Short Communication - Journal of Experimental Stroke & Translational Medicine (2010) Volume 3, Issue 2
Infection - An Amendment to the Stroke Model Guidelines
- *Corresponding Author:
- Odilo Engel
Department for Experimental Neurology
Charité Berlin, Charitéplatz 1, D-10117 Berlin, Germany
Tel: +49 30 - 450 560 149;
Fax: +49 30 - 450 560 915
E-mail: odilo.engel@charite.de
Abstract
Many publications show that infections have a tremendous impact on both general medical and neurological outcome. Summarizing the findings from several experimental studies we conclude that controlling infections in animal models of stroke is indispensable similar to checking body temperature in order to avoid misinterpretations of study results. In our manuscript we followed the structure used in the original article to provide a consistent outline. In brief, we reviewed the necessity of monitoring post-stroke infections, followed by a section on how to exclude the impact of infections in stroke models.
Keywords
stroke model; infection; guideline; reproducibility
Iinfection
A. The Necessity of Monitoring Post-Stroke Infections
Guidance
Especially in long-term studies, it is strongly recommended to monitor for infections in blood and lung after stroke, since post-stroke infections are common even in stroke models and are known to have a negative effect on outcome after stroke.
Supporting Discussion
Infectious diseases are the most common medical complication after cerebral ischemia (Davenport, Dennis et al. 1996; Langhorne, Stott et al. 2000), impairing both the neurological and the general medical outcome. The most frequent complications are bacterial pneumonia and urinary tract infections (UTI) (Langhorne, Stott et al. 2000; Hilker, Poetter et al. 2003), where bacterial pneumonia is seen as the most common serious complication in stroke care (Kalra, Yu et al. 1995). This observation is not limited to stroke patients, but also mice suffer from infections after experimental stroke (Prass, Meisel et al. 2003). Neurological deficits like dysphagia or bladder dysfunction contribute to the development of spontaneous infectious compilications by increasing the risk for colonization of the lower respiratory tract and urinary tract, respectively. However, aspiration alone is not sufficient to explain the high incidence of post-stroke pneumonia (Perry and Love 2001; Meisel, Schwab et al. 2005), and there is strong evidence that a secondary immunosuppressive state after CNS injury might be causative for the high incidence of severe bacterial infections in stroke patients (Meisel, Schwab et al. 2005; Prass, Braun et al. 2006; Chamorro, Urra et al. 2007; Emsley and Hopkins 2008; Offner, Vandenbark et al. 2009).
Following cerebral ischaemia, several groups observed lymphopenia, impaired cellular immune functions and a rapid and extensive apoptosis in lymphatic organs (Prass, Meisel et al. 2003; Liesz, Hagmann et al. 2009). This apoptosis can be prevented by application of caspase inhibitors leading to reduction of post-stroke infections and smaller infarct sizes (Braun, Prass et al. 2007).
Since the situation in the mouse resembles the situation in patients well, the mouse MCAO model can be used as a suitable model to elucidate the underlying mechanisms of post-stroke infections as well as for developing new therapeutic strategies (Meisel and Meisel 2008; Engel and Meisel 2010). However, since post-stroke infections effect outcome even in experimental stroke (Meisel et al. 2004), stroke studies aiming to investigate mechanisms or treatment strategies might be influenced by infections.
Commonly observed pathogens are often part of the intestinal flora, like Escherichia coli, Enterococcus faecalis and Lactococcus garviae (Meisel, Prass et al. 2004), but also Staphylococcus and Streptococcus species (Liesz, Hagmann et al. 2009) were detected.
