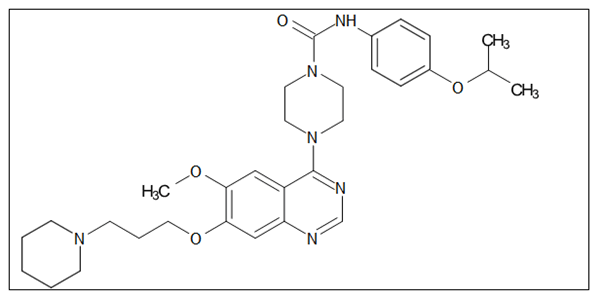
Platelet Derived Growth Factor Receptor (PDGFR): Four cystine-knot-type growth factors known as Platelet-Derived Growth Factors (PDGFs) (PDGF-A, B, C, and D) regulate the proliferation of connective tissue cells like fibroblasts and smooth muscle cells. While PDGF-PDGFR signaling is crucial for development, adulthood sees strict regulation of both PDGFs and PDGFRS expression. Except for when it occurs briefly during wound repair, increased PDGF-PDGFR signaling is typically regarded as abnormal and is a key component of several diseases involving proliferation, including many cancers, inflammation, pulmonary fibrosis and restenosis and most notably atherosclerosis. Figure 17 depicts the anticancer quinazolines 4-piperazino-substituted quinazoline derivative.
Figure 17. The anticancer quinazolines 4-piperazinosubstituted quinazoline derivative.
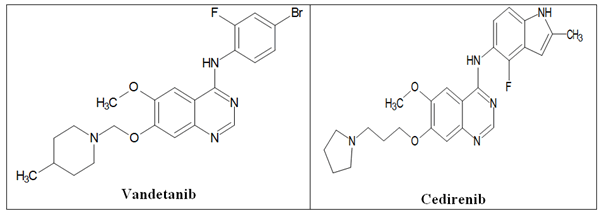
Vascular Endothelial Growth Factor Receptor (VEGFR): The most important active regulator of angiogenesis is the Vascular Endothelial Growth Factor (VEGF) pathway. It can encourage blood vessel development and vascular endothelial cell migration and proliferation. VEGFR-1, VEGFR-2 and VEGFR-3 are the three primary vascular endothelial growth factor receptors, which are the essential intermediates of tumor angiogenesis and new blood vessel formation and supply nourishment and oxygen for tumor growth. When VEGF binds to the receptor, the receptor undergoes homologous or heterogenous dimerization, the cell’s kinase region is phosphorylated, numerous important signaling pathways are activated and numerous physiological effects are produced. The primary effector of VEGF/VEGFR signal transduction in increasing tumor angiogenesis is Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2). It is expressed on blood vessel surfaces and is crucial for tumor angiogenesis. The Raf-1/MAPK/ERK signaling pathway is activated when VEGFR-2 is phosphorylated and this eventually causes angiogenesis, increased vascular permeability, tumor proliferation and tumor migration. Figure 18 depicts the anticancer quinazolines vandetanib and cediranib.
Figure 18. The anticancer quinazolines vandetanib and cediranib.
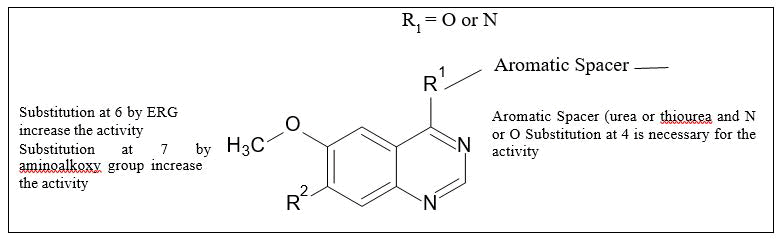
Structure Activity Relationship studies (SAR): Figure 19 shows SAR of VEGFR inhibitors.
Figure 19. The SAR of quinazolines as EGFR inhibitors.
