Research Article - International Journal of Clinical Rheumatology (2017) Volume 12, Issue 3
It is feasible to screen for important RA co-morbidities using patient completed questionnaires
- *Corresponding Author:
- Rosemary Waller
Great Western NHSFT
Swindon, UK
E-mail: rosiewaller@doctors.org.uk
Abstract
Background: To study the feasibility of pre-consultation questionnaires to screen for Rheumatoid Arthritis (RA) co-morbidities compared with face-to-face consultation. Methods and findings: Postal questionnaires were sent to all RA patients on our electronic database. Four domains were studied: Gastrointestinal (GI) symptoms, Cardiovascular (CVS) symptoms and risk factors; Foot; Anxiety and depression (Hospital Anxiety and Depression Scores (HADS)). Patients were then reviewed clinically, with a full history and examination undertaken and HAQ, DAS and QRISK2 scores were calculated for each patient. 597 questionnaires were sent, 301 (50%) completed and 129 patients were reviewed in clinic. The mean DAS score was 3.04. 32% of responders reported daily GI symptoms, with no extra symptoms of concern elicited at clinic review in any patient. 49% patients had high CVS risk as calculated by QRISK2. 5% patients assessed as low CVS risk by our questionnaire were re-categorised as high risk by QRISK2. 89% of patients reported foot symptoms in the past week. Mean foot score was 4/10 with correlation co-efficient 0.63 between postal and clinical scores. The mean anxiety score was 7.8 with 49% patients scoring 8 or more. The mean depression score was 9.3 with 64% patients scoring 8 or more. Conclusion: Pre clinic screening by questionnaire is a feasible way of identifying comorbidities. We found a high rate of co-morbidities in our RA population.
Keywords
rheumatoid arthritis, co-morbidities, health screening, questionnaires
Background
The ‘Treat to Target’ and the ‘early window of opportunity’ principles have led to improved joint related outcomes in patients with newly diagnosed Rheumatoid Arthritis (RA). However both newly diagnosed and well established RA patients continue to suffer from diverse associated co-morbidities which can reduce quality of life, longevity and work productivity [1,2]. Focus on joint related outcomes can divert a clinician’s attention away from the holistic approach required in the longer term management of this multisystem chronic disease [3]. There is a risk that the multisystem elements of disease not covered by a DAS will be missed and that the outlook for patients with more established disease will not improve in tandem with newly diagnosed patients.
The National Institute for Clinical Excellence (NICE), British Society for Rheumatology (BSR) and European League against Rheumatism (EULAR) guidelines all state that a multisystem review should form part of an annual review [4-6]. EULAR have recently launched a 93 point reporting form aiming to ‘improve the reporting and prevention of comorbidities in chronic inflammatory rheumatic diseases’ [7]. Attempting to assess all the important facets of holistic care in a single review appointment is daunting and time consuming. We wished to assess the feasibility of using patient completed questionnaires to facilitate this.
We have assessed four important domains: gastrointestinal (GI) health; cardiovascular health (CVS) exercise; foot symptoms; and psychological health. GI symptoms are particularly understudied but can have a large impact on, for example, treatment adherence. The excess mortality associated with cardiovascular disease in RA is well documented [3,8]. Feet are commonly affected by RA but are not included in the DAS 28, risking their assessment being considered a comorbidity. Psychological health problem affect overall quality of life and influence the tender joint count and VAS components of the Disease Activity Score (DAS).
Aims
We assessed the feasibility of using postal questionnaires to assess comorbidities by comparing results obtained from postal questionnaire with face to face consultation. We also documented the prevalence of extra-articular symptoms and comorbidities associated with RA in our patient population.
Methods
Postal questionnaire
A postal questionnaire was sent to all patients with a diagnosis of RA, confirmed by a consultant rheumatologist, on our electronic drug monitoring database (Supplementary File). They were also invited to participate in a general health screening research clinic and were asked to return a signed consent form with the questionnaire if they wished to attend this.
The questionnaire was divided into 4 sections:
• Section 1 asked 10 questions regarding GI health:
Patients were asked to indicate the frequency of 10 GI symptoms with a maximum score of 30.