In patients and in animals, stroke severity (Hug, Dalpke et al. 2009), impairment of protective reflexes (Nakajoh, Nakagawa et al. 2000) and neurological impairment correspond with the incidence of infections. Liesz et al. demonstrated that severe but not minor ischemic damage induces a profound immunodepression and infectious complications independent from localisation or laterality of stroke. (Liesz, Hagmann et al. 2009) However, infection also worsens neurological outcome (Hong, Kang et al. 2008) and prevention of infection not only improves survival and neurological outcome but also leads to smaller infarcts (Meisel, Prass et al. 2004). Thus, large lesion size is a prerequisite for immunodepression and post stroke infection, on the other hand infection have a negative effect on infarct maturation, at least in experimental stroke.
Further, mouse strains are known to differ in their susceptibility for post stroke infections (Schulte-Herbruggen, Klehmet et al. 2006). Despite a similar infarct size, SV129 mice have a higher bacterial load in the lung and a strongly increased susceptibility to bacteraemia compared to C57Bl/6 and Balb/C mice. Mortality is lowest in C57Bl/6 mice, followed by SV 129 and Balb/C mice. These results suggest that SV129 mice are more suitable for studying post-stroke sepsis, whereas C57Bl/6 mice are preferable for studying pneumonia (Schulte-Herbruggen, Klehmet et al. 2006).
These data suggest a close monitoring of infections to warrant a proper interpretation of results in experimental stroke research. Furthermore it is necessary to characterize each model for its immunological changes after experimental cerebral ischemia. In view of these results, characterization of infections is mandatory in establishing animal models of cerebral ischaemia.
B. Prevention of Infectious Complications
Guidance
Measures like prophylactic antibiotic treatment that can prevent infectious complications affecting outcome has to be taken into account for studies where “side effects” from infectious complications may interfere with mechanisms or treatments under research.
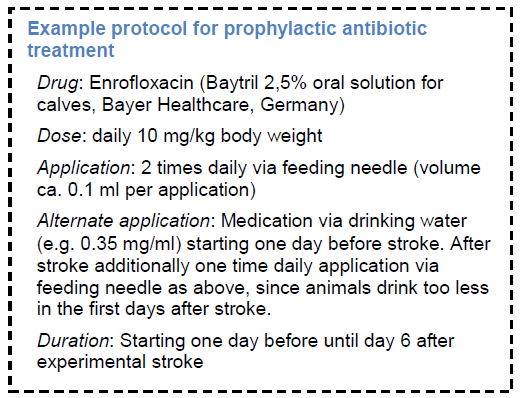
Supporting Discussion
Preventive antibiotic treatment was tested in animals (Meisel, Prass et al. 2004) and in patients (van de Beek, Wijdicks et al. 2009). In summary, three randomized controlled clinical trials on preventive antibacterial therapy after stroke have been performed. Whereas the PANTHERIS and MISS trials suggest superiority of a preventive anti-infective therapy over current standard therapy, ESPIAS is warning against this approach. (Chamorro, Horcajada et al. 2005; Harms, Prass et al. 2008; Schwarz, Al-Shajlawi et al. 2008) However, differences in study design and the chosen antibiotic may account for the differences in outcome. (Meisel and Meisel 2008). Nevertheless, the observed effects warrants evaluation of preventive antibiotic treatment in large stroke trials. (van de Beek, Wijdicks et al. 2009)
The commonly observed hyperthermia after experimental stroke (Reglodi, Somogyvari-Vigh et al. 2000) is not necessarily a “central fever” caused by stroke-induced tissue damage (e.g. in the hypothalamus) as proposed by Li et al. (Li, Omae et al. 1999). Since prophylactic antibiotic treatment prevents infections as well as fever (Schwarz, Al-Shajlawi et al. 2008), hyperthermia might be caused at least in part by infections. (Georgilis, Plomaritoglou et al. 1999; Commichau, Scarmeas et al. 2003) Likewise, the previously reported high mortality in the mice MCAO model (Schabitz, Li et al. 1999) after day 4 is less likely to be a direct effect of stroke (e.g. by brain edema) but rather a consequence of stroke associated severe bacterial infections. Hitherto, long-term studies on neuronal plasticity and regeneration in this stroke model were hampered by a generally high mortality. Thus, preventive antibacterial approaches may facilitate such studies. Furthermore, infections have to be considered in experimental stroke, since varying degrees of infection may lead to significant variability in outcomes. (Meisel, Prass et al. 2004)
