Review Article - Interventional Cardiology (2015) Volume 7, Issue 6
Fractional flow reserve: conundrums, controversies and challenges
- Corresponding Author:
- Divaka Perera
Cardiovascular Division, St Thomas’ Hospital Campus,
Kings College London, & the British Heart Foundation Centre of Excellence,
St Thomas’ Hospital, London, UK
Tel: +44 20 7188 1048
Fax: +44 20 7188 1097
E-mail: Divaka.Perera@kcl.ac.uk
Fractional flow reserve (FFR) is an important invasive physiological tool that has had a great impact on the management of coronary artery disease in the catheterization laboratory. It has been proven in several large randomized trials to be superior to angiography-guided percutaneous coronary intervention, paving the way for an era of ischemia-guided revascularization. Albeit straightforward to measure, the physiological derivation of FFR and its inherent assumptions are poorly understood, which can lead to its misinterpretation in routine clinical practice. The aim is, therefore, to discuss the key physiological principles of FFR, review clinical scenarios in which its application may challenge its evidence base and discuss future perspectives with respect to alternative physiological indices and the noninvasive measurement of FFR through CT.
Submitted: 2 June 2015; Accepted: 31 July 2015; Published online: 12 November 2015
Abstract
Fractional flow reserve (FFR) is an important invasive physiological tool that has had a great impact on the management of coronary artery disease in the catheterization laboratory. It has been proven in several large randomized trials to be superior to angiography-guided percutaneous coronary intervention, paving the way for an era of ischemia-guided revascularization. Albeit straightforward to measure, the physiological derivation of FFR and its inherent assumptions are poorly understood, which can lead to its misinterpretation in routine clinical practice. The aim is, therefore, to discuss the key physiological principles of FFR, review clinical scenarios in which its application may challenge its evidence base and discuss future perspectives with respect to alternative physiological indices and the noninvasive measurement of FFR through CT.
Keywords
coronary angiography, coronary artery disease, fractional flow reserve, pressure wire
The invasive functional assessment of coronary artery stenoses has been in the clinical arena for over two decades through the use of the pressure wire and subsequent calculation of fractional flow reserve (FFR). This was developed due to the well-documented limitations of the assessment of lesion severity on the basis of angiography alone (referred to as ‘lumenography’), particularly in visually intermediate lesions, where an assessment of its impact on myocardial flow is key in determining whether revascularization will improve patient outcomes.
Many physiological indices of lesion severity seek to estimate the impact of the latter on regional myocardial blood flow. While it is difficult to measure absolute coronary flow rates in the clinical setting, flow velocity is used as a surrogate of flow. Coronary flow reserve is calculated as the ratio of coronary flow velocity during stress (usually hyperemia) to flow velocity at rest. Its clinical utility has been limited by inherent practical difficulties in acquisition of consistent flow velocity signals and the fact that they are influenced by prevailing hemodynamic conditions [1,2]. In contrast, coronary pressure can be easily and reliably measured leading to the exploration of techniques, whereby flow could be derived from pressure measurements alone, which ultimately led to the description of FFR. Myocardial FFR is the ratio between maximal hyperemic myocardial flow in the stenotic territory to maximal hyperemic flow if the stenosis were not present. In clinical practice, this is calculated as a ratio between invasive aortic pressure (Pa) and distal coronary pressure (Pd) during maximal hyperemia, most commonly by an intravenous infusion of adenosine. This physiological tool was initially described by Pijls et al., and his group provided the first experimental data that validated this method in dogs and subsequently humans, whereby a cut off of 0.75 corresponded to ischemia demonstrated by noninvasive testing [3–5].
