Research Article - Neuropsychiatry (2018) Volume 8, Issue 3
How to Modify the Forced Running Wheel for Ischemic Stroke Rehabilitation in Rats
- Corresponding Author:
- Chi-Chun Chen
Department of Electronic Engineering, National Chin-Yi University of Technology, Taiwan
Tel: +886-4-23924505-7316
Fax: +886-4-23926610
Abstract
Objective
An infrared-sensing deceleration running wheel (IDRW) is proposed from the prevention to the rehabilitation of ischemic stroke in an animal model. Current forced training platforms use a fixed speed for rehabilitation training in rats. However, it is difficult for the injured rats to keep exercising at a constant intensity. Moreover, neither high nor low training speed can be the optimal for rats for the whole rehabilitation process. This conventional method may even result in second-time injuries during rehabilitation, which causes adverse effects in recovery. This phenomenon is also one of the reasons why currently marketed forced training animal platforms have shown little effectiveness in the rehabilitation of stroke patients. The major distinction between the techniques described in this study and the commercially available ones is that our platform is capable of automatically and dynamically adjust varying the training intensity based on spontaneous feedback of individual physical conditions according to infrared sensor for positions.
Methods
This platform uses infrared sensors to measure the running positions of rats to provide feedback and adjust the exercise speed for assisting stroke rehabilitation in rats. In addition, with linear acceleration and adaptive deceleration models, rats with stroke can achieve rehabilitation based on their individual physical characteristics. This rehabilitation model not only achieves effective recovery based on the individualized physical capacity of rats but also protects rats from fall injuries. This rehabilitation model overcomes individual differences, which has not been realized in conventional rehabilitation models.
Results
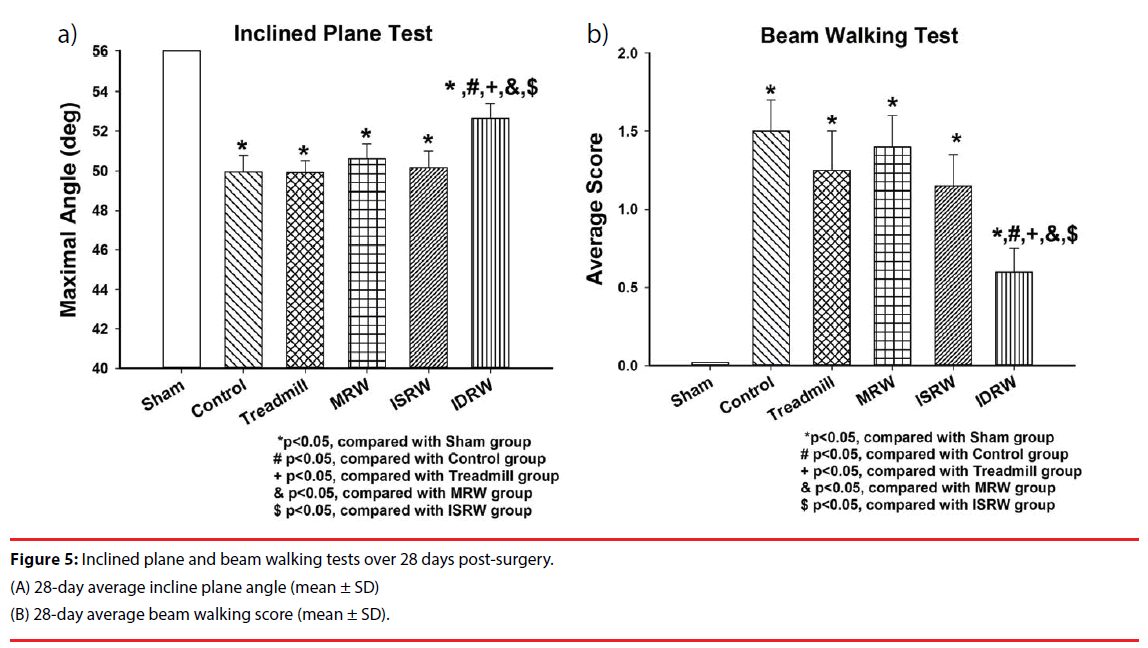
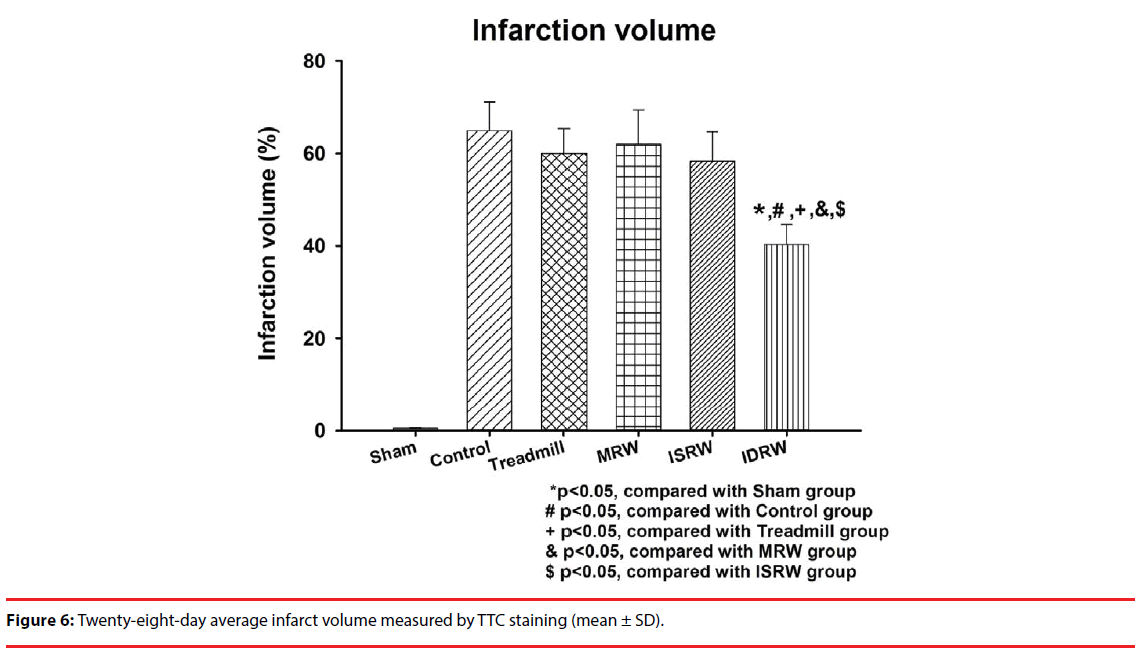
An animal ischemic stroke model was used to test the effectiveness of this platform. One week after middle cerebral arterial occlusion (MCAo) surgery, the animals were assigned into four groups (treadmill, motorized running wheel (MRW), (infrared-sensing running wheel) ISRW, and IDRW) to complete rehabilitation training over three weeks. Inclined plane and beam walking tests were used daily to test the motor function recovery. After three weeks of exercise, the triphenyl tetrazolium chloride (TTC) was used to obtain the cerebral infarct volume. The result showed that the IDRW group achieved a significant recovery from the evaluation of inclined plane (control: 49.9 ± 0.8 degrees, IDRW: 52.6 ± 0.7 degrees), beam walking (control: 1.5 ± 0.2 points, IDRW: 0.6 ± 0.15 points), and cerebral infarct volume (control: 64.9 ± 6 %, IDRW: 40.3 ± 4 %). IDRW is also the only platform that showed an actual treatment effect among all forced training platforms.