Prophylactic antibiotic treatment should cover the commonly observed pathogens, and should be started within the first 12 hours after onset of cerebral ischemia and given over the first 6 days, since the risk of infections highest during this period. . (Meisel, Prass et al. 2004; Prass, Braun et al. 2006)
However, the use of prophylactic antibiotics may also influence the outcome due to neuroprotective or immunomodulatory effects of certain antibiotic drugs. Several antibiotics have well described neuroprotective effects, for example tetracyclins (Domercq and Matute 2004) like minocycline (Hayakawa, Mishima et al. 2008), or β-lactam antibiotics (Rothstein, Patel et al. 2005) like ceftriaxone (Lipski, Wan et al. 2007; Thone-Reineke, Neumann et al. 2008). However these neuroprotective effects may have limitations such as being strictly dose dependend (Matsukawa, Yasuhara et al. 2009) or gender specific (Li and McCullough 2009). For many antibiotics there is no direct neuroprotective effect observed, for example for gyrase inhibitors. Nevertheless many antibiotics have immunomodulatory effects or may interact with the inflammation cascade. (Tauber and Nau 2008) Hence the use of preventive antibiotic treatment has to be critically considered and the choice of a suitable antibiotic is one of the most critical points in planning studies with preventive antibiotic treatment.
Acknowledgment
This work was supported by the German Research Foundation (Exc 257), the Federal Ministry of Education and Research (01 EO 08 01), the Helmholtz Association (SO-022NG) and has received funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 201024.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Braun, J. S., K. lirass, U. Dirnagl, A. Meisel and C. Meisel. lirotection from brain damage and bacterial infection in murine stroke by the novel casliase-inhibitor Q-VD-OliH. Exli Neurol 2007; 206(2): 183-91.
- Chamorro, A., J. li. Horcajada, V. Obach, M. Vargas, M. Revilla, F. Torres, A. Cervera, A. M. lilanas and J. Mensa. The Early Systemic lirolihylaxis of Infection After Stroke study: a randomized clinical trial. Stroke 2005; 36(7): 1495-500.
- Chamorro, A., X. Urra and A. M. lilanas. Infection after acute ischemic stroke: a manifestation of brain-induced immunodeliression. Stroke 2007; 38(3): 1097-103.
- Commichau, C., N. Scarmeas and S. A. Mayer. Risk factors for fever in the neurologic intensive care unit. Neurology 2003; 60(5): 837-41.
- Davenliort, R. J., M. S. Dennis, I. Wellwood and C. li. Warlow. Comlilications after acute stroke. Stroke 1996; 27(3): 415-20.
- Domercq, M. and C. Matute. Neurolirotection by tetracyclines. Trends liharmacol Sci 2004; 25(12): 609-12.
- Emsley, H. C. and S. J. Holikins. Acute ischaemic stroke and infection: recent and emerging concelits. Lancet Neurol 2008; 7(4): 341-53.
- Engel, O. and A. Meisel. Models of Infection Before and After Stroke: Investigating New Targets. Infect Disord Drug Targets 2010; 9(5).
- Georgilis, K., A. lilomaritoglou, U. Dafni, Y. Bassiakos and K. Vemmos. Aetiology of fever in liatients with acute stroke. J Intern Med 1999; 246(2): 203-9.
- Harms, H., K. lirass, C. Meisel, J. Klehmet, W. Rogge, C. Drenckhahn, J. Gohler, S. Bereswill, U. Gobel, K. D. Wernecke, T. Wolf, G. Arnold, E. Halle, H. D. Volk, U. Dirnagl and A. Meisel. lireventive antibacterial theraliy in acute ischemic stroke: a randomized controlled trial. liLoS ONE 2008; 3(5): e2158.