Fibroblast Growth Factor Receptor (FGFR): During embryonic development, organogenesis, and tissue maintenance, Fibroblast Growth Factors (FGF) and their tyrosine kinase receptors (FGFR) govern crucial cellular activities as cell proliferation, survival and migration. Their expression and activity are tightly regulated in healthy tissues and their dysregulation is typically seen at the onset of cancer. The cancer cells are given signals for survival and migration by FGF-dependent stimuli and these stimuli also have an impact on endothelial cells, promoting angiogenesis. As potential therapeutic targets for a number of cancers, the four FGFR genes and the numerous receptor proteins expressed from differently spliced FGFR-RNAS have come into focus.
DHFR inhibitors
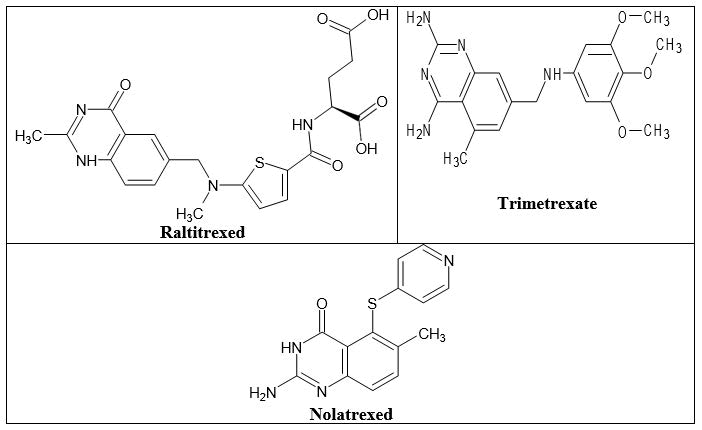
In both prokaryotic and eukaryotic cells, DHFR is involved in the synthesis of the raw materials for cell proliferation by catalyzing the reduction of dihydrofolate to tetrahydrofolate utilizing NADPH. Currently, several substances are being used in therapeutic, including the “classical antifolate” raltitrexed and the “non- classical antifolates” trimetrexate and nolatrexed (Figure 20). Classical antifolates are structurally similar to folate and have a glutamate tail, an aromatic ring and a pterin ring. They are actively transported by the decreased folate carrier system because they have a charged glutamate tail, which prevents them from passively diffusing across cell membranes. Non-classical antifolates are lipophilic compounds that passively diffuse into cells without the assistance of folate transport mechanisms. They are helpful for the creation of new cancer treatments since they can impede the proliferation of tumor cells.
Figure 20. The anticancer quinazolines as DHFR inhibitors.
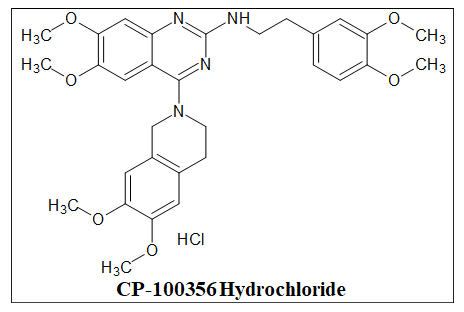
BCRP inhibitors
Among the ABC transporters, Breast Cancer Resistance Protein (BCRP) ABCG2-is a recently discovered and less well-characterized transporter that is also an essential MDR transporter. According to the Human Genome Organization, P-gp is the first member of the subfamily in the ABC transporter superfamily to be discovered to produce resistance to xenobiotics and anticancer drugs. It was also the first human ATP-Binding Cassette (ABC) transporter protein to be identified. A multidrug-resistant human breast cancer cell line was found to overexpress the transporter known as Breast Cancer Resistance Protein (BCRP). A dual MDR1 (P-gp)/BCRP inhibitor, CP-100356 Hydrochloride is orally active (Figure 21).
Figure 21. The anticancer quinazolines as BCRP inhibitors.
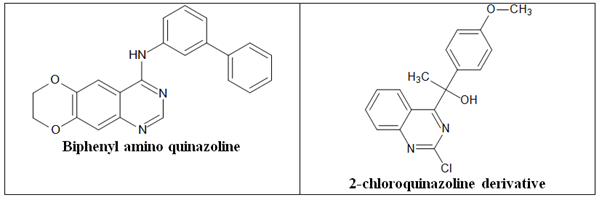
Tubulin polymerization inhibitors
Drugs that alter microtubule/tubulin dynamics are frequently used in chemotherapy. Instead of acting on the DNA, these medicines directly disrupt the microtubule machinery of the cell. Cytoskeletal elements called microtubules enable a cell to keep its form. They are necessary for the completion of cell division because they separate the chromosomes during mitosis and produce the mitotic spindle. Microtubules are hollow cylinders with a diameter of 24 nm that are made up of alpha and β-tubulin heterodimers. These structures are capable of rapid polymerization- induced growth and depolymerization-induced shrinkage. 2-Chloroquinazoline derivative and biphenylaminoquinazoline (Figure 22).