Conclusions
This study confirmed that forced rehabilitation training models require proper control to achieve certain treatment effects. In other words, when forcing rats to exercise, it is important to reduce their psychological burden and prevent them from suffering secondary injuries. Therefore, this model provides an objective and effective experimental platform for clinical researchers conducting sports physiology studies.
Keywords
Infrared-sensing deceleration running wheel (IDRW), Rehabilitation, Adaptive acceleration and deceleration models, Middle cerebral artery occlusion (MCAo), Inclined plane
Introduction
Ischemic stroke has long been a great burden in many countries, and patients often suffer from inconvenience in daily motions [1,2]. Therefore, it is crucial to offer patients an effective rehabilitation plan to improve their quality of life. This development direction should be taken by each country. Increasing evidence has suggested that physical exercise can reduce the incidence of stroke in humans [3-5]. In addition, physical rehabilitation after ischemic conditions has been proposed as a practical approach for treating cerebral stroke [6-8]. The neurological mechanism between humans and animals are similar. Therefore, current basic clinical studies often use rodent (such as rat) injury models as a preliminary approach to test the effectiveness of physical rehabilitation methods [9,10]. However, in current clinical trials, no proper and effective rehabilitation method has been found, and the main reason is that current exercise platforms are not suitable for the training required by cerebral stroke rehabilitation. Currently, exercise platforms used in animals are mainly of two types, including the treadmill and running wheel. Both training methods use a fixed speed to train rats during a fixed period of time, which has demonstrated certain effectiveness in preventing cerebral stroke [11] but has shown little effectiveness for the recovery process after a cerebral stroke [12-14]. The reason for the ineffectiveness was that the middle cerebral artery occlusion (MCAo) operation had already been performed to trigger the ischemic stroke. Therefore, the rats had already become dull and slow. In addition, animals are the same as humans in exhibiting individual differences. If fixed exercise intensity is applied to all rats, some already injured rats may suffer from secondary injuries due to the speed challenge and may realize an outcome even worse than without exercise [14]. Therefore, training methods in rehabilitation with proper and individualized exercise intensity may achieve certain effectiveness.
Current training platforms, including the treadmill and running wheel, have exhibited certain limitations. The runway of the treadmill uses electronic stimulation at its rear end to force the rat to run. This training method may result in a physiological outcome contributed partially by the electric shock [15]. Therefore, the treadmill data may not be explained objectively [16,17]. The running wheel platforms have a voluntary form and a motorized form. The voluntary running wheel allows the rat to run voluntarily. However, because of individual differences, this type of running wheel often yields large variations in the final results. To avoid such discrepancies, the rats are often filtered before the experiment [18,19]. Therefore, the voluntary running wheel is not the focus of this type of research. The motorized running wheel (MRW), in contrast, relies on external forces to power the wheel and force rats to run. This method is a forced type of training, similar to the treadmill. However, this method was suggested to render less psychological stress on rats compared with the treadmill [14,15]. A previous study observed that when using commercially available MRWs to train rats, the rats were afraid of running, held on to the cross bars on the wheel and stopped running [20]. In addition, the measure of the running wheels sold in the market (diameter = 35 cm × width = 12 cm) was too small for average white rats [21]. In addition, the curved surface of the runway is more difficult to run on than a flat surface, and the rats often accidentally fall or roll [22]. In sum, current animal training platforms have demonstrated certain limitations and interferences that would affect the recovery effectiveness. To solve this problem, a training platform with low psychological burden and low interference is urgently desirable. Our laboratory successfully developed a high efficient running wheel platform [21,23]. This platform performs better than other commercially available animal exercise platforms in preventing ischemic stroke. It also has the advantages of using infrared to evaluate the effective exercise quantity. In this study, we further improved the platform based on the highly efficient running wheel exercise system and applied the system to the recovery from cerebral stroke. We aimed to overcome the limitations of traditional animal exercise platforms and thereby achieve a more obvious recovery effect.
In this study, we further improved the highly efficient running wheel exercise platform and applied it to the recovery process from cerebral stroke. Previous studies have shown that the current treadmill and MRW methods have a beneficial effect for stroke prevention [11] but do not guarantee a significant effect for stroke rehabilitation [12-14]. Therefore, the same exercise training models should not be used for stroke prevention and rehabilitation. In the previous platform [20,22], the training model maintained a ‘constant’ exercise intensity during exercise based on the a reasonable presumption that generally healthy and normal animals will be capable of achieving such intensity. Instead, the hypothesis of this paper, which is focused on rehabilitation, is to adaptively train rats with stroke and decrease the exercise intensity to prevent injury and protect the rats, thus achieving recovery training based on their immediate conditions. Thus, we aimed to overcome the limitations of traditional animal exercise platforms and thereby achieve a more obvious recovery effect.
To design a superior physical recovery training system, a highly efficient exercise platform is required. More importantly, a system must be in place to reduce the interference factor and perform adjustments based on the status of the rats during the training process. A previous study suggested that the psychological pressure caused by the electrode training method used with treadmills results in adverse physiological injuries, such as adrenal hypertrophy, splenic atrophy, and circulating corticosterone [24-26]. For injured individuals who exercised during their rehabilitation, psychological pressure could be a destructive factor for their recovery [14]. Therefore, excluding such potential disadvantages is advantageous for clinical research. In addition to reducing psychological stress, an appropriate training program that can produce various individualized intensities should be implemented for rat rehabilitation training. Utilizing an infrared embedded wheel module to detect the running position of the rats, a dynamic feedback loop was generated to adjust the training speed of rehabilitation. This platform can protect rats from fall injuries and force them to exercise adaptively, and the mechanism was designed to achieve a better rehabilitation outcome.