• Section 2 assessed 8 CVS symptoms and risk factors:
The maximum CVS score was 10. Total CVS scores of 2 and 3 were compared as thresholds to indicate an increased risk of cardiovascular event. Patients were asked about type and frequency of exercise.
• Section 3 assessed foot symptoms using the 10 point Swindon Foot and Ankle Questionnaire (SFAQ):
A foot score out of 10 was assigned; with the answer no scoring 0 and yes scoring 1 (Supplementary File for more details on scoring).
• Section 4 contained a Hospital Anxiety and Depression (HAD) questionnaire [9,10].
HAD anxiety and depression scores were calculated with a maximum score of 21. The cut off of 8 or more was used to indicate patients at risk of anxiety or depression [11].
Readability
The questionnaires were designed to have a reading age of 11. This was to improve completion rates by patients, because 16% of adults in the UK are functionally illiterate, defined as a reading age at or below that of an 11 year old [12-14].
Face to face consultation
Patients underwent a comprehensive clinical review including:
1. Full GI history and examination, including for periodontal and perianal disease. GI score out of 30 was recalculated after clinical assessment.
2. QRISK2 score calculation (with blood pressure, blood sugar and cholesterol checked) along with a CVS examination [15]. A QRISK2 score of >20% CVS event risk at 10 years was considered as high risk.
3. Detailed foot examination (including completion of the validated Manchester Foot Pain and Disability Index (MFPDI) [16,17].
4. HAD was reassessed.
5. Activity of RA was documented using a Disease Activity Score (DAS) and an Overall Status in Rheumatoid Arthritis (OSRA) score [18,19]. Patients also completed a Health Assessment Questionnaire (HAQ), a Euroqol score was calculated and a medication history was taken, including use of complementary medications and therapies [20-22].
6. Demographic data was collected, including employment status.
Ethical approval was obtained from Wiltshire Research Ethic Committee and written consent obtained from all patients. Microsoft Excell 2007 was used to perform statistical analysis. All correlation co-efficients were calculated as Spearman rank correlation coefficients.
