Review Article - Interventional Cardiology (2014) Volume 6, Issue 3
Indigenous stents: examining the clinical data on new technologies
- Corresponding Author:
- Arramraju Sreenivas
Kumar
Chief Cardiologist, The Heart Centre
Continental Institute of Cardiovascular Sciences
Gachibowli, Hyderabad, India
E-mail: arramraj@yahoo.com
Abstract
Cardiovascular disease (CVD) is an important cause of mortality and morbidity in India. An estimated 47 million Indians had coronary artery disease (CAD) in 2010 and another estimated 2.3 million died from CAD compared with 404,000 in the USA. Approximately 50% of reported infarctions occur in Indian men under the age of 50 years, with 25% under the age of 40 years. In addition, some 30–40% of cardiovascular deaths occur between 35 and 64 years of age. An estimated 9.2 million productive years of life were lost to CVD in India in 2000, a number that is expected to increase to nearly 18 million by 2030 (ten-times the rate in the USA). The World Bank has concluded that in India, the disability adjusted life years lost due to ischemic heart disease is projected to more than double in the next 20 years. In 1990, coronary heart disease was responsible for 5.6 million disability adjusted life years lost in men and 4.5 million in women. This is projected to increase to 10.5 in men and 7.7 million in women by 2010. The economic impact was estimated to be US$9 billion in national income from premature deaths due to heart disease, stroke and diabetes in 2005 alone, with cumulative projected estimates of US$237 billion by 2015.Keywords
indigenous coronary stents, percutaneous coronary intervention
Cardiovascular disease (CVD) is an important cause of mortality and morbidity in India. An estimated 47 million Indians had coronary artery disease (CAD) in 2010 and another estimated 2.3 million died from CAD compared with 404,000 in the USA [1]. Approximately 50% of reported infarctions occur in Indian men under the age of 50 years, with 25% under the age of 40 years [2]. In addition, some 30–40% of cardiovascular deaths occur between 35 and 64 years of age. An estimated 9.2 million productive years of life were lost to CVD in India in 2000, a number that is expected to increase to nearly 18 million by 2030 (ten-times the rate in the USA) [3]. The World Bank has concluded that in India, the disability adjusted life years lost due to ischemic heart disease is projected to more than double in the next 20 years. In 1990, coronary heart disease was responsible for 5.6 million disability adjusted life years lost in men and 4.5 million in women. This is projected to increase to 10.5 in men and 7.7 million in women by 2010 [4]. The economic impact was estimated to be US$9 billion in national income from premature deaths due to heart disease, stroke and diabetes in 2005 alone, with cumulative projected estimates of US$237 billion by 2015 [4,5].
According to another estimate, the direct economic burden of heart disease in India could be 200 billion rupees (US$4.5 billion). Indirect costs would make the numbers even higher [4]. More than 80% of the population pays out of pocket for healthcare expenditure. As a result, 30 million are pushed into poverty every year [6,7]. India had the highest 15-month out-of-pocket CVD expenditures where the median income was international dollars (INT) $259/month and only 16% of the respondents had insurance coverage. The average out-of-pocket CVD expenditure was the equivalent of >6 months salary for all income groups [8]. According to the data from National Interventional Council India, only 152,332 percutaneous intervention procedures were performed during 2011 [9].
One of the reasons for the very low number of cardiac procedures carried out in India is poor affordability arising from the high cost of imported consumables. More than 90% of the consumables used in cardiac procedures are imported and are a drain on the precious foreign exchange [10]. Hence, there is a need to develop low cost indigenous devices that can be made available to all groups of the population at an affordable price.
Historical perspective
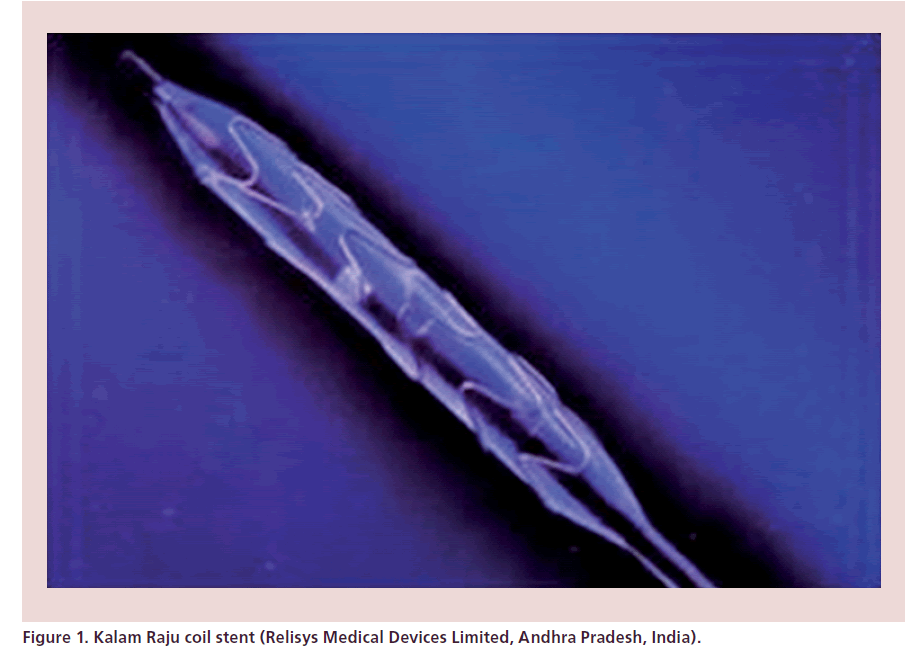
The first effort towards making an indigenous stent came in the late 1990s with the development of the Kalam Raju (KR) coil stent (Figure 1). The KR stent is a result of a coordinated effort of India’s Defense Research Organization and the medical community (CARE Foundation, India). The Society for Biomedical Technology was established to bring the advanced defense technology and medical knowledge together. For the first time, a delta ferrite-free matrix of authentic stainless steel was realized by the defense scientists. The technology developed to prevent the corrosion of naval ships was refined and adapted to attain the microrefined surface of the wire. The trade-off between minimal strut thickness to offer near laminar turbulence free blood flow and adequate metallic surface area of the helix to sustain arterial hoop stress resulted in the making of the KR stent. The first stent was implanted on 22 December 1996 at Mediciti Hospitals (Hyderabad, India) [11]. In a prospective observational study of primary angioplasty for acute myocardial infarction, a total of 100 patients who underwent primary angioplasty were analyzed. The majority of patients received a KR stent implantation, with a procedural success rate of 99%. None required emergency coronary artery bypass grafting (CABG) [12]. KR stents have been used in a number of centers all over the country and nearly 522 implantations have been successfully made with KR stents at CARE Hospital (Hyderabad, India) between 1997 and 2001. The development of the KR stent led to the crash of prices of imported coronary stents in the Indian market by more than 50%. For this historical effort, the CARE Foundation received of the Defense Technology Spin-off Award in 1998. Coil stents are no more in vogue as newer designs have been developed [10]. Since the development of the KR stent, a number of stents, both bare metal and drug-eluting, have been developed indigenously.
Indigenous bare metal stents
Cobal+C
Cobal+C (Relisys Medical Devices Limited, Andhra Pradesh, India) is bare metal stent with a cobalt chromium coronary stent system. It is made up of cobalt chromium alloy L-605. The strut thickness is 73 μm. The average foreshortening is less than 11% and average recoil is less than 5%. In a study conducted at CARE Hospitals, 283 patients with chronic stable angina or acute coronary syndrome were included in the Cobal+C registry. All of them received Cobal+C stents. A total of 261 patients out of 283 were followed clinically for a duration ranging from 9 to 18 months, out of which 80 patients had angiographic follow-up. At the end of 9 months, the major adverse cardiovascular events (MACE) was seen in 3.46%. Although the incidence of binary restenosis was 21%, the target lesion revascularization (TLR) was only 0.38% (CABG). The incidence of stent thrombosis was only 0.38% [13].