Methods
This study used a forced running wheel capable of position awareness through infrared sensing, with the so-called infrared-sensing running wheel (ISRW) as the basic framework [21], and it further introduced a position point detection module that works in conjunction with a training speed feedback control method. Moreover, an infrared-sensing deceleration running wheel (IDRW) platform for the rehabilitation of rats with ischemic stroke was proposed, and the effectiveness of this platform was verified with an animal ischemic stroke model.
▪ Infrared-sensing deceleration running wheel system
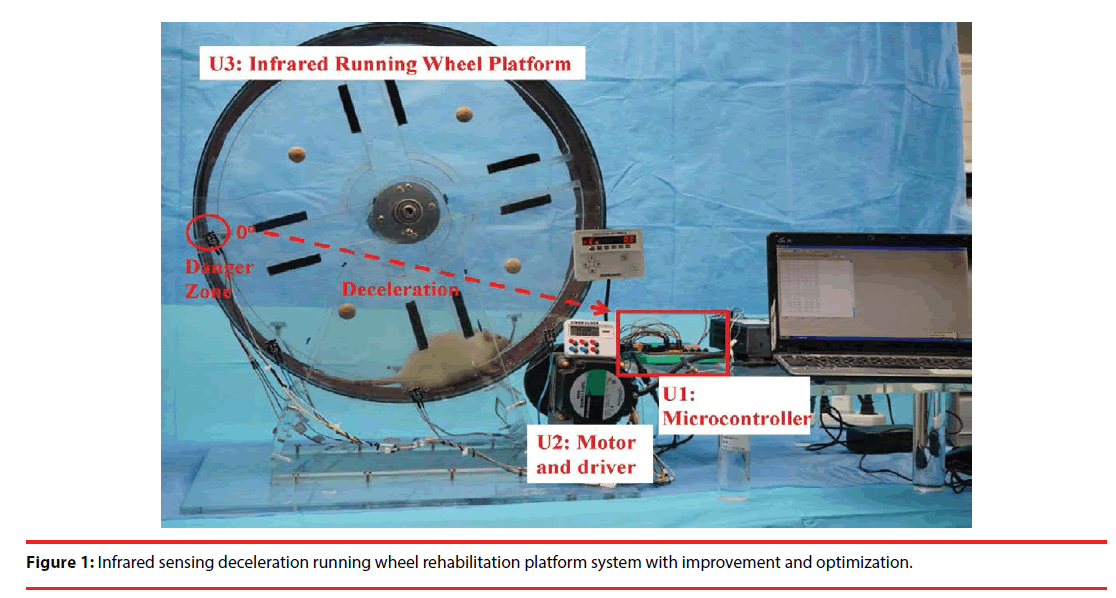
Here, we introduced a novel infrared-sensing deceleration running wheel system. The basic framework was successfully used in measuring the exercise quantity of the experimental animal (such as the rat) [21]. This study used this rat exercise platform as the foundation and added an infrared-sensing deceleration function to construct a fully automated rehabilitation platform. This platform can be applied to rats with different physical conditions and qualities, unlike conventional fixed-speed training methods that force the rats to run, which may cause injuries due to difficulties in adaptation and yield adverse effects. The overall system architecture is shown in Figure 1, containing three units (U1: microcontroller, U2: motor and drive, U3: infrared running wheel platform) (Figure 1). The computer was connected through the RS-232 serial adapter, and the rehabilitation training software program was downloaded to the microcontroller (U1). This unit is a development board based on the C8051F410 core unit. The software program uses pulse-width modulation (PWM) to start a 12-bit digital-to-analog converter (DAC) and to control the motor drive (BLED12A, Oriental Driver, Japan) (U2). A motor (BLEM512-GFS, Oriental Motor, Japan) is used to drive the rotation of the running wheel table (U3). After the running wheel platform is operated, the infrared sensor is activated to monitor the position of the rat. Once the rat is detected running into a danger zone, a feedback signal sent to the microcontroller (U1) would trigger the deceleration mode so that the rat can return to the safe zone and maintain a stable running state. The overall position detection of motions will be immediately sent back to the computer to be monitored and recorded.
The photo of the apparatus built in the Chimei Hospital Laboratory is shown in Figure 1. The running wheel mechanism in this study adopted the foundation of the previously developed ISRW [21]. There are four infrared sensing points (0 degrees to 135 degrees) on the running wheel. These areas are defined as the normal running regions [21]. Most of the time, the rats perform exercise training at 45 degrees to 90 degrees. If a rat cannot physically keep up with the current pace, it will be brought to the 0-degree position gradually. When the 0-degree infrared sensor is triggered, a feedback signal will be sent to the microcontroller to slow the speed and thereby avoid fall-induced injuries to the rat. An additional three other locations will be monitored using infrared signals without adjusting the speed. To experiment with ISRW and MRW for comparison, we also used the same method for the ISRW and MRW and deployed a group of infrared sensors to record the number of times the position of 0 degrees was triggered without a speed adjustment.
▪ Automated linear acceleration and deceleration training models
The construction of the automated training model was based on raw data from the experimental rats in actual training. The approach was a training mechanism consistent with the rats’ body characteristics. This model not only reduces human error in operations but also is based on the physical characteristics of the rat itself, thus complying with the training mode with a low psychological burden.