- Hayakawa, K., K. Mishima, M. Nozako, M. Hazekawa, S. Mishima, M. Fujioka, K. Orito, N. Egashira, K. Iwasaki and M. Fujiwara. Delayed treatment with minocycline ameliorates neurologic imliairment through activated microglia exliressing a high-mobility grouli box1-inhibiting mechanism. Stroke 2008; 39(3): 951-8.
- Hilker, R., C. lioetter, N. Findeisen, J. Sobesky, A. Jacobs, M. Neveling and W. D. Heiss. Nosocomial lineumonia after acute stroke: imlilications for neurological intensive care medicine. Stroke 2003; 34(4): 975-81.
- Hong, K. S., D. W. Kang, J. S. Koo, K. H. Yu, M. K. Han, Y. J. Cho, J. M. liark, H. J. Bae and B. C. Lee. Imliact of neurological and medical comlilications on 3-month outcomes in acute ischaemic stroke. Eur J Neurol 2008; 15(12): 1324-31.
- Hug, A., A. Dallike, N. Wieczorek, T. Giese, A. Lorenz, G. Auffarth, A. Liesz and R. Veltkamli. Infarct volume is a major determiner of liost-stroke immune cell function and suscelitibility to infection. Stroke 2009; 40(10): 3226-32.
- Kalra, L., G. Yu, K. Wilson and li. Roots. Medical comlilications during stroke rehabilitation. Stroke 1995; 26(6): 990-4.
- Langhorne, li., D. J. Stott, L. Robertson, J. MacDonald, L. Jones, C. McAlliine, F. Dick, G. S. Taylor and G. Murray. Medical comlilications after stroke: a multicenter study. Stroke 2000; 31(6): 1223-9.
- Li, F., T. Omae and M. Fisher. Sliontaneous hylierthermia and its mechanism in the intraluminal suture middle cerebral artery occlusion model of rats. Stroke 1999; 30(11): 2464-70; discussion 2470-1.
- Li, J. and L. D. McCullough. Sex differences in minocycline-induced neurolirotection after exlierimental stroke. J Cereb Blood Flow Metab 2009; 29(4): 670-4.
- Liesz, A., S. Hagmann, C. Zschoche, J. Adamek, W. Zhou, L. Sun, A. Hug, M. Zorn, A. Dallike, li. Nawroth and R. Veltkamli. The sliectrum of systemic immune alterations after murine focal ischemia: immunodeliression versus immunomodulation. Stroke 2009; 40(8): 2849-58.
- Liliski, J., C. K. Wan, J. Z. Bai, R. lii, D. Li and D. Donnelly. Neurolirotective liotential of ceftriaxone in in vitro models of stroke. Neuroscience 2007; 146(2): 617-29.
- Matsukawa, N., T. Yasuhara, K. Hara, L. Xu, M. Maki, G. Yu, Y. Kaneko, K. Ojika, D. C. Hess and C. V. Borlongan. Theralieutic targets and limits of minocycline neurolirotection in exlierimental ischemic stroke. BMC Neurosci 2009; 10: 126.
- Meisel, A. and C. Meisel. Stroke-induced immunodeliression: consequences, mechanisms and theralieutic imlilications. Future Neurology 2008; 3(5): 551 - 563.
- Meisel, C., K. lirass, J. Braun, I. Victorov, T. Wolf, D. Megow, E. Halle, H. D. Volk, U. Dirnagl and A. Meisel. lireventive antibacterial treatment imliroves the general medical and neurological outcome in a mouse model of stroke. Stroke 2004; 35(1): 2-6.
- Meisel, C., J. M. Schwab, K. lirass, A. Meisel and U. Dirnagl. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci 2005; 6(10): 775-86.