Indigenous bare metal stents (with limited clinical data)
Legend V
The Legend V (Relisys Medical Devices Limited) stent is a stainless steel coronary stent system with a novel stent geometry. The stent is made up of stainless steel 316LVM. It has a low strut thickness of 105 μm, with a high degree of radial strength and flexibility and unique crimping techniques for high stent dislodgment force and greater trackability and pushability [14].
Coronnium
Coronnium (Sahajanand Medical Technologies Pvt, Ltd, Surat, Gujarat, India) is a cobalt chromium alloy coronary stent system. It is made from a L-605 Co–Cr alloy. It is laser cut from seamless tubing in a serpentine pattern. The low strut thickness (0.0024”/60 μm) along with serpentine struts allows a homogenous stress distribution upon expansion. Preclinical studies have shown no stent thrombosis with complete endothelialization and no cytotoxic response [15].
M’ Sure-Cr chromium cobalt coronary stent
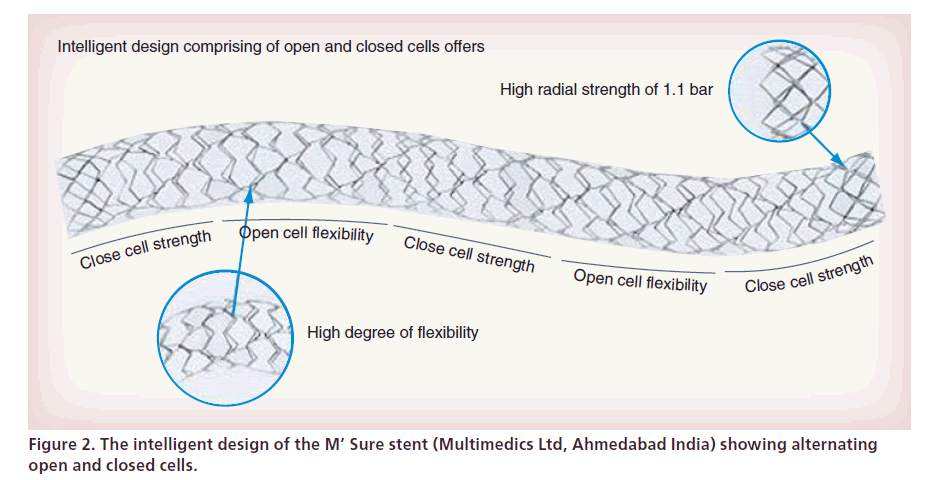
The M’ Sure-Cr chromium cobalt coronary stent (Multimedics Ltd, Ahmedabad, India) is made up of surgical grade chromium cobalt (L605), laser cut from seamless tubing in a serpentine pattern, which contains no molybdenum and only 10% nickel. The strut thickness is 59 μm with a high radial strength of 1.1 bars and a high degree of flexibility. The M’ Sure stent is a combination of closed cell and open cell design, which provides a high degree of flexibility, at the same time retaining adequate radial strength (Figure 2). During the bench testing, M’ Sure-Cr was found to have the lowest force recorded for either tracking the stent in a simulated model or pushing the stent through simulated vasculature [16].
M’ Sure-SS coronary stent
The M’ Sure-SS coronary stent (Multimedics Ltd) consists of surgical grade stainless steel (SS316L), laser cut from seamless tubing in a serpentine pattern. The 316L stainless steel has only 0.03% carbon. It is more resistant to general corrosion and pitting, than conventional stainless steel such as 302 or 304 [17].
NexGen cobalt chromium coronary stent system
The NexGen cobalt chromium coronary stent system (Meril Life Sciences Ltd, Gujarat, India) is made up of cobalt chromium L605. It has a unique hybrid cell design and provides high radial strength and flexibility (65-μm struts). It has an ultra high mirror finish and a low balloon overhang. The 6, 8 and 10 crown configuration on variable diameters allows for optimal scaffolding. The S-links and Y-connectors ensure no recoil and zero foreshortening [18].
Drug-eluting stents
A number of drug-eluting stents (DES) have also been developed indigenously (Table 1).
Paclitaxel DES
Release-T
Release-T (Relisys Medical Devices Ltd) paclitaxeleluting coronary stent system was the first indigenous paclitaxel-eluting coronary stent system to have completed multicenter clinical trials. It has a completely biodegradable, biocompatible polymer. The stent platform is The Legend V (Relisys Medical Devices Limited) stainless steel coronary stent system. The drug paclitaxel is a moderate-release formulation, with a uniform coating thickness of 8 μm. In a multicenter study conducted by Bhargava et al., the safety and efficacy of the indigenously developed paclitaxel-eluting Release-T stent was evaluated. One hundred patients who were undergoing angioplasty for various indications at four centers in India and who received the Release-T stent were included in the study. Patients were followed up for 6 months. There were two deaths: one patient had subacute stent thrombosis and the other patient had a sudden cardiac death. The 6-month rate of MACE was 4% and TLR rate was 2% [19].
Infinnium
The active ingredient in the Infinnium (Sahajanand Medical Technologies Pvt, Ltd) paclitaxel-eluting stent is paclitaxel. The paclitaxel concentration loaded on each stent was maintained to 1.4 μg/mm2. The drug is applied to the surface of a stainless steel (slotted tube design), balloon-expandable stent (Matrix, Sahajanand Medical Technologies Pvt Ltd) using biodegradable polymers (poly-l-lactide [PLLA], 50/50 poly-d-l-lactide- co-glycolide [PLGA], 75/25 poly-l-lactide-co-caprolactone and polyvinyl pyrrolidone [PVP]) in multiple layers. The drug is coated in three different layers of combination of drug and polymer. Each layer has a different release profile. The cumulative release of drug from the polymer is at 48 days after implantation. The stent is covered with a drug polymeric matrix all the way around the struts, providing efficient drug release into the vessel. The drug polymer’s coating thickness is 5–7 μm.
In a study by Vedat et al., 153 patients with native coronary artery lesions treated with the Infinnium paclitaxel-eluting coronary stent system were followed up for 1 year. This study was a single-center, nonrandomized retrospective study. Cumulative MACE rates were 5 (3.3%) in the first month, 6 (3.9%) in 3 months, 9 (5.9%) in 6 months and 15 (9.8%) in 1 year. At the end of the 1-year follow-up period, target vessel revascularization was 7.8% (12 patients), which included eight (5.2%) percutaneous coronary interventions (PCI) and four (2.6%) CABG patients. One-year survival without MACE was 90.2% [21]. In another study by Ostovan et al., 196 patients with symptomatic coronary disease who received the Infinnium paclitaxeleluting stents were studied prospectively. Cumulative MACE rates at the end of 12 months were: cardiac death 1%, myocardial infarction 5% (Q-wave 2.5%, non-Q-wave 2.5%) and repeat revascularization of the stented lesion 3%. The overall MACE rate was 8.1%. There were six (3%) stent thromboses; all occurred late after the procedure [22].
SIMPLE II was a multicenter, prospective registry study aimed at investigating the safety and efficacy of the Infinnium paclitaxel-eluting stent for the treatment of single de novo lesions in the native coronary arteries. A total of 103 patients with symptomatic CAD were treated for single de novo native coronary artery lesions using the Infinnium stent (paclitaxel concentration 1.4 μg/mm2 released over 48 days) in a multicenter, prospective study performed on three continents (Asia, Europe and South America). Hierarchical MACE at 30 days was 2.9%. At 9 months, 101 patients had clinical follow-up (one patient had died and one refused). There was one death (1.0%), one Q-wave myocardial infarction (Q MI) (1.0%), three non-Q MIs (2.9%), one clinically driven target lesion CABG (1.0%) and one clinically driven target lesion repeat PCI (1.0%). The overall event-free rate at 9 months was 93.2%. Quantitative coronary angiography revealed in-stent and in-segment late loss of 0.38 ± 0.49 mm and 0.18 ± 0.46 mm, resulting in binary restenosis rates of 7.3 and 8.3%, respectively. There was one case of late stent thrombosis in the patient experiencing the Q MI and subsequent repeat PCI [20].