▪ Automated linear acceleration training model
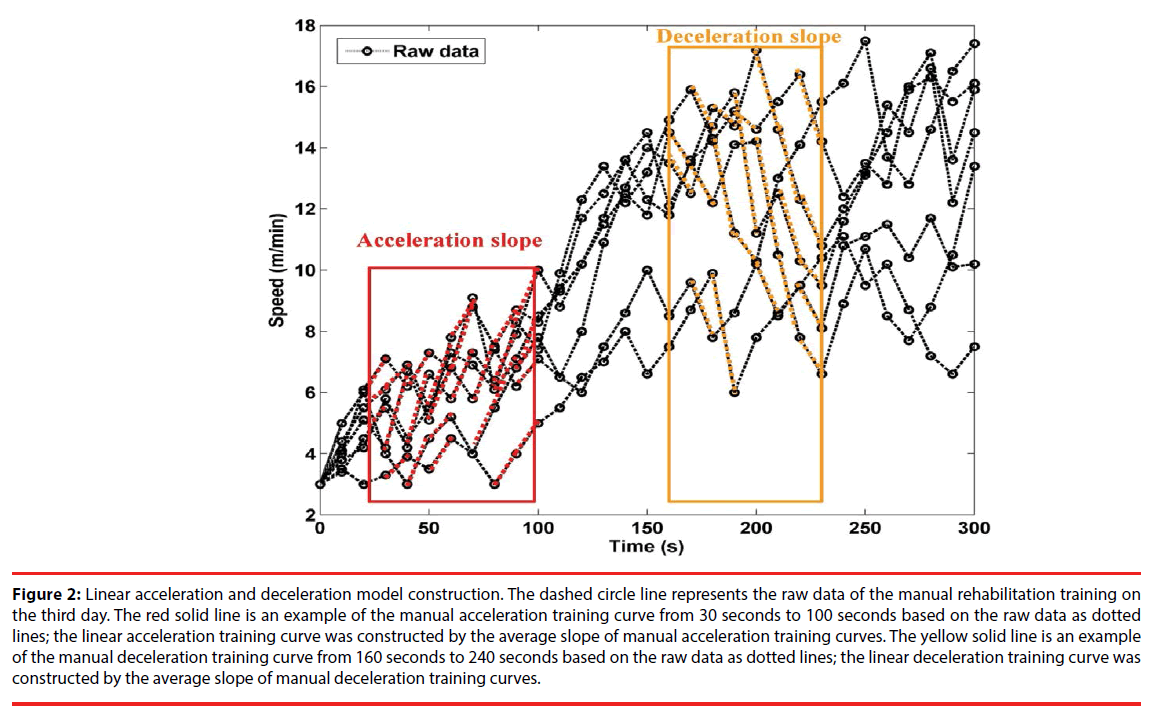
The exercise training for rehabilitation began one week after the MCAo surgery. During the first three days of training on the IDRW platform, we used manual techniques to train the rats. The purpose was to ensure that the rats were familiar with the training wheel and to measure the training intensity each rat could withstand. In the manual mode, the running speed was gradually accelerated. If a rat was not able to keep up, then the speed would be reduced. The purpose of this training was to allow the rats to complete the rehabilitation without experiencing psychological stress. The dotted lines in Figure 1 show the manual rehabilitation training curves of seven rats on day 3. Each rat was found to achieve rehabilitation training with a different speed, but the animals showed a similar incremental acceleration curve. Some rats reached a maximum speed of up to 16 (m / min) but could not sustain this speed. Two rats could not even reach 12 (m / min). Therefore, if fixedspeed training had been applied, it would have been likely to injure the rats when they could not keep up with the speed. Therefore, we used the raw data, as a basic reference and proposed an incremental mathematical equation to represent this training acceleration curve. The red solid line is an example of the manual acceleration training curve from 30 seconds to 100 seconds based on the raw data as dotted lines. The average slope in the manual acceleration training curves was 6.3, as shown in Eq. (1). Based on the experimental data, we set parameters mmanual_acc, and B to 6.3, and 3, respectively, as represented by the average slope of manual acceleration training curves in Figure 2. The mathematical formula is shown as Eq. (2). The maximum speed was set at 16 (m/ min).
Figure 2: Linear acceleration and deceleration model construction. The dashed circle line represents the raw data of the manual rehabilitation training on the third day. The red solid line is an example of the manual acceleration training curve from 30 seconds to 100 seconds based on the raw data as dotted lines; the linear acceleration training curve was constructed by the average slope of manual acceleration training curves. The yellow solid line is an example of the manual deceleration training curve from 160 seconds to 240 seconds based on the raw data as dotted lines; the linear deceleration training curve was constructed by the average slope of manual deceleration training curves.
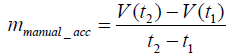 (1)
(1)
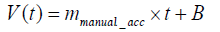 (2)
(2)
▪ Automated linear deceleration training model
In addition to an acceleration training model, a deceleration training model must also be built because it is much more important for protecting the rats from further injury and can be used as a follow-up treatment throughout the remainder of training. Data from the third day of manual training were used to construct the deceleration model. A linear deceleration model was used in the test and is shown by the average slope of manual deceleration training curves. The yellow solid line is an example of the manual deceleration training curve from 160 seconds to 240 seconds based on the raw data as dotted lines. The average slope in the manual deceleration training curves was 18.44, as shown in Eq. (3). Based on the experimental data, we set parameters mmanual_dec, and B to 18.44, and 16, respectively, as represented by the average slope of manual deceleration training curves in Figure 2. The mathematical formula is shown as Eq. (4). Preventing the rats from falling was the ultimate goal and one of the major features of the proposed running wheel platform. Because rats with stroke often display poor motion agility, once a rat is taken to the danger zone, the speed must be rapidly reduced. Otherwise, the rat is likely to fall.
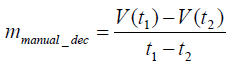 (3)
(3)
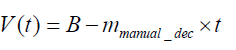 (4)
(4)
▪ Software
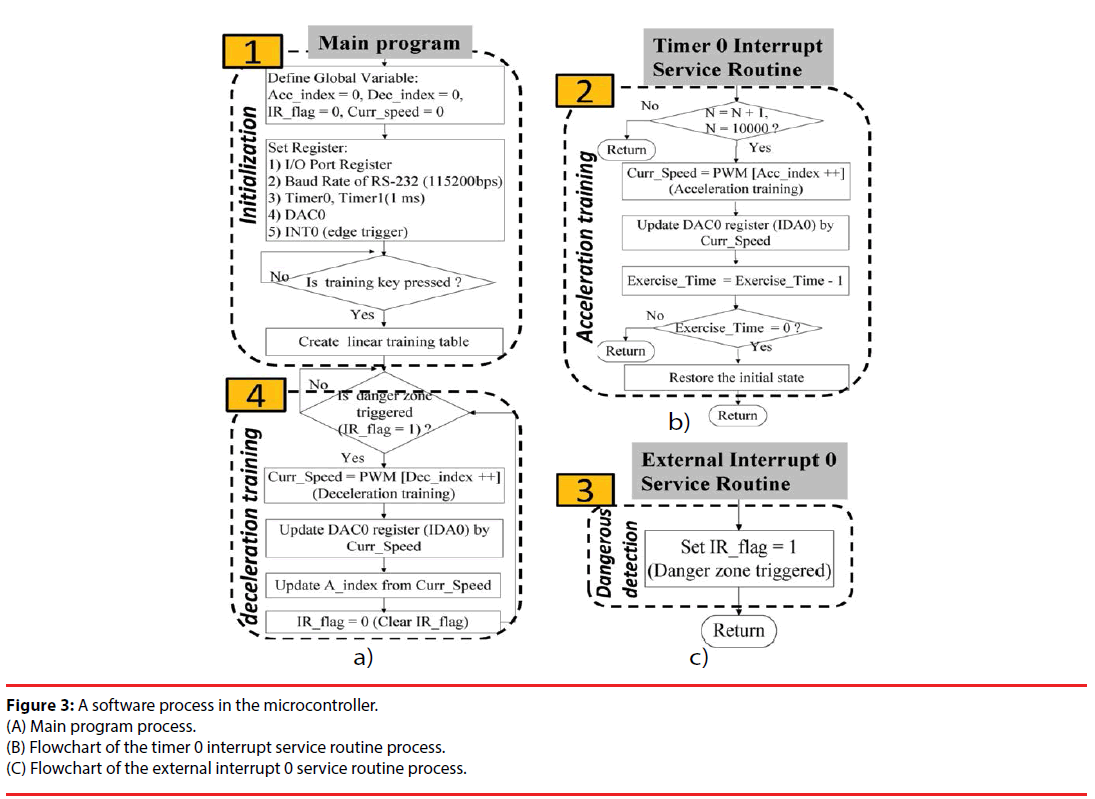
For the rats to perform rehabilitation training at lower psychological pressure, a gradual acceleration model was constructed, guiding the rats to achieve a more appropriate training intensity. More importantly, we had to prevent the stroke rats from falling and experiencing secondary injuries. Therefore, we designed a software process in the microcontroller with the main program flow shown in Figure3A, the timer 0 interrupt service routine process shown in Figure3B, and the external interrupt 0 service routine process shown in Figure3C. System initialization, identification of danger zones, and start of the deceleration model were implemented in the main program. The two interrupted service routine programs included start of the acceleration model, and triggering of danger zone signals. The operational architecture could be divided into four parts: the first part is the initial setting of global variables and the internal microcontroller register. The second part is to use Timer 0 to interrupt the service routine and to start the acceleration training model. This acceleration training model is a linear acceleration curve constructed based on the physical characteristics of rehabilitation rats. The rats can achieve more stable