Figure 22. The anticancer quinazolines as tubulin polymerization inhibitors.
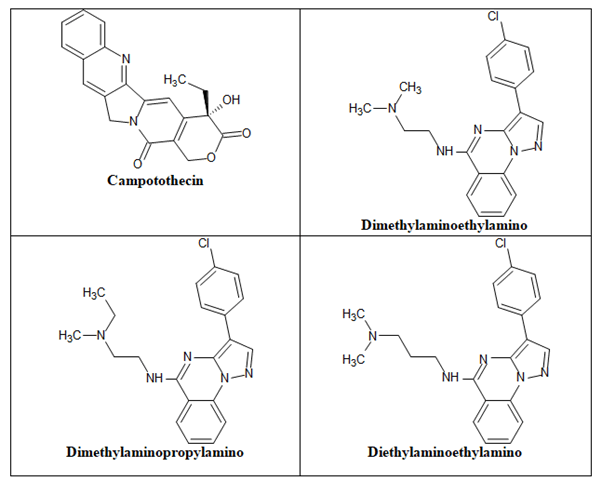
Topoisomerase I inhibitors
DNA enzymes called Topoisomerases (Topo) regulate the topology of the double-helix DNA supercoil during transcription and replication of cellular genetic material. Topo I and II are the two main subtypes of topoisomerases. Topo I starts the cleavage of one DNA strand, whereas Topo II starts the cleavage of both DNA strands. The buildup and stability of cleavable complexes and eventual cell death occur when this DNA replication machinery is partially or completely inhibited. As a consequence, it is assumed that Topo I inhibitors will be simple to target in cancers that express noticeably high quantities of the Topo I enzyme. The duration of exposure and less so the quantity of the Topo I inhibitor determine the cytotoxic effect of Topo I inhibition. Example of camptothecin, diethylaminoethylamino chain, dimethylaminoethylamino chain and dimethylaminopropylamino chain (Figure 23).
Figure 23. Anticancer quinazolines as topoisomerase I inhibitors.
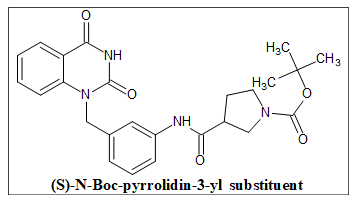
PARP 1 inhibitors
A key player in the regulation of several DDR pathways, including base excision repair, Homologous Recombination (HR), classical and alternative nonhomologous end joining, nucleotide excision repair, microhomology- mediated end joining, maintenance of replication fork stability and mismatch repair, is PARP [Poly(ADP-ribose) polymerase-1]. Additionally, it has been discovered that many cancers, including breast, ovarian and oral cancers, overexpress PARP in comparison to their healthy surrounding tissues. As a result, inhibiting PARP activity is a promising strategy for cancer therapeutics because it interferes with PARP functions, which impedes the DDR pathways of cancer cells. It is now a well- established strategy to use PARP inhibition for the treatment of breast or ovarian malignancies with BRAC1/2 mutations (deficiencies in the HR). A PARP inhibitor (PARP 1) is used to hinder the repair of single-strand breaks (SSBs), which causes SSBs to become double-strand breaks by slowing replication or speeding up fork elongation, necessitating HR for repair during S phase. The compound in (Figure 24) with the (S)-N-Boc-pyrrolidin-3-yl substituent shown the highest level of PARP-1 inhibitory activity.
Figure 24. The anticancer quinazolines as PARP 1 inhibitors.