In another study by Mimish et al., the safety and efficacy of Infinnium paclitaxel-eluting stent in Saudi Arabian patients was evaluated. The study was designed as an open-label, nonrandomized multicenter study. The study included 109 nonrandomized patients, with a total 176 Infinnium stents implanted (1.64 Infinnium/patient). The success rate of stent implantation was 98.4%. Acute stent thrombosis occurred in two patients, both of whom had sub-therapeutic anticoagulation (<150 s). One of these two patients died. There were three late thromboses (after 2 months), with two sudden deaths in the first year of follow-up, one of which is in a patient with a left ventricular ejection fraction of 25% [31].
Sirolimus-eluting stents
Supralimus-Core
Supralimus-Core (Sahajanand Medical Technologies Pvt Ltd) is sirolimus-eluting biodegradable polymer-coated cobalt chromium stent on the Coronnium bare metallic stent platform. It has a L605 Co–Cr alloy as its stent platform and a strut thickness of 60 μm, with biodegradable polymers and drug load of 1.4 μg/mm2. Approximately 70% of the drug is released within 7 days and the remaining drug is released over a period of 41 days. The coating layer comprises of drug sirolimus blended together with biodegradable polymeric matrix. This matrix includes different biodegradable polymers: PLLA, 50/50 PLGA and PVP, to control the drug elution from stent coating. After releasing the drug within 48 days, these polymers eventually degrade naturally and are excreted from the body in the form of their metabolites. The average coating thickness of the Supralimus-Core stent is between 5 and 6 μm.
The first-in-man study (MAXIMUS) was conducted to assess the safety and efficacy of the Supralimus-Core stent. This study was a single-center, prospective non-randomized study, which included clinical follow-up data that was collected at 1, 8 and 12 months after the procedure. The study included 105 patients with de novo native coronary artery lesions including multivessel disease treated with the Supralimus-Core stent. Repeat angiography was performed 8 months poststent implantation. At quantitative coronary angiography, 8-month luminal late loss was 0.39 ± 0.33 mm in-stent and 0.33 ± 0.35 mm in-segment. The incidence of any MACE at 30 days, 8 months and 12 months was 1 (1%), 6 (6%) and 7 (7%), respectively, thus demonstrating the safety and efficacy of the Supralimus-Core stent [23].
In the Supralimus-Core OCT study, the strut apposition and neointimal coverage of Supralimus- Core stent struts at 4 months after implantation was studied using optical coherence tomography. A total of 2870 struts and 1950 frames were analyzed from 15 stents. The apposed and covered struts were 2787 (97.11%), whereas malapposed and covered struts were three (0.10%), apposed and uncovered struts 49 (1.71%) and malapposed and uncovered struts 31 (1.08%). Mean neointimal hyperplasia thickness was 155.1 ± 55.2 μm. The study demonstrated that the Supralimus-Core stent has a favorable vascular healing pattern at 4 months after stent implantation in terms of stent–strut coverage and strut apposition [32].
The systemic exposure of sirolimus after Supralimus- Core coronary stent implantation was assessed in the Supralimus-Core PK study. A total of 20 patients with CAD who were treated with one, two or three newly designed metallic stents were included in the study. Blood samples were drawn at 14 time points to determine the pharmacokinetics of sirolimus. Whole blood concentrations of sirolimus were determined by using a sensitive validated high-performance liquid chromatography mass spectrometry/mass spectrometry method. Minimal measurable blood levels were detectable at 7 days. Across all dose levels, individual Tmax values ranged from 1.00 and 12.00 h; individual Cmax values ranged from 0.73 and 4.13 ng/ml. Thus confirming that the sirolimus concentration in systemic circulation is safe, well-tolerated and short-lived [33].
Supralimus
The Supralimus (Sahajanand Medical Technologies Pvt Ltd) stent is a sirolimus-eluting biodegradable polymer-coated stent. The Supralimus stent consists of an established balloon-expandable 316L stainless steel slotted-tube stent platform (Matrix, Sahajanand Medical Technologies Pvt Ltd) with two layers of degradable polymer coating; the base layer consists of 1.4 μg/mm2 sirolimus incorporated into a biodegradable polymer matrix consisting of PLLA, PLGA (50/50) and PVP, with a drug:polymer ratio of 35:65. The outer protective layer containing only PVP prevents premature drug release and is completely removed within 2 h after implantation. Following removal of this protective layer, an early burst phase releases 50% of the drug within the first 7 days to inhibit the inflammatory response and smooth muscle cell migration and proliferation. The remaining 50% of sirolimus is released within 41 days; thus, in an average of 48 days, the total drug content is released from the stent surface.
SERIES I is a prospective, nonrandomized, first-inman open-label study with the biodegradable polymerbased Supralimus sirolimus-eluting stent. In this study, 100 patients who were treated with 126 Supralimus stents were followed up for 30 months. MACE rates were 0% after 1 month, 6% at 9 months follow-up and 7% after 30 months follow-up, thus confirming the effectiveness of the Supralimus stent. There was no definite stent thrombosis and one case of probable and one case of possible stent thrombosis after the Academic Research Consortium definition. Angiographic follow-up in a prespecified subgroup of 60 patients at 6 months showed binary angiographic restenosis rates of 0% (in-stent) and 1.7% (in-segment). The in-stent late loss was 0.09 ± 0.37 mm [24].
The PAINT study was a multicenter three-arm randomized trial, which allocated patients to coronary intervention of de novo lesions with: Infinnium paclitaxel-eluting stent; Supralimus sirolimus-eluting stent; or control Millenium Matrix bare metal stent. A total of 274 coronary patients were randomly allocated to the Infinniumpaclitaxel-eluting stent, Supralimus sirolimus-eluting stent or the control Matrix bare metal stent (in a 2:2:1 ratio). The two DES used the same biodegradable polymers and were identical except for the drug. At 3 years, the pooled DES population had similar rates of cardiac death or myocardial infarction (9.0 vs 7.1; p = 0.6), but lower risk of repeat interventions (10.0 vs 29.9%; p < 0.01) than controls with bare stents. The cumulative 3-year incidence of definite or probable stent thrombosis in the pooled DES group was 2.3% (first year: 1.8%; second year: 0.4%; third year: 0). There were no significant differences in outcomes between paclitaxel- and sirolimus-eluting stents [34].
In the PAINT complex study, a subset of 185 patients (Infinnium: 77 patients; Supralimus: 70 patients; Matrix: 38 patients) from a total of 274 patients included in the PAINT trial, who had one or more of the following high-risk characteristics viz. small vessel disease (stent diameter: 2.5 mm); long lesion length (stent length: ≥23 mm); diabetes were analyzed with angiographic follow-up at 9 months and clinical follow-up at 12 months. The late loss in-stent and insegment late loss for the Matrix stent was 0.98 ± 0.49, 0.73 ± 0.54 mm, for the Infinnium stent 0.52 ± 0.45, 0.35 ± 0.44 mm and 0.33 ± 0.36 and 0.16 ± 0.36 mm, respectively, for the Supralimus stent. The incidence of binary restenosis at the end of 9 months was 30.6% for the Matrix stent, 9.6% for the Infinnium stent and 4.5% for the Supralimus stent. Thus, compared with bare metal stents, use of the Infinnium paclitaxeland Supralimus sirolimus-eluting stents in complex patients resulted in significantly lower angiographic neointimal hyperplasia that led to a reduction in the need for repeat revascularization. However, there were no significant differences in the rate of death, myocardial infarction and stent thrombosis between the three stent groups studied at 10 months follow-up. The head-to-head comparison between Infinnium and Supralimus (both with the same platform and polymeric coating) permitted to explore the isolate effect of sirolimus and paclitaxel. Although the sirolimus stent was associated with a significantly larger NIH inhibition than paclitaxel, the angiographic superiority was not translated into better clinical outcomes. Both stents had similar clinical outcomes, with a TLR rate of approximately 5% and a MACE rate of approximately 9% at 10 months [35].