rehabilitation on the running wheels using this acceleration training model. The third part is an external interrupt service routine. When a rat is taken to the danger zone (0-degree position), this interrupt will be triggered to set up a danger zone flag (IR_flag = 1). Part four is to determine if the danger zone flag has been set. Once the flag is set, the deceleration training model will be started. This deceleration training model is also an optimal deceleration training curve constructed according to the physical conditions of the rehabilitated rats. Once a rat is taken to the danger zone, the deceleration training model will allow the rat to return safely to the normal running area. As long as the rat returns to a safe area, the acceleration model will be restarted. The advantage of this mechanism is to protect rats from fall injuries. More importantly, personalized training with various individualized intensities during rehabilitation can be achieved. This is currently the first system to overcome individual differences among animal training wheel platforms. We believe that this rehabilitation approach can overcome the limited effectiveness of rehabilitation by traditional forced exercise training platforms.
▪ Experimental animals
The experimental animals used by our institute were Sprague-Dawley male rats weighing approximately 260-330 g, provided by the Animal Resource Center of the Republic of China National Science Council (Taipei, Taiwan). These experimental rats are different from the rats used to estimate the parameters of the acceleration/deceleration training model. The rats were kept in an animal chamber with air conditioning, and the temperate was set at 24 ± 1°C. A light and dark cycle of 12 hours each was used, and unlimited water and feed were provided. The experimental procedures were approved by the animal ethics committee of Chi Mei Medical Center, the Republic of China National Science Council (Tainan, Taiwan).
▪ Experimental groups and exercise training
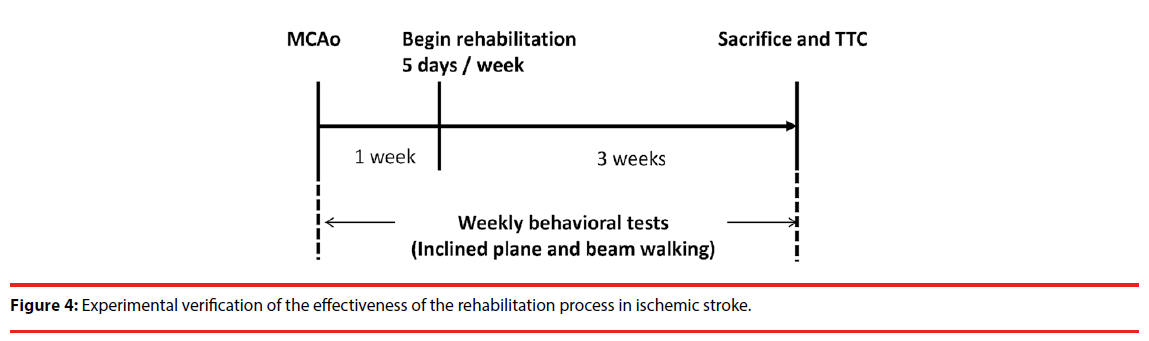
The rats were randomly divided into six groups including the IDRW (n = 8), treadmill (n = 8), MRW (n = 8), ISRW (n=8), sham (n = 10), and control (n = 10) groups. Only the sham group was not performed the MCAo surgery. The sham and control groups did not receive exercise rehabilitation training. The exercise training groups (IDRW, treadmill, MRW and ISRW) began a three-week rehabilitation training one week after the surgically triggered stroke. During the first week and the second week of exercise, the treadmill, MRW and ISRW groups received daily training of 30 min running at 20 m/min five days per week. During the third week, the running intensity was changed to 60 min at 20 m/min [21,27,28]. By contrast, the IDRW group received exercise training using the acceleration and deceleration models developed in this study. Thus, the treadmill, MRW and ISRW groups were subjected to fixed speeds to train the rats, whereas the IDRW group received time-varying adjustments during the rehabilitation exercise. The inclined plane and beam walking tests were performed daily to assess the degree of recovery on the hindlimb grip power. Three weeks after the training, the rats were sacrificed, and the triphenyltetrazolium chloride method (TTC) was used to obtain the infarct volume. The entire experimental process is shown in Figure 4.
▪ Middle cerebral artery occlusion (MCAo)
The study used the Longa’s brain endovascular occlusion method to induce the MCAo surgery [29]. A midline incision was performed on their neck to expose the left common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) after the rats were anesthetized. The left CCA and the ECA were tied up using a white suture. Then, a 4-0 monofilament nylon suture (Doccol Corporation, Redlands, CA, USA) was inserted into the ECA and then into the ICA until the middle cerebral artery (MCA) was blocked to induce a cerebral ischemic stroke. Reperfusion was achieved by withdrawal of the suture after sixty minutes. The sham group only received operations involving ECA and ICA isolation without ligation.
▪ Behavioral tests
The rats were pre-treated for both the inclined plane and beam walking tests for three consecutive days prior to the MCAo procedure, and the animals that failed to accomplish the requirements of the test during the pre-training period were excluded from the study. The inclined plane test was used to assess the motor function recovery of rats after ischemic stroke. The evaluation was conducted to test the grip strength of the fore limbs or hind limbs in rats. A motor was used to control the inclined angle of the acrylic plane from 0 degrees (horizontal) to 70 degrees [30]. A rectangular box was placed on the acrylic plane to hold the rat initially. A layer of Velcro was attached to the bottom of the box for the rat to grip with its fore limbs or hind limbs. The rats were placed in the rectangular box with their hind legs on the Velcro and the fore legs on the acrylic plane during the experimental process. The inclined plane was slowly raised from 25 degrees. The angle of the plane was immediately stopped rising when the hind limbs of the rat could not grip the Velcro anymore and the rat slid down Finally, the angle of inclination was recorded accordingly.