This tool has subsequently been tested in several large clinical trials including DEFER (Deferral Versus Performance of PTCA in Patients Without Documented Ischemia), FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) and FAME II (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation 2), showing that an FFR-guided revascularization approach in the catheter laboratory in single vessel and multivessel coronary artery disease is safe with no adverse effect on outcomes [6–9]. A recent meta-analysis of 19 studies has also confirmed the safety of deferring PCI in patients with nonischemic lesions assessed by FFR [10]. This body of evidence has led to its inclusion in current American [11] and European [12] revascularization guidelines and its use internationally in the catheter laboratory during routine angiography. Albeit an elegant and simple tool, several assumptions are made in its calculation, which can lead to a significant impact on clinical interpretation and subsequent management decisions. It is therefore important to understand its physiological basis in order to maximize its accuracy in determining whether a given stenosis can result in ischemia. Following from this, in part due to these assumptions and in part due to the validation studies being performed predominantly in a specific subset of patients, FFR can easily be misinterpreted in a variety of different clinical scenarios such as in left main stem disease, sequential stenoses, microvascular disease and left ventricular dysfunction. The aim of this review, therefore, is to address each physiological assumption and its impact on the diagnostic accuracy of FFR and review the evidence of the use of FFR in specific clinical situations, to enable the reader to maximize its clinical utility in everyday practice.
Physiological assumptions of FFR
Pressure is directly proportional to flow
FFR relies heavily on the assumption that pressure and flow within a coronary artery have a linear relationship when resistances are kept constant and minimal, thereby a drop in pressure leads to a drop in flow and vice versa. However, the relationship between pressure and flow is more complex. At lower perfusion pressures, the relationship between pressure and flow is concave, and in fact does not intercept at 0 pressure, but slightly above coronary venous pressure [1,13]. In a coronary artery that has a stenosis, the pressure drop across it is governed by energy losses due to friction through the stenosis, as well as acceleration of flow at the exit point, such the relationship between pressure and flow (velocity) is curvilinear [1]. This can be described by the quadratic equation ΔP= Av + Bv [2], where A and B are coefficients that are related to the morphology of the stenosis and blood characteristics. In the absence of a coronary stenosis, Bv [2] is often approximated to zero as the main contributor to this is the exit loss, resulting in the simplified equation ΔP = Av. FFR relies on the assumption that flow in nondiseased coronary arteries behaves the same as in diseased coronary arteries which is incorrect.
Microvascular resistance is constant & minimal
As described above, flow in a region of myocardium is approximated to the ratio of the pressure difference across the myocardial bed and the resistance of the microvasculature. If flow in a stenosed artery is referred to as QS and flow in the same (but undiseased) artery as QN, FFR equates to QS/QN, which in turn equates to the product of (Pd–Pv)/(Pa–Pv) and RN/RS. The second assumption that is made is that during either hyperemia, or in a specific time interval during late diastole (which underlies a newer index know as the instantaneous wave-free ratio, iFR, which is described later), microvascular resistance in the presence of a stenosed artery (RS) is the same as it would be within an undiseased artery (RN) and hence that the ratio of RN/ RS will be 1, or simply, that microvascular resistance can be omitted when calculating FFR. However this is not always the case and therefore has the ability to influence the accuracy of the value of FFR obtained. First and foremost, to keep resistances minimal and constant, maximal hyperemia should be obtained [14]. This is not always achievable due to either procedural or patient aspects. Effects of extrinsic factors (such as caffeine [15]), and intrinsic factors such as endothelial and microvascular dysfunction (present in a variety of different pathologies) have been shown to affect the ability of the coronary vasculature to vasodilate, which can subsequently affect the FFR value obtained.
