Mini Review - Interventional Cardiology (2021)
Galectin 3 in cardiovascular diseases: Do we look at the right ventricle?
- Corresponding Author:
- Beata Zaborska
Department of Cardiology,
Centre of Postgraduate Medical Education,
Grochowski Hospital,
Warsaw,
Poland,
E-mail: zaborska@kkcmkp.pl
Received date: February 26, 2021 Accepted date: March 12, 2021 Published date: March 19, 2021
Abstract
Galectin-3 (Gal-3) is a biomarker of fibrosis, inflammatory response and oxidative stress. It plays a role in heart remodelling. Most of the studies were focused on relationships with left ventricular geometry and function as well as prognostic usefulness of Gal-3. However, recently the associations between Gal-3 and Right Ventricular (RV) function and pressures are postulated. This review summarizes the relationship between Gal-3 and pulmonary hypertension, RV geometry and function in various populations, patients with pulmonary hypertension, heart failure and congenital heart disease.
Keywords
Galectin-3 • Heart failure • Right ventricular • Pulmonary hypertension • Biomarkers • Echocardiograph
Introduction
Galectin-3 (Gal-3) is the only chimera type member of the lectin family, widely expressed in human tissues, acting as a galactoside-binding protein involved in many biological processes, such as controlling cell-cell and cell-matrix interactions, adhesions, proliferation, apoptosis, immunity and inflammation. It is located predominantly in the cytoplasm, but also in the nucleus. It is secreted to cell surface and into biological fluids and into the circulation [1]. In the heart, Gal-3, released by activated cardiac macrophages and cardiac fibroblasts, stimulates fibrogenesis and pathological remodelling particularly by inducing fibroblast proliferation and collagen deposition [2]. It is also a marker of inflammatory response. Numerous studies have indicated that Gal-3 may be used as a diagnostic and prognostic biomarker for certain types of heart diseases, kidney diseases and cancer [1]. Since 2014 Gal-3 has been approved by the US Food and Drug Administration as a new biomarker for additive risk stratification in HF [3]. Moreover, Gal-3 measurements is recommended by the 2017 Guidelines of the American Heart Association for risk stratification and prognosis assessment in Heart Failure (HF) patients [4].
The biological role and molecular mechanism of Gal-3 in cardiovascular disease are vigorously investigated and reported in the literature. This mini-review is focused on the associations between Gal-3 and Right Ventricular (RV) function and pressures that are recently postulated. This issue is clinically relevant since RV function is a potent prognostic marker in several cardiovascular diseases.
Galectin 3 and Pulmonary Hypertension
Pulmonary artery hypertension is a progressive disease that affects pulmonary vasculature and leads to RV pressure overload. He et al. in a study on patients with pulmonary artery hypertension and an animal model found that Gal-3-mediated pulmonary artery hypertension led to RV remodelling by interacting with NADPH oxidase 4 and NADPH oxidase 4-derived oxidative stress [5]. The authors concluded that inhibition of the Gal-3 could alleviate the fibrosis. It is in concordance with findings reported by Hao et al. in an animal model. In this study, hypoxia-induced pulmonary artery hypertension was ameliorated due to Gal-3 inhibition, and the inflammatory response was reduced [6]. Calvier et al. revealed the role of elevated Gal-3 and aldosterone in different types of pulmonary artery hypertension: idiopathic and associated with connective tissue disease [7]. Gal-3 levels were predictive of RV dysfunction and correlated better with RV dysfunction parameters than NTproBNP in patients with chronic obstructive pulmonary diseaseassociated pulmonary hypertension [8]. Crnkovic et al. assessed fibrotic markers’ expression in the right ventricle of patients with pulmonary hypertension and in experimental animal models. Established fibrosis was found to be characterized by marked expression of Gal-3 and enhanced numbers of proliferating RV fibroblasts [9].