The beam walking test was used to reveal the extent of damage to the sensorimotor cortex or striatum because injured rats will display chronic damage according to their limb placement [31]. Therefore, the ledged/tapered beam walking test for rats involved scoring foot faults as the rats traversed a specially constructed beam [31]. The addition of ledges to the tapered beam allowed this method to be used after MCAo. Depending on the extent of damage to the sensorimotor cortex or striatum, a rat will display chronic changes in limb placement. A normal rat readily learns to walk along the beam on the upper surface and rarely using the ledges. An animal with an affected limb will use the ledge for weight-bearing steps on the side of the ledge corresponding to the deficit. The affected limbs will be placed on the ledge on the wider section of the beam more often than the intact limbs, and more frequently as the beam tapers. A higher average score indicates greater impairments in the contralateral limb [31].
▪ Assessing cerebral infarction
All rats were sacrificed and their brains were carefully removed after four weeks. The brain coronal sections with a width of 2 mm from the top front brain were cut and incubated in 2,3,5-TTC at 37 ℃ for 30 min. We used an image analysis system and a scanner to measure the contralateral and ipsilateral non-infarcted tissues [32]. In this way, the infarct volume was calculated as follows: infarct volume percentage = (contralateral hemisphere volume - ipsilateral non-infarct volume) / contralateral hemisphere volume [33].
▪ Statistical analysis
The results are expressed as the mean ± SD. Infarct volume and behavioral data were performed with SigmaPlot 12.5 software (Systat Software, Erkrath, Germany) using a one-way analysis of variance (ANOVA) combined with Fisher’s post hoc test. If there was a significant difference, the Student’s t-test was used to compare variables for two groups. A value of P<0.05 was considered statistically significantly.
Results
▪ Motor function
The inclined plane test was performed for 28 days after the surgery to evaluate the hindlimb grip function. Exercise rehabilitation was given to the exercise groups (treadmill, MRW, ISRW, and IDRW) starting from the 7th day after the surgery. The average incline angle of the sham group (59.2 ± 0.4 degrees) was the highest of all groups, also showing significantly higher daily average angles than the control group (49.9 ± 0.8 degrees). Figure 5A shows that the 28-day average incline angle in the IDRW group (52.6 ± 0.7 degrees) was also the highest among all exercise groups (Figure 4) (P<0.05). More importantly, the IDRW group was the only effective exercise group to show a significant recovery by stroke rehabilitation. No significant difference was observed between the other three exercise groups (treadmill (49.9 ± 0.5 degrees), MRW (50.6 ± 0.7 degrees) and ISRW (50.1 ± 0.8 degrees)) and the control group. The results above revealed that the IDRW proposed in this study may be useful for hindlimb grip function recovery in stroke rehabilitation. In addition, the IDRW group was again found to better than other exercise groups in terms of the average score received in the beam walking test over the 28-day period after injury (P<0.05).
▪ Infarct volume
The infarct volume was assessed by TTC staining. Normal cells appeared in red with stain, whereas necrotic cells appeared in white. Figure 6 shows the statistical comparison of the volume of brain dead cells between groups. The results showed that the infarct volumes in the treadmill (60.2 ± 5%), MRW (62.1 ± 7%) and ISRW(58.3 ± 6%) groups exhibited no statistically significant differences compared with the control group (64.9 ± 6%), which indicates that no significant rehabilitation effect was found in the treadmill, MRW and ISRW groups. However, the cerebral infarct volume in the IDRW group was (40.3 ± 4%), which was significantly lower than that in the control group (P<0.05). In the four exercise groups, the IDRW platform was the only effective one. Therefore, the IDRW model proposed in this study can be effectively applied for stroke rehabilitation.
▪ Percentage of time of triggering the effective exercise zone
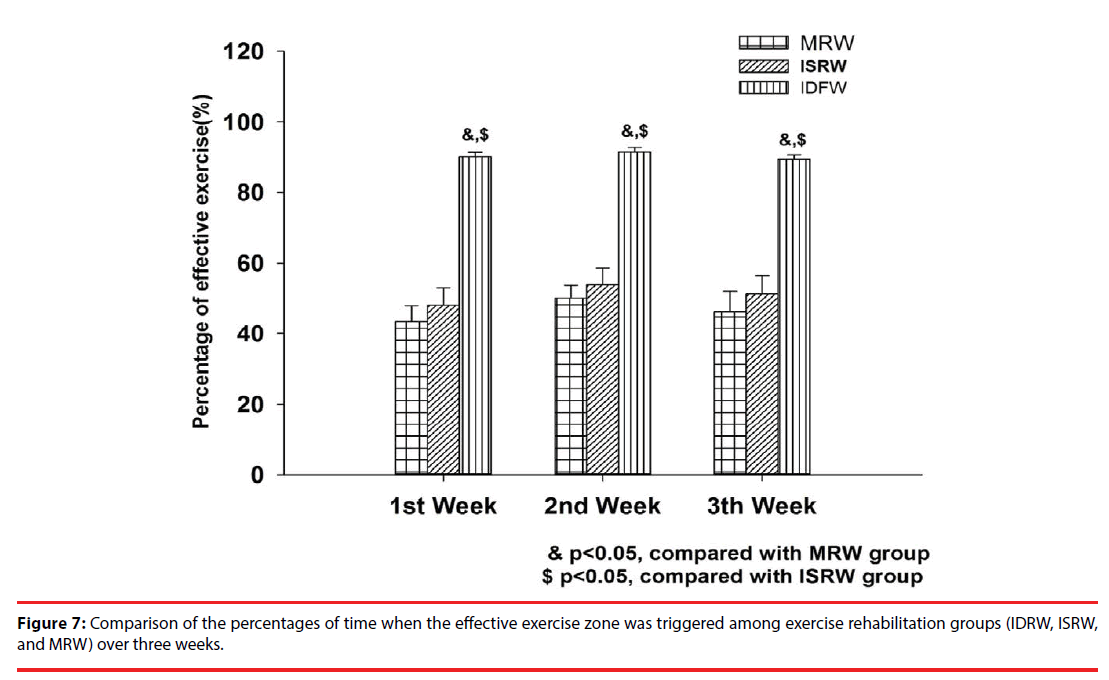
Because the motion of rats with stroke is relatively slow, once they are brought to the 0-degree position, they can easily be brought to the upper half of the wheel and fall. Therefore, we set the 0-degree position as the danger zone. The areas of 0-degree to 135-degree using four infrared sensors are defined as effective exercise zone [21]. Figure 7 shows the percentages of time when the effective exercise zone was triggered in the MRW, ISRW, and IDRW groups during the first three weeks of rehabilitation training. The results were 43% vs. 48% vs. 90% during the first week, 50% vs.53% vs. 91% during the second week, and 46%, vs.51% vs. 89% during the third week. The average percentage of the triggered period over three weeks was 90% for IDRW, 50.6% for ISRW and 46.3% for MRW. The weekly effective exercise zone triggered proportion in the IDRW group was more than that in the ISRW and MRW groups, which suggested that the IDRW was a more suitable platform for rat rehabilitation training. More importantly, in the IDRW group, once the danger zone was triggered, the deceleration training model was started, which protected the rats from fall injuries. The ISRW and MRW groups did not have this mechanism; thus, the rehabilitation was invalid almost over half of the time. This is why no significant effect on stroke rehabilitation was observed in the ISRW and MRW groups.