Venous pressure can be ignored
On the basis of the first two assumptions, FFR = (Pd– Pv)/(Pa–Pv). Yet, venous (or right atrial) pressure is rarely measured in everyday clinical practice, mainly due to time constraints and the additional vascular access required, particularly when coronary angiography is performed via the radial artery. However, it has been shown in a small cohort study that ignoring venous pressure (or assuming an arbitrary fixed value such as 5 or 10 mmHg) results in significant errors in FFR measurement, especially at lower values of Pd and hence FFR [16]. The investigators found that the sensitivity of detecting a significant stenosis (using a dichotomous threshold of 0.75) with FFR was reduced to 64% when venous pressure was assumed to be zero. Others have found similar results in prospective studies [17,18]. The adoption of a higher threshold (0.8) does reduce the likelihood of misclassification due to omission of venous pressure, but it should be borne in mind, especially in conditions where venous pressure may be elevated (such as left ventricular dysfunction), when calculation of true FFR would be preferable to Pd/Pa. With respect to LV dysfunction, these patients by definition will have raised left ventricular end diastolic pressures (LVEDP) and also right atrial pressures. Leonardi et al. performed an invasive hemodynamic study in 17 patients to investigate the impact of LVEDP on FFR (20 coronary arteries) [19]. In a multivariate model LVEDP was positively associated with FFR, therefore the higher the LVEP the higher the FFR, particularly in those lesions with an FFR < 0.8. This led the authors to conclude the sensitivity of an FFR < 0.8 for the identification of ischemia may be reduced when the LVEDP is high. Of note patients with normal LV function were recruited into this study, with LVEDP changes induced by nitroprusside infusion, which may have impacted the FFR results obtained. What can be concluded from these subsets of patients, however, is that the evidence of FFR is lacking in patients with LV dysfunction, and any measurements taken should be interpreted cautiously.
The FFR ‘gray-zone’
An initial validation study comparing FFR with noninvasive ischemia tests found that FFR had a high specificity of 100% and a high sensitivity of 88% [4] using an FFR cut-off of 0.75. This value was tested as a result of previous studies that had shown that lesions with FFR values below 0.75 demonstrated ischemia in noninvasive tests [20,21]. Outcomes were subsequently compared in both DEFER, which used a cut off of 0.75, and the FAME trials, which both used cut offs of 0.80. This leaves an FFR ‘gray-zone’ between 0.75 and 0.8. The shift from 0.75 to 0.80 occurred following the design and publication of the FAME trials, as in previous studies there were patients who had coronary lesions with FFR values of between 0.75 and 0.8 that demonstrated ischemia in noninvasive testing. As a result of this 0.8 was mandated as the cut off.
There are several apparent problems with this. First, the initial validation studies were performed on small numbers of patients comparing FFR with different noninvasive ischemia tests, that themselves have in built errors that can impact on their sensitivity and specificity in detecting ischemia. Second, the seminal trials have not used a consistent ischemic threshold to assess for impact on outcomes. They have confirmed its utility in guiding or deferring revascularization, but have not provided a consistent cut-off. Although the use of dichotomous values has likely aided its adoption, it is often the group of patients with intermediate stenoses where an FFR is key in deciding a management plan, that often have values within this ‘grayzone’. And third, physiological indices including FFR are inherently variable, both within and between subjects due to various factors. Petraco et al. reviewed the data from the DEFER trial and calculated how much the test–retest variability in FFR measurement could affect the certainty of its results [22]. In summary, they found that at extremes ends of the disease spectrum, with FFR less than 0.75 and FFR greater than 0.85, the repeated FFR measurements were the same in 100% of cases. Within 0.75 and 0.85 however, this agreement is much less, and is least around the 0.8 cut off. The authors suggested that in patients with FFR values of between 0.75 and 0.85 further clinical judgement and review of other noninvasive testing should be taken into account before a further management decision is made. A meta-analysis performed by Johnson et al. sought to investigate from the existing outcome data the relationship between FFR numeric values and their associated prognostic value [23]. They in found that the lower the FFR value the larger the absolute benefit received from revascularization. They found that the optimal threshold for a composite of death, MI and revascularization occurred at 0.67, rising to 0.76 after adjustment for percent diameter stenosis. Further randomized studies need to be performed to assess the outcome of patients that fall within the 0.75–0.8 FFR range, and using other thresholds, to guide practice in these differing subset of patients. However, until these studies are performed and published a cut off of 0.8 should be used due to the large body of prognostic data that support it.
The use of FFR in differing coronary & LV pathologies
Left main stem disease
Left main stem disease historically has been treated with coronary artery bypass grafting, however, PCI has become attractive in certain subsets of patients with an unprotected left main stem (ULMS) lesion. The large multicentre trial SYNTAX found that in patients with a ULMS lesion that was either ostial or in the main shaft not involving the bifurcation primary outcomes were comparative to surgery [24], with similar results found in the recently published 5-year results of the PRECOMBAT trial [25]. A cut off of 50% diameter stenosis angiographically has historically been deemed significant in the left main stem; however, the inaccuracies of assessing lesion severity by angiography alone hold most true in these subset of patients. This is due to the left main stems short length, the lack of a reference vessel and the presence of daughter vessels that can often overlie and distort its appearance.