Galectin 3 and Heart Failure
Heart failure remains one of the most prevalent and challenging medical condition, characterized by high morbidity and mortality. Galectin-3 is involved in HF’s pathophysiology mainly because of its role in fibrosis and cardiac ventricular remodelling. Although, not only impact on Left Ventricular (LV) function should be considered. To determine the relationship between Gal-3 concentration and cardiac structure and function in patients with acutely decompensated HF Shah et al. studied 115 patients presenting to the emergency department with acute dyspnoea [10]. Gal-3 levels were significantly associated with echocardiographic markers of LV filling, diastolic function, valvular regurgitation, and declined renal function, higher NT-proBNP and C-reactive protein. Moreover, the authors noticed a striking relationship between indices of RV function and Gal-3 levels. Higher levels of Gal-3 significantly correlate with worsted RV systolic function, expressed as fractional area change, more severe tricuspid regurgitation and higher RV systolic pressures. The authors discussed elevated LV filling pressure and direct RV pathology due to fibrosis, remodelling or hypertrophy as the possible explanations. In this study Gal-3, assessed during admission predicted 4-years mortality, independently of echocardiographic markers.
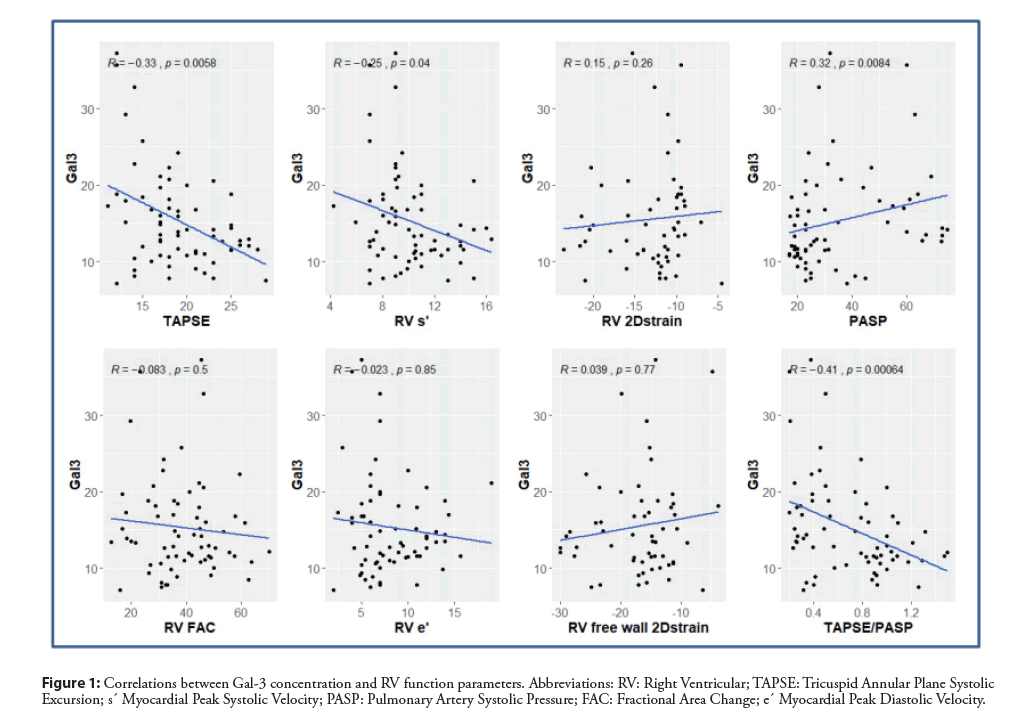
Recently we described the relationship between Gal-3 and RV dysfunction in HF patients with reduced ejection fraction [11]. We prospectively assessed consecutive patients with HF. Seventyfive per cent of them had ischaemic cardiomyopathy. Despite optimal medical therapy, all patients were symptomatic, in NYHA class II or III. However, all the patients presented severe LV systolic dysfunction with a mean ejection fraction of 26 ± 6%, and mean global longitudinal strain of -7 ± 3%, but the wide range of RV function was observed, from normal to severely depressed. Serum Gal-3 concentration was measured with a mean value of 15.3 ± 6.4 ng/mL and a median 13.5 ng/mL (IQR 11.1-18.1). The group with Gal-3 concentrations greater than or equal to the median had significantly worse RV systolic function parameters, higher pulmonary artery systolic pressure, more advanced tricuspid regurgitation and lower RV-to-pulmonary circulation coupling index. Simultaneously no significant differences were found in LV parameters comparing the group with low versus high Gal- 3 concentration. Moreover, the group with Gal-3 concentrations greater than or equal to the median achieved significantly lower parameters in cardiopulmonary exercise testing: exercise time, oxygen uptake at peak exercise and anaerobic threshold, and heart rate at peak exercise expressed as a percentage of maximal predicted heart rate. Significant negative correlations were found between Gal-3 concentration and RV parameters (Figure 1). The analysis of partial correlations between Gal-3 and echocardiographic parameters of LV and RV function and exercise capacity after adjustment for