Results
Of 597 questionnaires sent, 301 (50.3%) were returned. 129 patients (43% of questionnaire responders and 22% of total RA population sent screening questionnaires) were seen in research clinic.
Of the 301 patients returning questionnaires 202 (67%) were female, with an age range of 31– 91 (median 62). Of the 129 patients reviewed in clinic, 90 (61%) were female, with an age range of 38–89 (median 61) and median disease duration of 13 years (range 1–49).
Gastrointestinal health
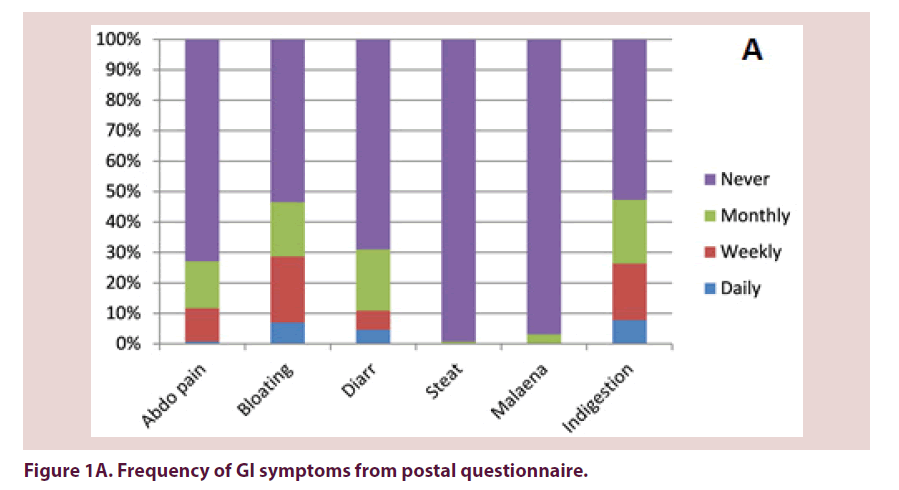
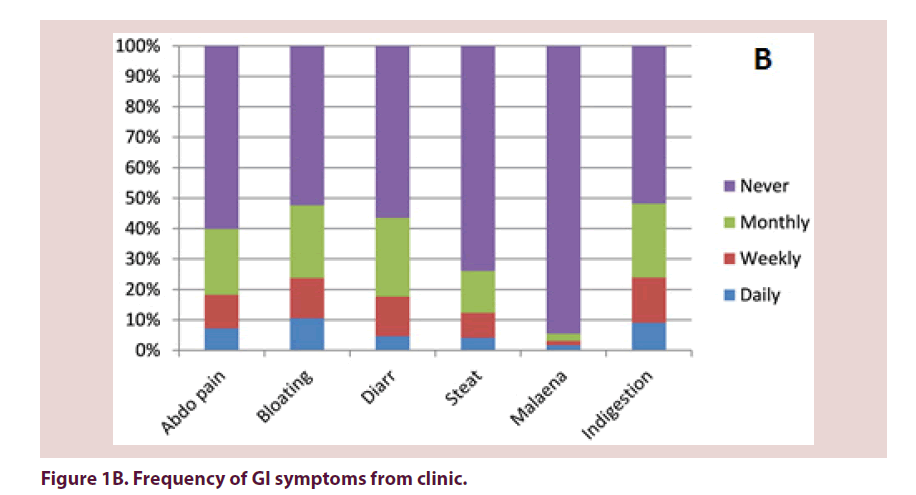
32% of questionnaire responders and 18% of patients seen in clinic reported at least 1 daily GI symptom (Table 1). 17% and 9% of patients respectively reported no GI problems. There were no statistically significant differences in frequency of GI symptoms reported on questionnaire compared with clinic. Out of the symptoms reported on at least a weekly frequency, indigestion, bloating and abdominal pain were the most commonly reported (Figures 1A and 1B).
| Symptom frequency | Number of symptoms | |||||
|---|---|---|---|---|---|---|
| 1 or more | 2 or more | 3 or more | ||||
| Questionnaire 301 pts |
Clinic 129 pts |
Questionnaire 301 pts |
Clinic 129 pts |
Questionnaire 301 pts |
Clinic 129 pts |
|
| Daily | 96 (32%) |
23 (18%) |
35 (12%) |
5 (4%) |
13 (4%) |
2 (2%) |
| At least weekly | 160 (53%) |
68 (53%) |
96 (32%) |
39 (30%) |
52 (17%) |
15 (12%) |
| At least monthly | 246 (82%) |
119 (92%) |
205 (68%) |
85 (66%) |
157 (53%) |
59 (46%) |
| Never | 50 (17%) |
11 (9%) |
||||
Table 1. Frequency of GI symptoms.
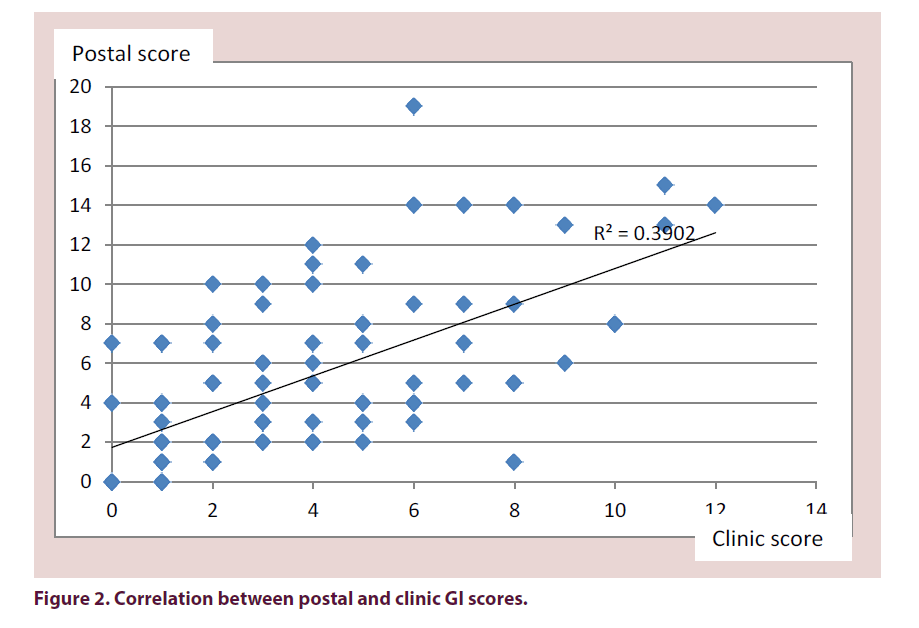
The mean total GI score of all 301 questionnaires was 5 (range 0–21). The mean score at clinic review was 4 (range 0–12). Of the patients seen in clinic, the mean GI score from their questionnaires was 5 (range 0–19). The correlation coefficient of their clinic score compared with their questionnaire score in those patients who were assessed in clinic was 0.64 (Figure 2).