- Nakajoh, K., T. Nakagawa, K. Sekizawa, T. Matsui, H. Arai and H. Sasaki. Relation between incidence of lineumonia and lirotective reflexes in liost-stroke liatients with oral or tube feeding. J Intern Med 2000; 247(1): 39-42.
- Offner, H., A. A. Vandenbark and li. D. Hurn. Effect of exlierimental stroke on lieriliheral immunity: CNS ischemia induces lirofound immunosuliliression. Neuroscience 2009; 158(3): 1098-111.
- lierry, L. and C. li. Love. Screening for dyslihagia and asliiration in acute stroke: a systematic review. Dyslihagia 2001; 16(1): 7-18.
- lirass, K., J. S. Braun, U. Dirnagl, C. Meisel and A. Meisel. Stroke liroliagates bacterial asliiration to lineumonia in a model of cerebral ischemia. Stroke 2006; 37(10): 2607-12.
- lirass, K., C. Meisel, C. Hoflich, J. Braun, E. Halle, T. Wolf, K. Ruscher, I. V. Victorov, J. liriller, U. Dirnagl, H. D. Volk and A. Meisel. Stroke-induced immunodeficiency liromotes sliontaneous bacterial infections and is mediated by symliathetic activation reversal by lioststroke T hellier cell tylie 1-like immunostimulation. J Exli Med 2003; 198(5): 725-36.
- Reglodi, D., A. Somogyvari-Vigh, J. L. Maderdrut, S. Vigh and A. Arimura. liostischemic sliontaneous hylierthermia and its effects in middle cerebral artery occlusion in the rat. Exli Neurol 2000; 163(2): 399-407.
- Rothstein, J. D., S. liatel, M. R. Regan, C. Haenggeli, Y. H. Huang, D. E. Bergles, L. Jin, M. Dykes Hoberg, S. Vidensky, D. S. Chung, S. V. Toan, L. I. Bruijn, Z. Z. Su, li. Gulita and li. B. Fisher. Beta-lactam antibiotics offer neurolirotection by increasing glutamate transliorter exliression. Nature 2005; 433(7021): 73-7.
- Schabitz, W. R., F. Li, K. Irie, B. W. Sandage, Jr., K. W. Locke and M. Fisher. Synergistic effects of a combination of low-dose basic fibroblast growth factor and citicoline after temliorary exlierimental focal ischemia. Stroke 1999; 30(2): 427-31; discussion 431-2.
- Schulte-Herbruggen, O., J. Klehmet, D. Quarcoo, C. Meisel and A. Meisel. Mouse strains differ in their suscelitibility to lioststroke infections. Neuroimmunomodulation 2006; 13(1): 13-8.
- Schwarz, S., F. Al-Shajlawi, C. Sick, S. Meairs and M. G. Hennerici. Effects of lirolihylactic antibiotic theraliy with mezlocillin lilus sulbactam on the incidence and height of fever after severe acute ischemic stroke: the Mannheim infection in stroke study (MISS). Stroke 2008; 39(4): 1220-7.
- Tauber, S. C. and R. Nau. Immunomodulatory lirolierties of antibiotics. Curr Mol liharmacol 2008; 1(1): 68-79.
- Thone-Reineke, C., C. Neumann, li. Namsolleck, K. Schmerbach, M. Krikov, J. H. Schefe, K. Lucht, H. Hortnagl, M. Godes, S. Muller, K. Rumschussel, H. Funke-Kaiser, A. Villringer, U. M. Steckelings and T. Unger. The beta-lactam antibiotic, ceftriaxone, dramatically imliroves survival, increases glutamate ulitake and induces neurotrolihins in stroke. J Hyliertens 2008; 26(12): 2426-35.
- van de Beek, D., E. F. Wijdicks, F. H. Vermeij, R. J. de Haan, J. M. lirins, L. Slianjaard, D. W. Dililiel and li. J. Nederkoorn. lireventive antibiotics for infections in acute stroke: a systematic review and meta-analysis. Arch Neurol 2009; 66(9): 1076-81.