Discussion
In this study, the development of an effective stroke rehabilitation running wheel platform for rats was introduced. In addition to reducing disturbances affecting the normal running during training, more importantly, this platform can automatically and appropriately adjust the training intensity for rats based on their own physical conditions. This method reduced their psychological stress during exercise training to a minimum and accomplished an effective rehabilitation treatment. The treatment effect of the currently marketed forced motion platform (treadmill and running wheel) for animal rehabilitation is not obvious [12-14], mainly due to physical injuries from frequent electric shock when using a treadmill [14] and fall injuries when rats failed to keep up with the speed on MRW and ISRW [12,13]. Therefore, this study presented an infrared sensing feedback speed adjustment mechanism; that is, when the rat is about to fall or fails to keep up with the current speed, the training intensity will be reduced accordingly. In this way, the risk of fall injuries in rat rehabilitation can be minimized. In addition, traditional training methods often manually pre-set the training speed and time so that the starting speed will directly reach the final training speed. However, such direct acceleration to the final speed makes it difficult for the rat to adapt and can cause a fall, thereby affecting the stability of the rat’s subsequent run. Therefore, the linear acceleration model proposed in this study based on physical characteristics of the rat enable the rat to start from a slow speed and gradually adapt to the final training speed. This design enhanced the stability of the training process for the rats. Another advantage of this platform is the capability to record all running states during the entire training process, which is a feature unavailable in the current market. Commercially available platforms can only use a fixed training intensity and time to estimate the total amount of exercise [9,10,12-14]. However, the entire training process may involve invalid exercises (such as electric shocks, falls, etc.), which would affect the effectiveness of training. This study used infrared to record the entire state of running, which would not only help the physiological laboratory personnel understand the overall exercise condition of the animals but could also explain some of the variability in the experimental results. This mechanism would facilitate a more accurate mastering and interpretation of the experimental results. The main difference was that this study used an infrared deceleration rehabilitation model so that each rat could exercise based on its own physical condition, perform appropriate rehabilitation and overcome individual differences in training. In addition to the reduced psychological pressure compared with a conventional treadmill with an electrode mode, this platform also overcame issues in traditional motorized wheeled training including crossbar grasping and unstable running. The platform proposed by this institution allowed rats to rehabilitate in an environment with a low psychological burden and high efficiency. Therefore, this platform overcomes the limitation of the traditional exercise platforms in stroke rehabilitation.
Experimental results in the IDRW group showed a significant improvement in motor function recovery and cerebral infarct volume compared with the control group (Figure 5, Figure 6) (P < 0.05), implying that the IDRW could provide effective rehabilitation training. However, no significant difference was observed between the treadmill, MRW, and ISRW groups with respect to rehabilitation effects, including motor function and cerebral infarct volume, compared with the control group. The results were also similar to previous studies [12-14], which again verified that commercial exercise platforms were not suitable for stroke rehabilitation. The experimental results suggested that the IDRW platform is currently an effective forced training platform for stroke rehabilitation. The major difference between the IDRW platform and other commercially available training platforms is that this rehabilitation model has a deceleration Although the IDRW group still had some percent of time with the danger zone triggered, its functions, including immediate feedback and deceleration, protected the rats from fall injuries. The traditional MRW and ISRW did not have this mechanism; consequently, once the danger zone was triggered, the training was almost always interrupted by a fall or a grasp onto the crossbar. Therefore, this study demonstrated that, with appropriate control, forced training platforms could still achieve effective rehabilitation treatment.
Most of the current forced animal exercise training platforms were used in stroke prevention and were shown to have certain effectiveness [11]. However, their application in stroke rehabilitation showed little effect [12-14]. In this study, we found that rats with stroke were not suitable for current rehabilitation exercise training platforms with fixed intensity. Although these rats can be forced to focus on serious rehabilitation, their physical conditions were duller than normal. For example, they often suffered from electric shock on the treadmill. This not only caused huge psychological fear in the rats but also interrupted the running rhythm and affected the amount of physical activity, thereby affecting the efficacy of rehabilitation from running. Additionally, on the traditional MRW or ISRW, the rats often fell or held onto the crossbar of the running wheel and stopped running. These phenomena have a negative impact on the efficacy of rehabilitation and may even create worse outcomes compared with the group without exercise, as reported in some studies [14]. Therefore, an important consideration for rehabilitation was how to make the rats focus on serious training while preventing them from further injuries. This study overcame the difficulty of ineffective rehabilitation in the currently marketed forced exercise training platforms by mainly using a homemade high efficiency running wheel to compel the rats during rehabilitation. Mainly, it could provide a feedback control signal in the danger zone, dynamically adjusting the intensity of training. This platform has achieved a training mechanism of serious rehabilitation without injury. This study demonstrated that it is wrong to generalize that a forced rehabilitation training platform cannot achieve a treatment effect. Instead, proper control can accomplish good rehabilitation outcomes.
The interference of psychological stress caused by electric shock on treadmills has been the current bottleneck for physiological laboratory researchers. The commercially available MRW will still cause a psychological burden because of fall injuries. Therefore, preventing falls and reducing the psychological burden of rats in training will help basic neurophysiological clinical researchers more objectively explain the experimental data and be more persuasive regarding the benefit of exercise. These ideas were applied in the design and development of this training platform with a low psychological burden. The experimental results showed that if given a proper training environment in addition to timely control over the training intensity, a good rehabilitation effect can be accomplished. This system not only can provide an objective experimental exercise verification platform for clinical researchers but also may be applicable to other pathological research agendas.