The only significant difference in specific symptom reporting between the questionnaire and clinic was steatorrhoea, with no patients experiencing true steatorrhoea on questioning in clinic but 4% reporting it daily, 8% weekly and 13% monthly on questionnaire.
Extra information obtained at clinic review:
1. Dental disease:
• 109 (84%) had fillings;
• 11 (<1%) patients had normal dentition;
• 28 (22%) had poor gum health, including loose teeth or gingivitis;
• 70 (54%) patients had a dry or coated tongue;
• 42 (32%) of patients reported mouth ulcers.
2. 36 patients (28%) had haemorrhoids.
3. Use of medication with commonly reported GI side effects (GI toxic drugs):
• 34 (26%) of patients were on bisphosphonates;
• 12 patients (9%) were on prednisolone;
• 64 patients (50%) were on NSAIDS;
• 15 patients (12%) were on at least 2 of the above.
Patients taking potentially GI toxic drugs were more likely to suffer GI symptoms on at least weekly basis–49% vs. 34% (Table 2).
| GI toxic med | No GI toxic meds | |||
|---|---|---|---|---|
| Weekly GI symptoms | 37/75 (49%) | 18/53 (34%) | ||
| + PPI | No PPI | +PPI | No PPI | |
| Weekly GI symptoms | 16/31 (52%) | 21/44 (47%) | 10/21 (48%) | 8/32 (25%) |
Table 2. GI symptoms and PPIs.
4. 52 patients (40%) were on a Proton Pump Inhibitor (PPI). 31/52 (60%) were taking at least 1 GI toxic drugs. Co-prescription of a PPI did not reduce the reporting rate of GI symptoms, whereas those patients taking a PPI but no GI toxic medication did have a lower GI symptoms rate.
Cardiovascular disease screening
121 patients had a both a postal score and Qrisk® 2 score in clinic calculated. The mean and median postal score was 2 (range 0- 6). Using 2/10 postal score as the threshold for high CVS risk, 85 (70%) patients were deemed high risk. If 3/10 postal score was used, 56 (46%) patients were deemed high risk. 51 (41%) patients were stratified as high risk by Qrisk® 2.
76 patients (63%) were classified in the same risk group using a postal cut off of 2 or more compared with Qrisk® 2 score. 77 patients (64%) were classified in the same risk group using a postal cut of 3 or more (Table 3).
| Risk category | QRISK2 (clinic) Number (%) |
Postal score 2 or more Number (%) |
Postal score 3 or more Number (%) |
|---|---|---|---|
| High | 85(70%) | 56(46%) | 51(41%) |
| Low | 36(30%) | 65(54%) | 70(59%) |
| Both scores high | 43(36%) | 30(25%) | |
| Both scores low | 33(27%) | 47(39%) | |
| Postal high, QRISK2 low | 40(33%) | 25(21%) | |
| Postal low, QRISK2 high | 5(4%) | 22(18%) |
Table 3. Comparison of CVS scores.
5 patients (4%) deemed low risk on postal questionnaire using a cut off of 2 were reclassified as high risk by Qrisk® 2 (false negatives). 22 patients (18%) patients using a cut off of 3 were deemed low risk by postal questionnaire but were high risk on Qrisk® 2. 40 patients (33%) deemed high risk on postal questionnaire using a cut off of 2 were reclassified as low risk by Qrisk® 2 (false positive). 25 (21%) patients deemed high risk using a cut off of 3 were reclassified as low risk on Qrisk® 2 score.