Conclusion
From hazardous non-selective medicines to less toxic selective agents, the discovery of new anticancer drugs has advanced. The primary drawbacks of the existing chemotherapy drugs are their lack of selectivity and excessive toxicity. As a result, the treatment of many life-threatening illnesses requires focused therapy that acts on a particular biological target. Quinazoline and its derivatives are being investigated as a targeted anticancer agent as a result of ongoing research efforts in the field of drug discovery. Different quinazoline used against cancer having different targets (modes of action). The effectiveness of quinazolines as focused anticancer medicines was demonstrated through scientific trials. The FDA approved several molecules of the anticancer quinazolines, are now commercially available for the treatment of several cancer diseases. To create the anticancer activity, most quinazoline modifications involves the substituents at C-4, C- 6, and C-7 from the quinazoline structure. The selectivity, potency and anticancer activity of the quinazoline system were drastically altered by small chemical alterations. This review provides a clear view and comprehensive understanding for developing quinazolines as specific chemotherapeutic drugs.
References
- Negi JS. Science and action of quinazoline moiety: An efficient audit study. Int J Pharm Investig. 7, 61-65 (2020).
- Choi HJ, Jeong YJ, Kim J et al. EGFR is a potential dual molecular target for cancer and Alzheimer’s disease. Front Pharmacol. 14, 1238639 (2023).
[Crossref] [Google Scholar] [PubMed]
- Zayed MF. New fluorinated quinazolinone subordinates as anticonvulsant specialists. Diary Taibah College Clin Sci. 9, 104-109 (2014).
- Ghorab MM, Abdel-Kader MS, Alqahtani AS et al. Synthesis of some quinazolinones inspired from the natural alkaloid L-norephedrine as EGFR inhibitors and radiosensitizers. J Enzyme Inhib Med Chem. 36, 218-238 (2021).
[Crossref] [Google Scholar] [PubMed]
- Zayed MF. Union and natural assessment investigations of novel quinazolinone subsidiaries as antibacterial and mitigating specialists. Saudi Pharm J. 22, 157-162 (2014).
[Crossref] [Google Scholar] [PubMed]
- Zayed MF. Plan, union and organic assessment investigations of novel quinazoline subsidiaries as cytotoxic specialists. Med Exp. 63, 210-215 (2013).
- Zayed MF. Plan, blend, and natural assessment investigations of novel quinazolinone subordinates as anticonvulsant specialists. Ther Sci Exp. 22, 5823-5831 (2013).
- Alam MJ. A survey: Late examinations on quinazoline framework. Global Diary Cutting Edge Expl. 3, 1656-1664 (2015).
- Connolly DJ, Cusack D, O'Sullivan TP et al. Synthesis of quinazolinones and quinazolines. Tetrahedron. 61, 10153-1202 (2005).
- Meyer JF, Wagner EC. The Niementowski reaction. The use of methyl anthranilate or isatoic anhydride with substituted amides or amidines in the formation of 3-substituted-4-keto-3, 4-dihydroquinazolines. The course of the reaction. J Org Chem. 8, 239-252 (1943).
- Lansky ES, Lansky S, Paavilainen HM. Harmal: The genus peganum. CRC Press. (2017).
- Hameed A. Quinazoline and quinazolinone as significant restorative platforms: A similar patent survey (2011-2016). Well-Qualified assessment on Helpful Licenses. 28, 281-297 (2018).
- Auti PS, George G, Paul AT. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. RSC Adv. 10, 41353-41392 (2020).
[Crossref] [Google Scholar] [PubMed]
- Gupta T, Rohilla A, Pathak A et al. Current perspectives on quinazolines with potent biological activities: A review. Synth Commun. 48, 1099-1127 (2018).
- Bhavyesh D, Soliya S, Konakanchi R et al. The recent advances in ironâcatalyzed c (sp3)−h functionalization. Chem Asian J. 19, e202301056 (2024).
[Crossref] [Google Scholar] [PubMed]
- Chahar A. Synthetic carcinogenesis: A short survey on system and digestion. Oral Med Oral Pathol Oral Radiol. 6, 120-124 (2020).
[Crossref]
- Peters JM, Gonzalez FJ. The evolution of carcinogenesis. Toxicol Sci. 165, 272-276 (2018).
[Crossref] [Google Scholar] [PubMed]
- Sell S. How vaccines work: Immune effector mechanisms and designer vaccines. Expert Rev Vaccines. 18, 993-1015 (2019).