In the E-SERIES registry (in complex patients), which is a prospective, multicenter, international, nonrandomized, web-based registry, a total of 1146 patients who received the sirolimus-eluting Supralimus stent were followed up. Preliminary clinical outcomes at 6 months demonstrated 2.4% overall death, 2.2% TLR and 0.8% stent thrombosis [36]. At the end of 2 years, the MACE-free survival was 86.7%. The definite/probable stent thrombosis rate was 0.6% [25]. In the E-SERIES registry (in diabetic patients), the safety and clinical effectiveness of Supralimus bioabsorbable sirolimus-eluting DES in diabetic patients was assessed. A total of 423 patients who received the Supralimus bioabsorbable sirolimus-eluting stent and completed 6 months were evaluated at 6 months follow-up; clinical outcomes were comparable in diabetics versus nondiabetics, including an overall MACE <6% and TLR <2% [37].
In the SELL study, the safety, efficacy and procedural outcomes of extra long sirolimus-eluting Supralimus stents were studied. In this study, a total of 50 patients treated with a 39-mm-long single Supralimus sirolimus-eluting stent for treatment of single de novo lesions (vessel diameter: 2.5–4.0 mm) at three Indian centers were retrospectively analyzed. Clinical success, technical success and in-hospital MACE rates were studied. Technical success/failure was assessed on primary intention-to-treat basis. Technical success was defined as successful implantation of stent with <20% residual diameter stenosis. Procedural difficulty was assessed based on grading by the operators. The deployment failure was observed in 4% due to angulation/angulation plus calcification. No case of acute or subacute stent thrombosis was reported and there was no in-hospital MACE. Average procedural difficulty was 3.5 on a scale of 5. Use of single extra long sirolimus-eluting stents gave a significant cost advantage and avoided concerns about long-term outcomes of overlapping DES [38].
The Manipal-S registry was designed to evaluate the safety and effectiveness of the biodegradable polymercoated Supralimus sirolimus-eluting coronary stent for the treatment of CAD, across a wide range of patients who are treated in real-life clinical practice. In total, 116 patients underwent single-vessel or multiple vessel PCIs with the use of Supralimus sirolimus-eluting stents. Patients were clinically followed up at 1, 9, 12 and 24 months postprocedure. In the 116 patients, a total of 126 lesions were implanted with 144 stents, which had an average stent length of 25.8 ± 8.0 mm. The incidences of any major adverse cardiac and cerebral events at 1, 9, 12 and 24 months were 0, 5 (4.3%), 8 (6.9%) and 10 (8.6%), respectively, thus providing evidence for the safety and effectiveness of the Supralimus sirolimus-eluting coronary stent system with the biodegradable polymer in real-life patients [26].
Indolimus stent
The Indolimus (Sahajanand Medical Technologies Pvt Ltd) stent is another sirolimus-eluting stent system with an open cell design. In the Indolimus registry, the safety and efficacy of the biodegradable polymercoated Indolimus sirolimus-eluting coronary stent for the treatment of CAD across a wide range of patients treated in routine clinical practice, including those with high-risk characteristics and complex lesions was evaluated. A total of 530 consecutive unselected patients who underwent PCI with the Indolimus stent were included in the registry. The 30 days and 6 months follow-up of all 530 patients was obtained. The incidence of any MACE at in-hospital, at 30 days and at 6 months were 5 (0.94%), 8 (1.52%) and 18 (3.40%), respectively. Long-term follow-up of this registry is awaited [27].
Superia
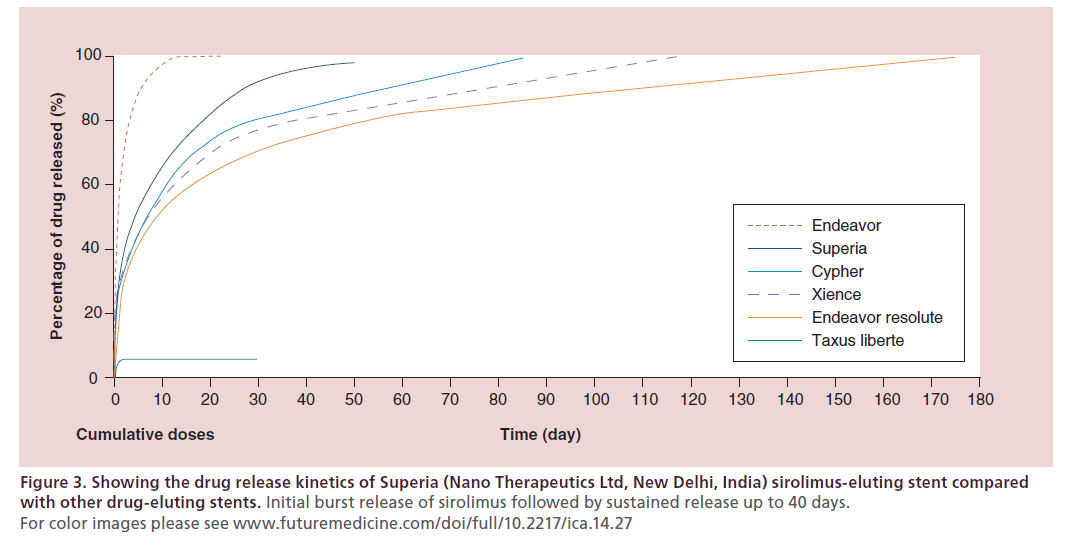
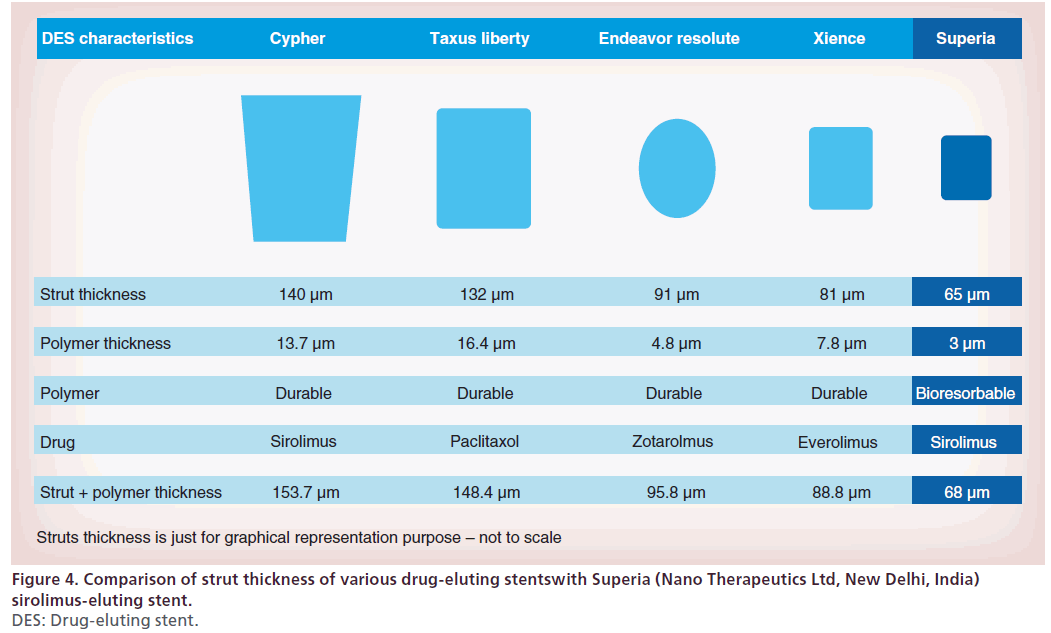
The Superia (Nano Therapeutics Ltd, New Delhi, India) sirolimus-eluting stent is a sirolimus-eluting stent on a balloon-expandable L605 cobalt chromium Flexia coronary stent platform (Nano Therapeutics Ltd). It has a uniform sinusoidal strut design offering uniform drug delivery throughout stent length. It has an initial burst release of sirolimus followed by sustained release up to 40 days (Figure 3). It has a strut thickness of 65 μm (Figure 4), with a fully bioresorbable polymer. In a prospective, multicenter, postmarketing surveillance study, 150 patients who received the Superia sirolimuseluting stent were evaluated. Evaluation of study end points at 180 days follow-up shows 100% procedural success with no MACE except 2% restenosis [28].