Although there is strong outcome data with the use of FFR in guiding revascularization from several large randomized controlled trials, all these trials excluded patients with ULMS disease. There have been small observational studies suggesting the efficacy of FFR in assessing the functional significance of ULMS disease [26,27]. Hamilos et al. [28] reported the outcomes of 213 patients who had equivocal ULMS lesions assessed with FFR. Those who had an FFR less than 0.8 underwent revascularization with coronary artery bypass grafting, and those who had an FFR greater than or equal to 0.8 were treated medically. There was no significant difference in event-free survival rates between the two groups. They also found that angiographic assessment of ULMS lesions often leads to underestimation of lesion severity, with a quarter of patients with a ULMS stenosis of less than or equal to 50% had an FFR of less than 0.8. Conversely 6% of patients had a ULMS stenosis greater than 50% whose lesions were hemodynamcially insignificant. A recent meta-analysis performed of six prospective cohort studies also found that in patients with indeterminate LMS lesions who had revascularization deferred on the basis of a negative FFR had similar outcomes to the revascularized group [29]. However, it is important to note that there is no randomized data to support the use of FFR in LMS disease. Furthermore, it is unclear whether a diagnostic threshold of 0.75 or 0.80 would apply to patients with LMS disease, or whether even higher values may be prognostically important, given the greater mass of subtended myocardium. Also, many distal left main stem lesions involve the bifurcation, and further downstream disease in the LAD and circumflex arteries, which pose additional challenges when interpreting FFR measurements and evaluating the contribution of the LMS lesion itself. In these cases, it is important that a steady pullback is performed to look for discrete step-ups in order to assess the hemodynamic significance of a ULMS lesion. In the case of single vessel (either LAD or circumflex) involvement, the normal vessel should be used for assessment of FFR, although it is important to be aware that any disease in the other vessel may also impact the value of the FFR obtained.
Sequential & diffuse coronary lesions
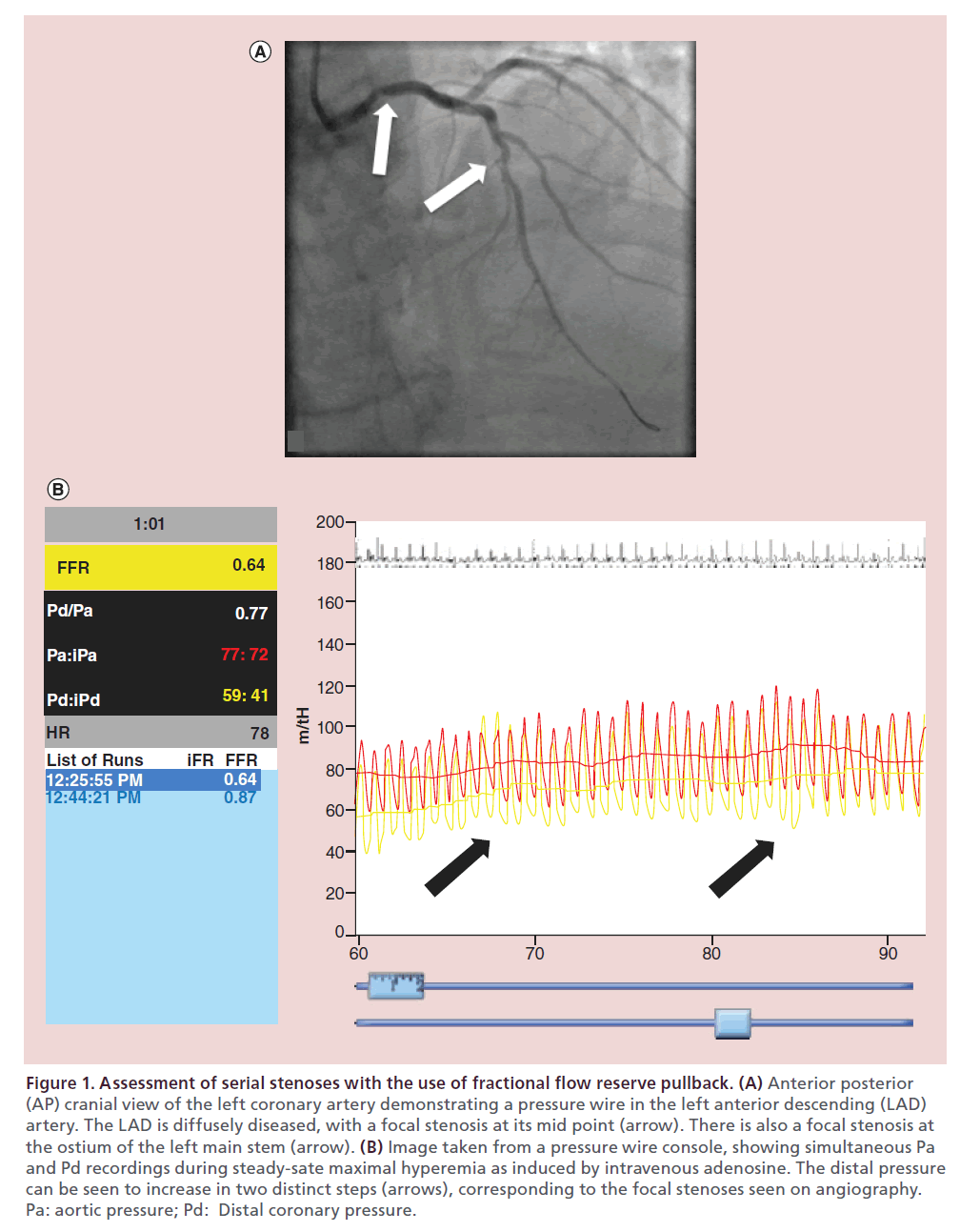