284 patients answered the questions about exercise on the postal questionnaire. Of these, 86 patients (30%) never exercised, 21 (7%) exercised monthly, 75 (26%) weekly and 103 (36%) daily. 14 patients did both cardiovascular and stretching exercises. 76 performed stretching only, 42 performed cardiovascular only, 67 used walking and 4 used gardening as their main exercise.
7 patients were found to have previously undiagnosed hypertension. 32 patients (26%) were found to have cholesterol of greater than 4. 42 patients (35%) were taking a statin.
12 patients were taking oral steroids and 64 patients were taking NSAIDs. 23 of these patients (32%) had a high Qrisk® 2 score compared with 16 patients (28%) not taking these medications, with a relative risk of 1.14.
Feet
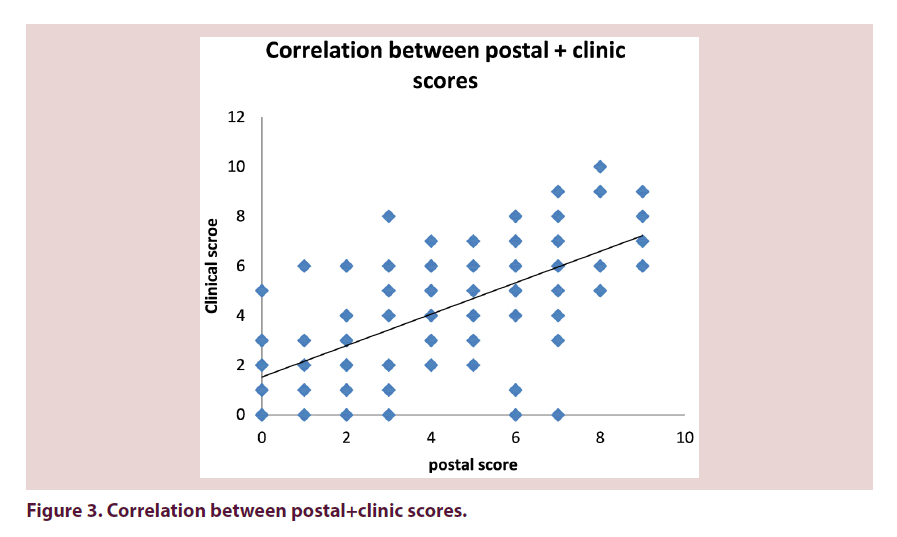
From 301 questionnaires returned the mean Swindon Foot and Ankle Questionnaire Score (SFAQ) was 4 (range 0–10). 16 patients (11%) scored 0. The mean SFAQ foot score from 139 patients seen in clinic was 4 (range 0–10). The correlation between postal score and clinic score was r=0.63. The correlation between clinic score and the Manchester Foot score was 0.67 (Figure 3).
92% of patients assessed in clinic had some form of foot or ankle problems documented and 66% had more than one problem (Table 4).
| Diagnosis | Number of patients | % patients | ||||
|---|---|---|---|---|---|---|
| Hallux valgus | 56 | 41 | ||||
| Callous | 27 | 20 | ||||
| MTP synovitis | 25 | 18 | ||||
| Ankle varus | 22 | 16 | ||||
| Claw toes | 21 | 15 | ||||
| Moretons neuroma | 20 | 15 | ||||
| Posterior Tibial tendonitis | 16 | 12 | ||||
| Peroneal tendonitis | 16 | 12 | ||||
| Hind foot pathology | 15 | 11 | ||||
| Ankle pathology | 14 | 10 | ||||
| Hammer toes | 14 | 10 | ||||
| Other | 10 | 7 | ||||
| Previous surgery | 5 | 4 | ||||
| Cross over | 3 | 2 | ||||
| Achilles tendonitis | 1 | 1 | ||||
| Plantar fasciitis | 2 | 1 | ||||
| Number of diagnoses(%) | 0 | 1 | 2 | 3 | 4 | ≥5 |
| 12 (9) | 35 (26) | 58 (42) | 21 (15) | 10 (7) | 2 (1) | |
Table 4. Prevalence of foot pathologies.