Figure 3: Showing the drug release kinetics of Superia (Nano Therapeutics Ltd, New Delhi, India) sirolimus-eluting stent compared with other drug-eluting stents. Initial burst release of sirolimus followed by sustained release up to 40 days. For color images please see www.futuremedicine.com/doi/full/10.2217/ica.14.27
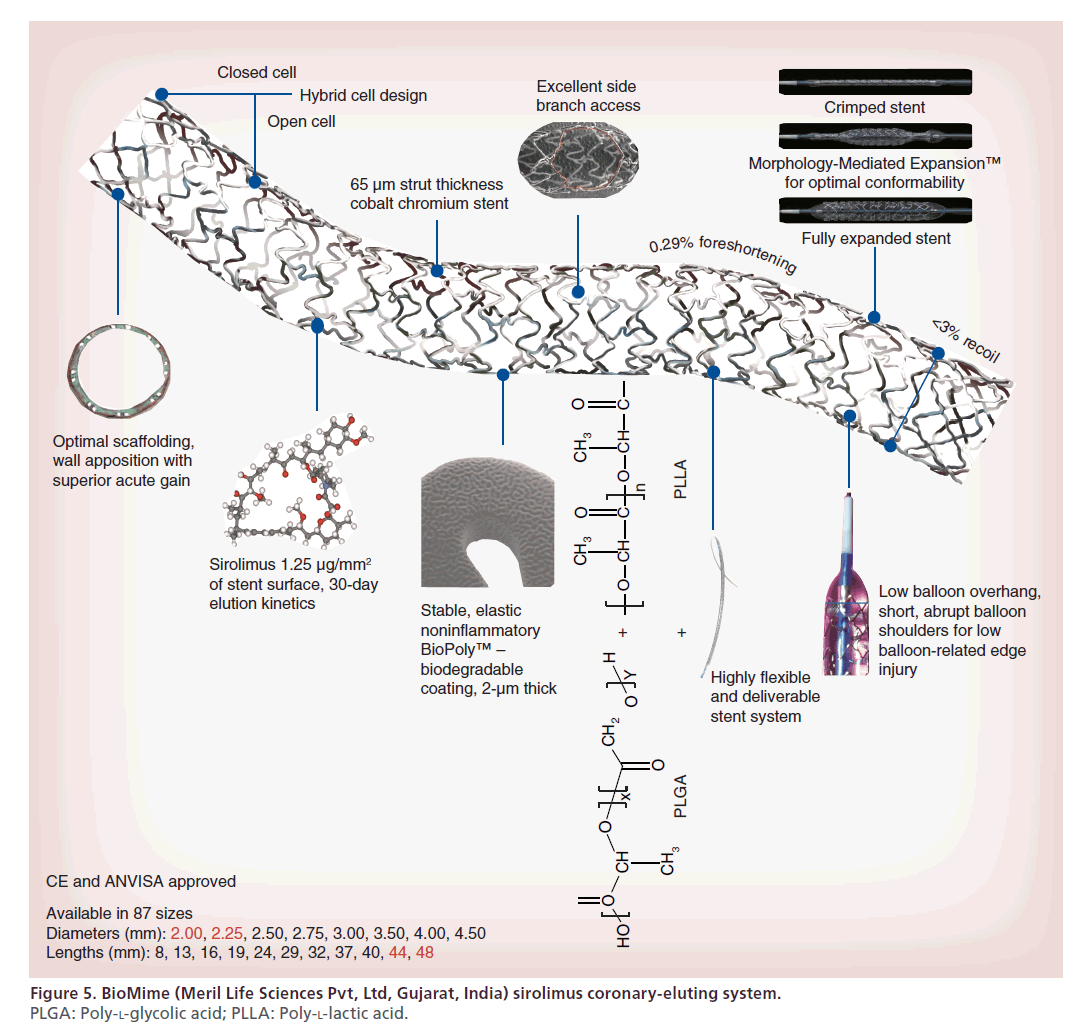
BioMime
The base stent in BioMime (Meril Life Sciences Pvt, Ltd) sirolimus coronary-eluting system is the NexGen L605 cobalt chromium stent platform (Figure 5). It has a strut thickness of 65 μm. Its novel design incorporates an intelligent mix of open and closed cells allowing for morphology-mediated expansion of the stent. It has a unique strut width variability that ensures a <3% recoil and 0.29% foreshortening. A special electro-polishing technique eliminates surface nickel oxides. This hybrid stent has a high radial strength and comes premounted on a flexible delivery system that maintains shortabrupt balloon shoulders to minimize balloon-related edge injuries. The entire stent surface is coated with a 2-μm mix of known biodegradable polymers: PLLA and PLGA, along with the active antiproliferative drug sirolimus (1.25 μg/mm2). The drug is timed to elute over a period of 30 days, while the polymeric mixture degrades soon via hydrolysis and eventual elimination as CO2 and H2O.
The Merit-1 trial was a prospective, nonrandomized, single-arm, first-in-man clinical evaluation of the performance of the novel BioMime sirolimus-eluting stent in the treatment of noncomplex coronary lesions. The objective was to provide preliminary safety, feasibility and efficacy evaluations of this new technology in humans with CAD treated at a single clinical institution. A total of 30 patients who received the BioMime sirolimus-eluting stent were evaluated. Angiographic follow-up was available in 86.75% of patients (26/30). Median in-stent late luminal loss was 0.15 mm. There were no cases of binary restenosis reported, as well as no exaggerated neointimal hyperplasia at the stent edges or outside the stent [39].
In the Merit-2 study, 242 patients who received a total of 363 BioMime sirolimus coronary-eluting systems were followed up for 12 months. There were no MACE at the end of 30 days. The late lumen loss at the end of 8 months (n = 132) was 0.15 mm. Cumulative rates of MACE in 12 months were 5.7% (0.5% cardiac deaths: 4.7% TLR). There were three cases of stent thrombosis: one acute, one subacute and one late thrombosis [29].
In the angiographic subanalysis of combined Merit-1 and Merit-2 trials, a total of 280 patients with 385 de novo lesions were analyzed. The late lumen loss was 0.12 mm. Binary restenosis was found in only 5.7% patients (4.5% in-stent). The focal pattern was the common pattern of restenosis [40].
MeRes bioresorbable scaffold
MeRes bioresorbable scaffold (Meril Life Science Pvt, Ltd) is a bioresorbable scaffold, which is cut from the radial-expanded tubing using a femtosecond laser. Strut width and thickness are <200 μm. Three platinum markers are placed at each end, which allow for clear visibility of the scaffold postdeployment. It has a new PLA backbone formulation for enhanced radial strength. It employs 1.25 μg/mm2 of sirolimus, timed to elute over 90 days from noninflammatory biodegradable polymers. Preliminary engineering tests have demonstrated sound mechanical characteristics of this novel bioresorbable scaffold platform. MeRes is currently under preclinical studies.
M’ Sure-S
The M’ Sure-S (Multimedics Ltd) sirolimus-eluting coronary stent system uses a validated formulation of low dose sirolimus (1.30 μg/mm2) timed to elute in 30 days from a biodegradable and biocompatible polymer base (PLLA and PLGA), which degrades simultaneously. The M’ Sure-S stent is built on a cobalt chromium platform. It has an ultra-low strut thickness of 59 μm with 0.29% foreshortening and <3% recoil. The thickness of drug and polymer coating is 2 μm.