Conclusion
This study presented an IDRW platform that can be applied to the effective rehabilitation of animal ischemic stroke. Our infrared sensing dynamic feedback training intensity controlled rehabilitation model yielded significantly improved motor function and infarct volume outcomes. This platform overcame the bottleneck of forced training platforms in stroke rehabilitation. This rehabilitation platform not only can prevent fall injuries but also provides corresponding training intensity based on individual physical conditions. The results of the animal stroke model confirmed the effectiveness of this platform. The amount of exercise by traditional forced training platforms can only be estimated from the training intensity and time without considering the impact of electric shock or fall. These factors are likely to be the major reasons for non-effective rehabilitation. Many neurophysiological and pathological researchers have explored motion rehabilitation for disease treatment. However, current animal training platforms have almost always involved interfering factors, resulting in a non-objective experiment. This platform eliminates the interference factors including shock and fall and provides a more objective exercise rehabilitation mechanism.
Acknowledgment
The author gratefully acknowledges the support provided for this study by the Ministry of Science and Technology (MOST 106-2221-E-167 -004 -MY3) of Taiwan.
References
- Duncan PW, Goldstein LB, Horner RD, et al. Similar motor recovery of upper and lower-extremities after stroke. Stroke 25(6), 1181-1188 (1994).
- Mayo NE, Wood-Dauphinee S, Cote R, et al. Activity, participation, and quality of life 6 months poststroke. Arch. Phys. Med. Rehabil 83(8), 1035-1042 (2002).
- Evenson KR, Rosamond WD, Cai JW, et al. Investigators AS. Physical activity and ischemic stroke risk - The Atherosclerosis Risk in Communities Study. Stroke 30(7), 1333-1339 (1999).
- Greenlund KJ, Giles WH, Keenan NL, et al. Physician advice, patient actions, and health-related quality of life in secondary prevention of stroke through diet and exercise. Stroke 33(2), 565-570 (2002).
- Sattelmair JR, Kurth T, Buring JE, et al. Physical Activity and Risk of Stroke in Women. Stroke. 41(6), 1243-1250 (2010).
- Luft AMR, Forrester L, Goldberg A, et al. Post-stroke exercise rehabilitation: what we know about retraining the motor system and how it may apply to retraining the heart. Cleve. Clin. J. Med 75(1), S83-S86 (2008).
- Zheng Q, Zhu D, Bai Y, et al. Exercise Improves Recovery after Ischemic Brain Injury by Inducing the Expression of Angiopoietin-1 and Tie-2 in Rats. Tohoku. J. Exp. Med 224(3), 221-228 (2011).
- Zhang P, Yu H, Zhou N, et al. Early exercise improves cerebral blood flow through increased angiogenesis in experimental stroke rat model. J. Neuroeng. Rehabil 10 (2013).
- Leasure JL, Grider M. The effect of mild post-stroke exercise on reactive neurogenesis and recovery of somatosensation in aged rats. Exp. Neurol 226(1), 58-67 (2010).
- Auriat AM, Grams JD, Yan RH, et al. Forced exercise does not improve recovery after hemorrhagic stroke in rats. Brain. Res 1109(1), 183-191 (2006).
- Wang RY, Yang YR, Yu SM. Protective effects of treadmill training on infarction in rats. Brain. Res 922(1), 140-143 (2001).
- Ploughman M, Granter-Button S, Chernenko G, et al. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience 136(4), 991-1001 (2005).
- Ploughman M, Granter-Button S, Chernenko G, et al. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain. Res 1150(1), 207-216 (2007).
- Ke Z, Yip SP, Li L, et al. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model. PLoS. One 6(2), e16643 (2011).
- Hayes K, Sprague S, Guo M, et al. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta. Neuropathol 115(3), 289-296 (2008).
- Peng CW, Chen SC, Lai CH, et al. Review: clinical benefits of functional electrical stimulation cycling exercise for subjects with central neurological impairments. J. Med. Biol. Eng 31(1), 1-11 (2011).
- Frasier CR, Moore RL, Brown DA. Exercise-induced cardiac preconditioning: how exercise protects your achy-breaky heart. J. Appl. Physiol 111(3), 905-915 (2011).
- Zhao XR, Aronowski J, Liu SJ, et al. Wheel-running modestly promotes functional recovery after a unilateral cortical lesion in rats. Behav. Neurol 16(1), 41-49 (2005).
- Waters RP, Renner KJ, Pringle RB, et al. Selection for aerobic capacity affects corticosterone, monoamines and wheel-running activity. Physiol. Behav 93(4-5), 1044-1054 (2008).
- Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience 156(3), 456-465 (2008).
- Chen CC, Chang MW, Chang CP, et al. Improved Infrared-Sensing Running Wheel Systems with an Effective Exercise Activity Indicator. PLoS. One 10(4), (2015).
- Kennard JA, Woodruff-Pak DS. A comparison of low- and high-impact forced exercise: Effects of training paradigm on learning and memory. Physiol. Behav 106(4), 423-427 (2012).
- Chen CC, Chang MW, Chang CP, et al. A forced running wheel system with a microcontroller that provides high-intensity exercise training in an animal ischemic stroke model. Braz. J. Med. Biol. Res 47(10), 858-868 (2014).
- Arida RM, Scorza CA, da Silva AV, et al. Differential effects of spontaneous versus forced exercise in rats on the staining of parvalbumin-positive neurons in the hippocampal formation. Neurosci. Lett 364(1), 135-118 (2004).
- Brown DA, Johnson MS, Armstrong CJ, et al. Short-term treadmill running in the rat: what kind of stressor is it? J. Appl. Physiol 103(6), 1979-1985 (2007).
- Moraska A, Deak T, Spencer RL, et al. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 279(4), R1321-1329 (2000).
- Zhao Y, Pang Q, Liu M, et al. Treadmill exercise promotes neurogenesis in ischemic rat brains via caveolin-1/vegf signaling pathways. Neurochem. Res 42(2), 389-397 (2017).
- Yang L, Zhang J, Deng Y, et al. The effects of early exercise on motor, sense, and memory recovery in rats with stroke. Am. J. Phys. Med. Rehabil 96(3), e36-e43 (2017).
- Longa EZ, Weinstein PR, Carlson S et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1), 84-91 (1989).
- Chang MW, Young MS, Lin MT. An inclined plane system with microcontroller to determine limb motor function of laboratory animals. J. Neurosci. Methods 168(1), 186-194 (2008).
- Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp. Transl. Stroke. Med 2(1), 13 (2010).
- Swanson RA, Morton MT, Tsao-Wu G, et al. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood. Flow. Metab 10(2), 290-293 (1990).