Anxiety and depression
From 296 postal questionnaires, the mean anxiety score was 7.8 (range 0–21), with 146 patients (49%) scoring 8 or more. From 296 postal questionnaires, the mean depression score was 9.3 (range 0–21), with 188 patients (64%) scoring 8 or more. The anxiety and depression scores correlated with each other, with R=0.76 but did not correlate with other measures of disease outcome, including HAQ, DAS, GI score, foot score or exercise (Table 5A).
| (A) | Anxiety | Depression | |
|---|---|---|---|
| HAQ | 0.28 | 0.38 | |
| DAS | 0.14 | 0.03 | |
| GI score | 0.18 | 0.16 | |
| Foot score | 0.02 | 0.05 | |
| Exercise | 0.13 | 0.16 |
| (B) | Correlation co-efficient | |
|---|---|---|
| HAQ | 0.09 | |
| Foot score | 0.29 | |
| HAD anxiety | 0.14 | |
| HAD depression | 0.03 | |
| GI score | 0.1 | |
| QRISK2 score | -0.02 |
Table 5. (A and B) HADS correlations.
DAS28
Mean DAS score was 3.04 (range 1.1–6.19) and mean HAQ score was 1.25 (range 0–3).
The DAS28 did not correlate with any of the other domains assessed. The correlation coefficient for DAS28 and SFAQ was 0.29 (Table 5B).
Discussion
High rate of comorbidities once again demonstrated
We present the results of a screening exercise in a group of patients with RA, whose demographics are compatible with a typical DGH population. 301 patients completed the postal questionnaire and 129 patients were seen in clinic. The results demonstrate that our patients with RA have a high rate of self- reported extra articular diseases and symptoms, in all the domains we studied. Nearly one third of patients reported daily GI symptoms. Nearly half of our patients had a 10 year cardiovascular event risk of greater than 20%. 90% of patients reported some form of foot pathology or symptom in the past week, consistent with results of other studies [23-29]. Similarly half of our patients had an anxiety or depression score indicating that they were at risk of problems. This compares with the findings of recent meta-analysis of RA where 34% of patients had a HAD score greater than 8 and 15% greater than 11 [30]. Our results highlight the importance of assessing the global health of our RA patients, particularly in light of the wellestablished link between RA and increased risk of cardiovascular disease and the on-going excess mortality associated with RA despite treatment advances.
Correlation between postal and clinic results
The correlation between postal results and clinic results were moderate in all domains, suggesting that self-completed questionnaires completed prior to a clinic appointment, either by post sent out with an appointment letter or completed in the waiting room on the day of appointment could be a useful means of highlighting to the assessing physician the health concerns of the patient, which could help to guide and focus the subsequent consultation. Few new symptoms or problems of concern were elicited at clinic that was missed by our questionnaires.
Simple screening tools are feasible
Currently there are no freely available tools to simultaneously assess several facets of health in patients with RA. There are several patient reported outcome measures, each covering separate domains both specifically for use in RA and in the wider medical population [31]. ‘Questionnaire fatigue’ is a real problem for both patients and clinicians because of the length of some assessment tools and the problems in interpreting questionnaire results.
Our postal questionnaire was designed to be simple and brief to both complete and interpret. It is important that any screening tools take account of the average UK reading age of 11, are brief enough to maximise full completion, whilst covering all domains. By balancing all of these factors, the maximum amount of valid information can be obtained. There is very limited published data on the readability of currently available medical questionnaires and none regarding tools specifically used in the assessment of RA. To our knowledge, our postal questionnaire is the first assessment tool used in RA to consider the readability of the tool and to document the reading age results.
Tensions and barriers to multisystem review
An early treat to target approach in RA has improved disease outcomes. It is important however, that the focus upon achieving a threshold DAS does not distract completely from the assessment of the multisystem effects and comorbidities associated with RA. In particular there is a growing concern that feet are being considered comorbidity rather than an integral feature of RA by use of the DAS28. A recent survey found that 50% of clinicians did not examine feet as they were not included in DAS [32]. Time constraints, rising patient referral number s and the pressure to adhere to new to follow up ratios are all factors leading to a perceived difficulty in ensuring holistic care.