In the PRISM pilot trial, 32 patients who received the M’ Sure-S sirolimus-eluting coronary stent system were followed for 6 months. At the end of 6 months, an in-stent late lumen loss of 0.05 mm and an in-segment late lumen loss of 0.05 mm with 0% binary restenosis were noted. No stent thrombosis was observed at 6-month follow-up. The PRISM – Indian Subcontinent (100 patients), Euro – PRISM (100 patients) and PRISM – Global (1000 patients) are ongoing trials to evaluate the safety and efficacy of the M’ Sure-S [41].
Abluminus
The Abluminus (Envision Scientific Pvt, Ltd, Surat, Gujarat, India) sirolimus-eluting stent system has a unique coating. It is an abluminal surface only coated stent. The stent, as well as the balloon outer surface, is coated (i.e., the stent is coated after crimping on balloon). With this, it provides a directional release of the formulation to the vessel wall. Abluminus avoids inner surface coating, which aids better endothelialization and eliminates delayed healing of the arterial tissue. The formulation is delivered from a cobalt chromium stent platform, which has hybrid design – a mix of open and closed cells.
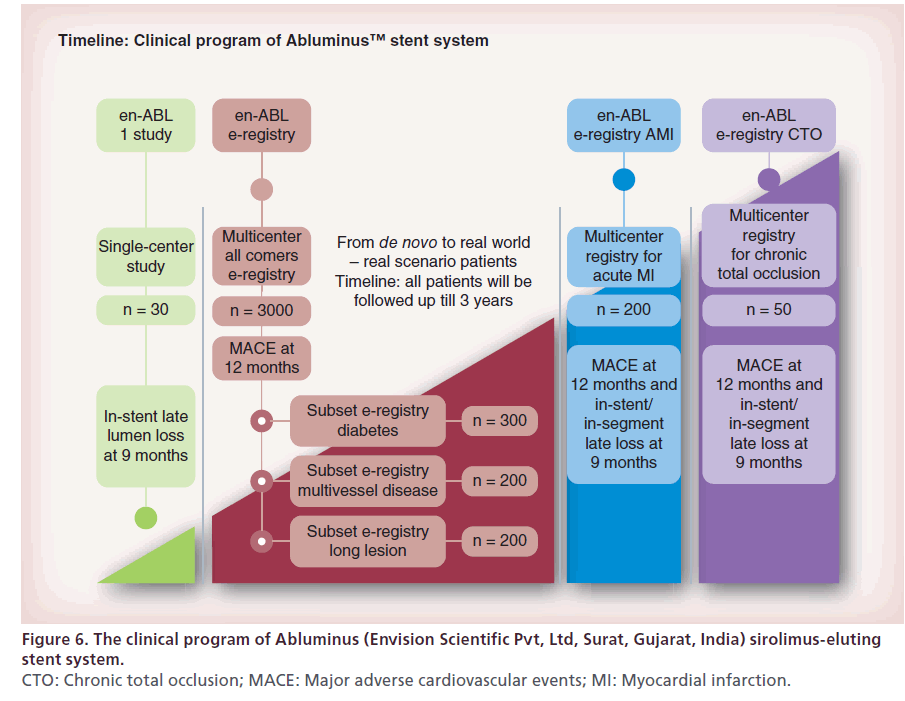
The en-ABL 1 study, en-ABL e-registry, en-ABL e-registry AMI and en-ABLe-registry CTO are ongoing studies evaluating the safety and efficacy of the Abluminus stent in various settings (Figure 6) [42].
Figure 6: The clinical program of Abluminus (Envision Scientific Pvt, Ltd, Surat, Gujarat, India) sirolimus-eluting
stent system.
CTO: Chronic total occlusion; MACE: Major adverse cardiovascular events; MI: Myocardial infarction.
The FOCUS np stent (Envision Scientific Pvt, Ltd) platform has a novel carrier: a phospholipid two-layer nanoparticle that encapsulates sirolimus. Drug at the nanometer size is encapsulated into an excipient-based carrier. The encapsulated carrier is also at a nanometer size range allowing for better biodistribution and biocompatibility of the drug molecule. The encapsulated sirolimus is coated on the surface of the stent and the balloon (108 μg of sirolimus on a 3.0 × 16.0 mm stent system). Nanoactive technology utilizes a spray coating mechanism, which is precisely metered. The coating is applied on a crimped stent system. An abluminal surface coating (coating on the surface of the stent only) is helpful in faster and better healing of endothelial tissue. Avoiding drug on the inner surface allows cells to grow more uniformly and helps in advancing healing at the target site. Sirolimus is programmed to be completely released within 28 days; however, the tissue concentration of sirolimus peaks within the first 24 h. Preclinical studies have demonstrated the safety and efficacy of the nanocarrier coating, with the FOCUS np stent showing similar late lumen loss and inflammation scores to that seen in the Cypher sirolimus-eluting stent at 28 days and at 90 days. The Nano Active FIM-IN clinical study (n = 100) and Nano Active e-registry (n = 1000) and Nano Active EU study are ongoing clinical trials to study the safety and efficacy of the FOCUS np stents [43].
Yukon Choice PC
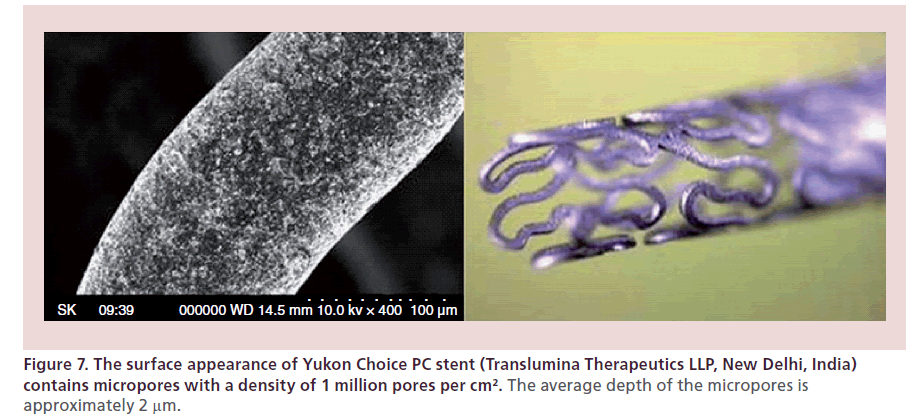
Yukon Choice PC (Translumina Therapeutics LLP, New Delhi, India) is a third-generation sirolimuseluting stent system that combines the synergistic effect of surface modification and biodegradable polymer to create optimum release. Yukon Choice PC is a combination of sirolimus, biodegradable polymer resomer R202S coated abluminally on the microporous stent surface with a top coat of shellac resin. Yukon Choice PC’s stent surface is coated abluminally with no drug or polymer on the luminal side of the stent for enhanced safety, which ensures faster endotheliazation. Yukon Choice PC is the first product where a proven technology of DES has been sourced from Europe and manufactured in India using international standards, high-end machines and layouts created as per the European Guidelines. The microporous surface of Yukon Choice PC is well studied for its efficacy and safety when compared with an electropolished surface (Figure 7). A microporous surface shows a trend in reduced rate of binary restenosis with equivalent safety, which proves that it is safe and feasible to release drug from a microporous surface. The microporous stent surface, called pearl surface, favors better endothelialization, which is essential in avoiding thrombosis and restenosis. The biodegradable polymer on the stent degrades in 90 days and the stent becomes a bare metal stent.
The biodegradable polymer and permanent polymer DES were compared in the ISAR Test 4 trial. The trial was a randomized clinical trial with broad inclusion criteria, enrolling 2603 patients at two clinics in Munich, Germany. Patients were randomized to either biodegradable polymer (n = 1299) or permanent polymer stents (n = 1304); patients treated with permanent polymer stents were randomly allocated to everolimus-eluting stent (n = 652) or sirolimus-eluting stent (n = 652). The trial was designed as an all-comers trial, with a high proportion of diabetic, acute myocardial infarction and prior myocardial infarction patients, as well as a significant number of patients with multivessel disease. The primary end point was the composite of cardiac death, target vessel-related myocardial infarction or TLR.