glomerular filtration rate was performed. However, data on the association between Gal-3 and renal function were known, in our study all founded correlations, regarding clinical and statistical significance, have persisted [11,12]. After adjusting for the NT-pro BNP, a significant correlation was retained for long-axis systolic RV function, expressed as Tricuspid Annular Plane Systolic Excursion (TAPSE) and non-invasive index of RV-to-pulmonary circulation coupling (TAPSE/PASP). No significant correlation was found between Gal-3 and LV function parameters. Some parameters of exercise capacity correlated with Gal-3. Multivariate regression analysis revealed that TAPSE and heart rate at peak exercise were independently related to galectin-3 concentration. Advanced heart failure is a complex clinical syndrome and condition of other target organs like liver must be taken into account, especially in patients with coexisting RV dysfunction. There are data in the literature, that Gal-3 is a good marker of fibrosis in cirrhosis and toxic hepatitis, which reflects the stage of liver damage [13]. Therefore, liver function parameters were included into the backward multivariate linear regression analysis model: total bilirubin, ALT, and scores Fibrosis-4 and Model for End Stage Liver Disease excluding INR (MELD-XI). MELD-XI score was chosen, because it is relevant concerning oral anticoagulant therapy and its prognostic utility in patients with end-stage heart failure. None of the analysed liver function parameters or scores was significant [11].
Figure 1:Correlations between Gal-3 concentration and RV function parameters. Abbreviations: RV: Right Ventricular; TAPSE: Tricuspid Annular Plane Systolic Excursion; s´ Myocardial Peak Systolic Velocity; PASP: Pulmonary Artery Systolic Pressure; FAC: Fractional Area Change; e´ Myocardial Peak Diastolic Velocity.
We concluded that elevated galectin-3 concentration in HF patients with reduced ejection fraction might indicate concomitant RV dysfunction and exercise intolerance [11].
Galectin 3 and Congenital Heart Diseases
Pulmonary hypertension and RV function are essential issues for pathophysiology and prognosis in the majority of congenital heart diseases. Shen et al. evidenced that Gal-3 could aggravate pulmonary artery hypertension by activating the immune response in congenital heart disease patients [14]. Kowalik et al. assessed Gal- 3 levels in patients with extremely high-pressure overload of the RV due to congenitally corrected TRansposition of the Great Arteries (ccTGA) and Eisenmenger syndrome [15]. Gal-3 concentrations are significantly higher compared to healthy subjects, especially in Eisenmenger syndrome. In ccTGA patients, Gal-3 correlated with clinical status, echocardiographic parameters of systemic RV function, left atrial area, and systemic atrioventricular valve regurgitation, therefore, might reflect remodelling of systemic RV. Gal-3 values in Eisenmenger syndrome might reflect extensive noncardiac fibrosis secondary to desaturation and chronic hypoxia.