There is tension between the overlapping roles of primary and secondary care. A recent multi-national cross-sectional study found that rates of detecting co-morbidities were variable [31]. The Arthritis Research UK Adult Inflammatory Arthritis Clinical Studies Group looking at co-morbidities discussed the current status of care in the UK and how best to address the gap between the standards expected by national guidance bodies and current practice [33]. They concluded that patient reported outcomes were as important as data collected by hospital staff. Current tools specifically developed to look at co-morbidities in RA focus mainly upon detecting risk factors of excess mortality rather than factors affecting morbidity as well.
There was no correlation between the scores in different domains. In particular, a high anxiety and/or depression score did not predict a high score in other domains as an explanation for high levels of self-reported symptoms. This suggests that problems in one area of global health were independent of another. There was also no correlation between high scores in the extra-articular domains we examined and the severity of RA as assessed by DAS score. This was true even of those patients with an elevated anxiety and depression score. This is in contrast to other studies which have demonstrated a relationship between high self-reported anxiety and depression and VAS and tender joint counts [34]. The lower weightings given to these components of the DAS score may account for the lack of association seen in our study. It highlights that anxiety and depression are not merely a reaction to active inflammation.
Study limitations
Our return rate of questionnaires was 50%, which is similar to the response rate of other postal surveys. It is possible that there was a selection bias, in that those patients returning questionnaires and consenting to a clinic review were more likely to report symptoms. There was however, no statistically significant difference in scores comparing those patients seen in clinic, with those patients who returned questionnaires only [35].
We have not been able to cover every domain of extra-articular disease in RA and sleep, fatigue and joint stiffness are important omissions from our study. There was also limited patient involvement in the development of the questionnaire, both in terms of which domains were covered but also in terms of question design.
These results are from a single centre at one time point in 2010 and so caution should be given in more widely interpreting results. However, 6 years after our data collection, assessment of extra-articular features and co-morbidities in RA remains relevant. The rate of comorbidities detected in our studies is comparable with other published data.
Conclusion
Pre clinic screening by questionnaire is an efficient, effective and feasible way to identify co-morbidities and direct treatment. Important symptoms and red flags were generally not missed by this approach.
This study highlights both the high rate of significant co-morbidities associated with RA, and the multi-system nature of the disease. We found clinically acceptable correlation between postal questionnaires results and the clinic findings.
This simple practical working model can be used prior to clinic appointments to allow clinicians to cover a comprehensive range of RA co-morbidities plus focus consultations towards important patient concerns. We anticipate that these questionnaires will be adapted to an electronic format for use in the waiting room prior to consultations.
References
- Koduir G, Norton S, Cox N et al. Co-morbidities in an inception cohort of RA. A study of baseline prevalence and 15 year cumulative incidence of co existent disease and the predictive value of a co-morbidity index for mortality. Ann. Rheum. Dis. 69(s3), 522–528 (2010).
- Young A, Prouse P, Williams P et al. Effect of Rheumatoid Arthritis on job loss and work productivity. Results from an inception cohort with 10 year follow up. Rheumatology. 48(21), 271 (2009).
- Aviña-Zubieta JA, Choi HK, Sadatsafavi M et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 59(12), 1690–1697 (2008).
- https://www.nice.org.uk/guidance/cg79/resources/guidance-rheumatoid-arthritis-pdf
- http://www.rheumatology.org.uk/includes/documents/cm_docs/2009/m/management_of_rheumatoid_arthritis_after_first_2_years.pdf
- M JL, Peters D, Symmons PM et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann. Rheum. Dis. 69, 325–331(2010).
- Baillet A, Gossec L, Carmona L et al. Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: a EULAR initiative. Ann. Rheum. Dis. 75, 965–973 (2016).
- Westlake S, Colebatch A, Baird J et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: A systematic literature review. Rheumatology. 49(2), 295–307 (2010).
- Waller R, Manuel P, Williamson L. The Swindon Foot and Ankle Questionnaire: Is a Picture Worth a Thousand Words? ISRN Rheumatol. 2012, 105479 (2012).
- http://www.glassessment.co.uk/products/hospital-anxiety-and-depression-scale-0
- Olsson I, Mylkletun A, Dahl A. The hospital anxiety and depression rating scale: A cross-sectional study of psychometrics and case finding abilities in general practice. BMCPsychiat. 5, 46 (2005).