The primary end point ( a combination of cardiac death, myocardial infarction related to the target vessel or TLR) of the study at 12 months of the bioerodable polymer stent was noninferior to the combined permanent polymer stents (Cypher/Xience) group. Similarly, no differences were seen for the secondary end points of cardiac death or myocardial infarction or TLR. Rates of stent thrombosis were numerically, but not statistically, significantly different between the two groups. Angiographically, late lumen loss was roughly 0.25 mm in both groups, while binary restenosis rates were 12% in both groups. No statistically significant differences were seen between any of the study end points, and the criteria for noninferiority were met [44].
Clinical events continued to accrue at a low rate out to 3 years in all groups. Overall, there was no significant difference between biodegradable polymer and permanent polymer DES with regard to the primary end point (20.1 vs 20.9%; hazard ratio [HR]: 0.95; 95% CI: 0.80–1.13; p = 0.59). Rates of definite/probable stent thrombosis were also similar in both groups (1.2 vs 1.7%, respectively; HR: 0.71; 95% CI: 0.37–1.39; p = 0.32). In patients treated with permanent polymer stents, everolimus-eluting stent were comparable to sirolimus-eluting stent with regard to the primary end point (19.6 vs 22.2%, respectively; HR: 0.87; 95% CI: 0.68–1.11; p = 0.26), as well asdefinite/probable stent thrombosis (1.4 vs 1.9%; HR: 0.75; 95% CI: 0.32–1.78; p = 0.51) [30].
Stefanini et al., pooled individual patient data from three large-scale multicenter randomized clinical trials (ISAR-TEST 3, ISAR-TEST 4 and LEADERS) comparing biodegradable polymer DES with durable polymer sirolimus-eluting stent and assessed clinical outcomes during follow-up through 4 years. The efficacy end point of interest was TLR and the safety end point of interest was definite stent thrombosis. Out of 4062 patients included in the present analysis, 2358 were randomly assigned to treatment with biodegradable polymer DES (sirolimus-eluting: n = 1501; biolimus-eluting: n = 857) and 1704 patients to durable polymer sirolimus-eluting stent. At 4 years, the risk of TLR was significantly lower among patients treated with biodegradable polymer DES versus durable polymer sirolimus-eluting stent (HR: 0.82; 95% CI: 0.68–0.98; p = 0.029). In addition, the risk of stent thrombosis was significantly reduced with biodegradable polymer DES versus durable polymer sirolimus-eluting stent (HR: 0.56; 95% CI: 0.35–0.90; p = 0.015), driven by a lower risk of very late stent thrombosis (HR: 0.22; 95% CI: 0.08–0.61; p = 0.004). In keeping with this, in landmark analysis between 1 and 4 years, the incidence of myocardial infarction was lower for patients treated with biodegradable polymer DES versus durable polymer sirolimus- eluting stent (HR: 0.59; 95% CI: 0.73–0.95; p = 0.031) [45].
Peripheral
Periflex
The Periflex (Nano Therapeutics Ltd) cobalt chromium peripheral stent is a balloon expandable L605 cobalt chromium alloy stent, premounted onto a balloon delivery catheter (over the wire and rapid exchange delivery system), which can be deployed by inflating the balloon. Upon balloon inflation, the stent achieves a cylindrical shape and it is a permanently implantable device. The stent design has circumferential open cells with multiple connectors. The strut thickness is 110 μm.
Curatis
The Curatis (NanoTherapeutics Ltd) cobalt chromium renal and biliary stent is a balloon-expandable L605 cobalt chromium alloy stent, premounted onto a rapid exchange delivery catheter, which can be deployed by inflating the balloon. Upon balloon inflation, the stent achieves a cylindrical shape and it is a permanently implantable device.
Conclusion
PCI in India has entered an exciting phase with newer stent technologies being developed and evaluated, and long-term data of existing indigenous stents demonstrate results comparable with the US FDA-approved stents.
Future perspective
Much of the clinical data available for indigenous stents are from registries, short-term studies, nonrandomized studies and few randomized studies. Newer technologies should reach the people at an affordable cost. At the same time, safety and efficacy should not be compromised. This is an era of evidence-based medicine. Hence, it is very important to generate good scientific evidence to say that indigenous products are not only cost effective, but also efficacious and are of equally good standards. Although the indigenous stent technology is advanced, there is a paucity of clinical data. Accordingly the Indian Council of Medical Research is planning to sponsor a randomized controlled, noninferiority multicentric clinical trial comparing the identified indigenous stents with a standard FDA-approved stent with the best outcomes data. Based on the results of the study, the clinicians practicing in India will be able to make informed decisions while choosing between the indigenous and standard FDA-approved DES. The Indian Council of Medical Research will evaluate two latest-generation Indian stents against one FDA-approved stent through a sponsored trial, with a view to give fillip to the development of indigenous technologies, products and gather data about safety and efficacy [46].
Executive summary
• The Kalam Raju stent was the first indigenous stent to be developed in India.
• The introduction of indigenous stents led to a significant drop in the prices of all stents in India.
• The available clinical data with indigenous bare metal stents (cobalt chromium coronary stents) showed good results with low major adverse cardiovascular events and a low incidence of stent thrombosis.
• The indigenous drug-eluting stents with a biodegradable polymer showed excellent clinical outcomes similar to the permanent polymer stents.
• The indigenous drug-eluting stents with bioresorbable scaffold are also in the pipeline.
• A randomized controlled, noninferiority multicentric clinical trial comparing the identified indigenous stents with a standard US FDA-approved stent is planned by the Indian Council of Medical Research. The trial data will further provide evidence regarding the indigenous stent technologies.
References
- Enas EA, Singh V, Gupta R et al. Recommendations of the Second Indo-US Health Summit for the prevention and control of cardiovascular disease among Asian Indians. Indian Heart J. 61, 265–274 (2009).
- Enas EA, Senthilkumar A. Coronary artery disease in Asian Indians: an update and review. Internet J. Cardiol. 1, 2 (2002).
- DeMaria AN. Cardiology in India. J. Am. Coll. Cardiol. 56, 678–679 (2010).
- Gupta R. Coronary heart disease in India: absolute numbers and economic burden. Rapid response to Ghaffar A, Reddy KS, Singhi M. Burden of non-communicable diseases in South Asia. BMJ 328, 807–810 (2004).
- World Health Organization. Prevention of Cardiovascular Disease: A Vital Investment. World Health Organization, Geneva, Switzerland (2007).
- Gupta R, Guptha S, Joshi R, Xavier D. Translating evidence into policy for cardiovascular disease control in India. Health Res. Policy Syst. 9, 8 (2011).
- Van Doorslaer E, O’Donnell O, Rannan-Eliya RP et al. Effect of payments for health care on poverty estimates in 11 countries in Asia: an analysis of household survey data. Lancet 368(9544), 1357–1364 (2006).
- Huffman MD, Rao KD, Pichon-Riviere A et al. A crosssectional study of the microeconomic impact of cardiovascular disease hospitalization in four low- and middle-income countries. PLoS ONE 6(6), e20821 (2011).
- Chakraborthy R, Ramakrishnan S. Coronary intervention data year 2011. Presented at: Interventional Council of India Mid Term Annual Meeting. Kochi, India (2012). www.slideshare.net/saketsinghi/nic-ptca-registry-coronarydata- presentation-by-dr-rabin-chakraborthy
- CARE foundation. Kalam Raju Stent. www.carefoundation.org.in/index.php/technology/ kalamrajustenttech.html
- Kalam Raju stent. Relysis Medical Devices Ltd. Hyderabad India. www.sbmtindia.org/activities/kalam_raju_coronary_stent/ index.html
- Reddy NK, Raju PR, Kapoor S et al. Prospective observational study of primary angioplasty of the infarct-related artery for acute myocardial infarction. Indian Heart J. 51(2), 167–172 (1999).