Galectin-3 Normal Value-Unsolved Issue
The question of values of Gal-3 concentration, normal and abnormal, remains open and mainly depends on the tested population. The data on values of Gal-3 in healthy subjects are relatively scarce and results are also sometimes contradictory, with regard to age and sex. Agnello et al. recruited 706 blood donors and found that median Gal-3 concentration was 14.3 (IQR 11.9-16.7) ng/mL with 97.5th percentile URL 26.1 ng/mL and it was related to age [16]. Mueller et al. also examined blood donors concluded that 97.5th percentile URL for Gal-3 was 16 ng/mL in males and 17 ng/mL in females [17]. The development of detection methods of Gal-3 is required to improve the sensitivity, accuracy and consensus between different laboratories, and to provide supports for its clinical utility.
Conclusion
Gal-3 has been known for its significant role in mediating cardiac fibrosis and inflammation. Several studies found the clinically relevant relationship between Gal-3 and pulmonary hypertension and RV dysfunction, which is essential for understanding pathophysiological mechanisms and risk stratification. For future direction, Gal-3 as a potential, novel target in therapeutics would be of great importance.
References
- Dong R, Zhang M, Hu Q, et al. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy. Int J Mol Med. 41(2): 599-614 (2018).
- Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110(19): 3121-3128 (2004).
- Sun RR, Lu L, Liu M, et al. Biomarkers and heart disease. Eur Rev Pharmacol Sci. 18(19): 2927-2935 (2014).
- Chow SL, Maisel AS, Anand I, et al. Role of biomarkers for the prevention, assessment and management of heart failure: A scientific statement from the American Heart Association. Circulation. 135(22): e1054-e1091 (2017).
- He J, Li X, Luo H, et al. Galectin-3 mediates the pulmonary arterial hypertension-induced right ventricular remodeling through interacting with NADPH oxidase 4. J Am Soc Hypertens. 11(5): 275-289 (2017).
- Hao M, Li M, Li W. Galectin-3 inhibition ameliorates hypoxia-induced pulmonary artery hypertension. Mol Med Rep. 15: 160-168 (2017).
- Calvier L, Legchenko E, Grimm L et al. Galectin-3 and aldosterone as potential tandem biomarkers in pulmonary artery hypertension. Heart. 102(5): 390-396 (2016).
- Agoston-Coldea L, Lupu S, Petrovai D, et al. Correlations between echocardiographic parameters of right ventricular dysfunction and Galectin-3 in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Med Ultrason. 17(4): 487-495 (2015).
- Crnkovic S, Egemnazarov B, Damico R et al. Disconnect between fibrotic response and right ventricular dysfunction. Am J Respir Crit. Care Med. 199(12): 1550-1560 (2019).
- Shah RV, Chen-Tournoux AA, Picard MH, et al. Galectin-3, cardiac structure and function, and long term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail 12: 826-832 (2010).
- Zaborska B, Sygitowicz G, Smarż K, et al. Galectin-3 is related to right ventricular dysfunction in heart failure patients with reduced ejection fraction and may affect exercise capacity. Scient Rep 10: 16682 (2020).
- Gopal DM, Kommineni M, Ayalon N, et al. Relationship of plasma galectin-3 to renal function in patients with heart failure: Effects of clinical status, pathophysiology of heart failure, and presence or absence of heart failure. J Am Heart Assoc. 1(5): e000760 (2012).
- Gudowska M, Gruszewska E, Cylwik B, et al. Galectin-3 concentration in liver diseases. Ann Clin Lab Sci. 45(6): 669-73 (2015).
- Shen Q, Chen W, Liu J, et al. Galectin-3 aggravates pulmonary arterial hypertension via immunomodulation in congenital heart disease. Life Sci. 232: 116546 (2019).
- Kowalik E, Kuśmierczyk-Droszcz B, Wróbel A, et al. Galectin-3 plasma levels in adult congenital heart disease and the pressure overloaded right ventricle: Reason matters. Biomark Med. 14(13): 1197-1205 (2020).
- Agnello L, Bivona G, Lo Sasso B, et al. Establishing the upper reference limit of Galectin-3 in healthy blood donors. Biochem Med (Zagreb). 27: 1-7 (2017).
- Mueller T, Egger M, Leitner I, et al. Reference values of galectin-3 and cardiac troponins derived from a single cohort of healthy blood donors. Clin Chim Acta. 456:19-23 (2016).