- http://www.literacytrust.org.uk/assets/0000/3816/FINAL_Literacy_State_of_the_Nation_-_30_March_2010.pdf
- http://www.officialdocumentsgov.uk/document/other/0118404792/0118404792.pdf
- Doak C, Doak L, Root J. Teaching patients with low literacy skills (2nd edn.). Philadelphia: J. P. Lippincott Company. 1996.
- Collins G, Altman D. An independent and external validation of QRISK2 cardiovascular disease risk score: a prospective open cohort study. BMJ. 340, c2442 (2010).
- Cook C, Cleland J, Pietrobon R et al. Calibration of an item pool for assessing the disability associated with foot pain: an application of item response theory to the Manchester Foot Piand and Disability Index. Physiology. 93, 89–95 (2007).
- Menz HB, Tiedemann A, Kwan MM et al. Foot pain in community-dwelling older people: an evaluation of the Manchester Foot Pain and Disability Index. Rheumatology (Oxford). 45(7), 863–867 (2006).
- Prevoo ML, van't Hof MA, Kuper HH et al. Modified disease activity scores that includes twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. ArthritisRheum. 38(1) 44–48 (1995).
- Harrison MJ, Hassell A, Dawes PT et al. The overall status in rheumatoid arthritis (OSRA) measure--further evidence to support its use in clinical practice. Rheumatology (Oxford). 46(5), 849–855 (2007).
- Bruce B, Fries JF. The stanford health assessment questionnaire: dimensions and practical applications. Health Qual. Life Outcomes. 1, 20 (2003).
- Brooks R. EuroQol: The current state of play. Health Policy. 1996, 37(1), 53–72.
- Luo N, Chew LH, Fong KY et al. Validity and reliability of the EQ-5D self-report questionnaire in English-speaking Asian patients with rheumatic diseases in Singapore. Qual. Life. Res. 12(1), 87–92 (2003).
- Kerry RM, Holt GM, Stockley I. The foot in chronic rheumatoid arthritis: a continuing problem. Foot. 4(4), 201–203 (1994).
- Rome K, Gow PJ, Dalbeth N et al. Clinical Audit of foot problems in patients with rheumatoid arthritis treated at Counties Manukau District Health Board, Auckland, New Zealand. J. Foot Ankle Res. 2, 16 (2009).
- Wickman AM, Pinzur MS, Kadanoff R et al. Health-related quality of life for patients with rheumatoid arthritis foot involvement. Foot Ankle Int. 25(1), 19–26 (2004).
- Rojas-Vilarraga, Bayona J, Zuluaga N et al. The impact of rheumatoid foot on disability in Colombian patients with rheumatoid arthritis. BMC Musculoskelet. Disord. 10, 67 (2009).
- Bal A, Aydog E, Aydog ST et al. Foot deformities in rheumatoid arthritis and relevance of foot function index. Clin. Rheumatol. 25, 671–675 (2006).
- Scott DL, Smith C, Kingsley G. Joint damage and disability in rheumatoid arthritis: an updated systemic review. Clin. Exp. Rheumatol. 21(Suppl 31): S20–S27 (2003).
- van der Leeden M, Steultjens M, Dekker JH et al. The relationship of disease duration to foot function, pain and disability in rheumatoid arthritis patients with foot complaints. Clin. Exp. Rheumatol. 25 (2), 275–280 (2007).
- Matcham F, Rayer L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatol. 52(12), 2136–2148 (2013).
- Dougados M, Soubrier M, Antunez A et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann. Rheum. Dis. 73(1), 62–68 (2014).
- De Souz S, Williams R, Lemp P. Patient and clinician views on the quality of foot health care for rheumatoid arthritis outpatients: a mixed methods service evaluation. J. Foot. Ankle. Res. 9, 1 (2016).
- http://www.arthritisresearchuk.org/research/our-clinical-study-groups-and-researchstrategies/adult-inflammatory-arthritis/workshops.aspx
- Imran MY, Kham EA, Ahmad NM et al. Depression in Rheumatoid Arthritis and its relation to disease activity. Pak. J. Med. Sci. 31(2), 393–397 (2015).
- Matcham F, Norton S, Scott D et al. Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis: secondary analysis of a randomized controlled trial. Rheumatology. 55, 268–278 (2016).