- Cobal+ C registry. Relisys Medical Devices Ltd. Bare metal stent systems, Cobal+C. www.relisysmedicaldevice.com/Cobalc4.html
- Relisys Medical Devices Ltd. Bare metal stent systems, Legend V. www.relisysmedicaldevice.com/Legend-V2.html
- Sahajanand Medical Technologies Pvt Ltd. Coronnium stent. www.smtpl.com/coronnium.html
- Multimedics Ltd Ahmedabad India. Bare metal stents. M’ Sure-Cr chromium cobalt coronary stent. http://multimedicsllc.com/msure_cr.html
- Multimedics Ltd Ahmedabad India. Bare metal stents. M’ Sure-SS coronary stent. http://multimedicsllc.com/msure.html
- Meril Life Sciences Ltd. Cardiovascular bare metal stents: NexGen. www.merillife.com/nexgen.aspx
- Bhargava B, Karthikeyan G, Narag R et al. Clinical evaluation of an indigenous paclitaxel-eluting stent in de novo coronary artery stenosis. Indian Heart J. 58(1), 38–41 (2006).
- Vranckx P, Serruys PW, Gambhir S et al. Biodegradablepolymer- based, paclitaxel-eluting Infinnium stent: 9-month clinical and angiographic follow-up results from the SIMPLE II prospective multi-centre registry study. EuroIntervention 2(3), 310–317 (2006).
- Vedat A, Selcuk G, Refik E. Treatment of patients with coronary artery disease with biodegradable polymer based paclitaxel-eluting Infinnium coronary stent system: results of 1-year clinical follow-up a single center experience. Indian Heart J. 61(3), 254–257 (2009).
- Ostovan MA, Mollazadeh R, Kojuri J, Mirabadi M. Experience with paclitaxel-eluting Infinnium coronary stents. Asian Cardiovasc. Thorac. Ann. 16(6), 454–458 (2008).
- Seth A, Chandra P, Chouhan NS, Thakkar AS. A firstin- man study of sirolimus-eluting, biodegradable polymer coated cobalt chromium stent in real life patients. Indian Heart J. 64(6), 547–552 (2012).
- Dani S, Kukreja N, Parikh P et al. Biodegradable-polymerbased, sirolimus-eluting Supralimus stent: 6-month angiographic and 30-month clinical follow-up results from the series I prospective study. EuroIntervention 4(1), 59–63 (2008).
- Costa JR Jr, Abizaid A, Machado B et al. Supralimus® bioabsorbable sirolimus-eluting stent technology in patients with acute coronary syndrome: two -year results of the prospective, multicenter, E-SERIES registry. J. Am. Coll. Cardiol. 55, A208 (2010).
- Shetty R, Vivek G, Thakkar A et al. Experience with biodegradable polymer coated sirolimus-eluting coronary stent system in ‘real-life’ percutaneous coronary intervention: 24-month data from the manipal-s registry. J. Clin. Diagn. Res. 7(9), 1959–1963 (2013).
- Rajasekhar D, Vanajakshamma V, Shashank C, Srinivasakumar ML, Sivasankara C. The real world experience of the biodegradable polymer -coated sirolimuseluting coronary stent system: results from an ‘all-comers’ clinical experience. Catheter Cardiovasc. Interv. doi:10.1002/ ccd.25246 (2014) (Epub ahead of print).
- Chandra P. A prospective, multicenter, post marketing surveillance study to evaluate the safety and effectiveness of the Superia sirolimus eluting coronary stent system (SSECSS) implanted during routine clinical practice in India. http://nano-therapeutics.net/forclinicians.html
- Abreu-Silva E, Costa R, Seth A et al. TCT 650 Impact of the new Biomime sirolimus eluting stent in complex patients of daily practice – Merit-2 study. J. Am. Coll. Cardiol. 60, 17 (2012).
- Byrne RA, Kastrati A, Massberg S et al. Biodegradable polymer versus permanent polymer drug-eluting stents and everolimus- versus sirolimus-eluting stents in patients with coronary artery disease: 3-year outcomes from a randomized clinical trial. J. Am. Coll. Cardiol. 58(13), 1325–1331 (2011).
- Mimish L, Anwar M, Hassan T et al. Experience with Infinnium paclitaxel in Saudi Arabia. Heart Views 7(1), 2–14 (2006).
- Abhyankar A, Prajapati J, Reddy S, Reddy S. Early vascular healing with biodegradable polymer coated sirolimuseluting coronary stent implantation: assessed by optical coherence tomography results at 4-month follow-up. Minerva Cardioangiol. 61(3), 313–322 (2013).
- Thakkar AS, Abhyankar AD, Dani S et al. Systemic exposure of sirolimus after coronary stent implantation in patients with de novo coronary lesions: Supralimus-Core® pharmacokinetic. Indian Heart J. 64(3), 273–279 (2012).
- Lemos PA, Moulin B, Perin MA et al. Late clinical outcomes after implantation of drug-eluting stents coated with biodegradable polymers: 3-year follow-up of the PAINT randomised trial. EuroIntervention 8(1), 117–119 (2012).
- Lemos PA, Moulin B, Perin MA et al. Biodegradablepolymer Infinnium® paclitaxel-eluting and Supralimus® sirolimus-eluting stents reduce neointima proliferation in patients at low risk of restenosis: findings from the 3-arm paint randomized controlled trial. The PAINT Complex Poster presentation in e-poster no: 4139 ESC Congress-2008. www.smtpl.com/paint_complex.html
- Costa AR, Abizaid A, Abizaid SA et al. Complex patients with coronary artery disease treated with the novel Supralimus® sirolimus-eluting stents. Preliminary results of the prospective, multicenter, non-randomised E-Series trial. EuroIntervention 5(E), E-44 (2009).
- Siqueira AD, Ricardo A, Costa AR et al. Supralimus® bioabsorbable sirolimus-eluting stent technology in diabetic patients undergoing percutaneous coronary intervention (PCI): preliminary findings from the prospective, international, multicenter E-SERIES registry. Am. J. Cardiol. 102, 12–17 (2008).
- Abhyankar A, Dani S, Desai D et al. Evaluation of safety, efficacy and procedural outcomes of extra long sirolimuseluting stent with novel polymeric technology for treatment of long de novo coronary lesions. Catheter Cardiovasc. Interv. 68, S1 (2006).
- Dani S, Costa RA, Joshi H, Shah J, Pandya R, Virmani R, Sheiban I, Bhatt S, Abizaid A. First-in-human evaluation of the novel BioMime sirolimus-eluting coronary stent with bioabsorbable polymer for the treatment of single de novo lesions located in native coronary vessels – results from the Merit-1 trial. EuroIntervention 9(4), 493–500 (2013).
- Costa AR, Abizaid A, Dani S et al. TCT-212 efficacy of the novel BioMime sirolimus-eluting stents with a biodegradable polymer in the treatment of de novo coronary lesions: an angiographic subanalysis of the combined Merit-1 and Merit-2 prospective clinical trials. J. Am. Coll. Cardiol. 62(18 Suppl. 1), B68 (2013).
- Multimedics Ltd. PRISM basket of clinical trials. http://multimedicsllc.com/clinical_overview.html
- Envision Scietific Pvt Ltd, Abluminus sirolimus eluting coronary stent system.
- Kaul U. FOCUS np: a nanoparticle-based siroliumuseluting stent and balloon transcatheter cardiovascular therapeutics (2013). www.tctmd.com
- Byrne RA, Kastrati A, Kufner S et al. Randomized, noninferiority trial of three limus agent-eluting stents with different polymer coatings: the intracoronary stenting and angiographic results: test efficacy of 3 limus-eluting stents (ISAR-TEST-4) trial. Eur. Heart J. 30(20), 2441–2449 (2009).
- Stefanini GG, Byrne RA, Serruys PW et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur. Heart J. 33(10), 1214–1222 (2012).
- Alexander J. ICMR to conduct comparative study of Indian drug eluting stents with US FDA approved stent soon. www.pharmabiz.com/