Although lesions seen on diagnostic angiography often appear isolated, what has been found histologically is that these lesions are commonly part of a more diffuse atherosclerotic process, occurring as either diffuse narrowing throughout the arterial tree or as sequential stenoses. With the advent of intravascular imaging techniques, we are increasingly seeing this at the index catheterization. What is often seen on FFR with diffuse disease is a gradual drop in pressure when invasively measured, and although not necessarily classed as ischemic with respect to FFR cut offs, has been shown to be prognostically important. Similarly assessment of sequential disease can be a challenge, due to the impact of each stenosis on flow and pressure. This was highlighted by mathematical modeling and then validation in an animal study performed by De Bruyne et al. [30]. His group found that although FFR can be used to determine the hemodynamic significance of both stenoses, it cannot be used to predict the FFR of each individual stenoses. They highlighted the importance of integrating coronary wedge pressure into the equation, which takes into account collateral flow. However, in clinical practice this is often impractical, so a pullback of the pressure wire can be performed across the stenosis during maximal hyperemia, allowing qualitative appraisal of the relative contributions of serial lesions (as demonstrated in Figure 1). This approach is still to be validated in a randomized study.
Figure 1. Assessment of serial stenoses with the use of fractional flow reserve pullback. (A) Anterior posterior
(AP) cranial view of the left coronary artery demonstrating a pressure wire in the left anterior descending (LAD)
artery. The LAD is diffusely diseased, with a focal stenosis at its mid point (arrow). There is also a focal stenosis at
the ostium of the left main stem (arrow). (B) Image taken from a pressure wire console, showing simultaneous Pa
and Pd recordings during steady-sate maximal hyperemia as induced by intravenous adenosine. The distal pressure
can be seen to increase in two distinct steps (arrows), corresponding to the focal stenoses seen on angiography.
Pa: aortic pressure; Pd: Distal coronary pressure.
Bifurcation disease
Bifurcation disease also poses a challenge to interventional cardiologists, and over the years several strategies and techniques have been used to manage these lesions. Currently the favored approach is a strategy of performing PCI on the main vessel, with balloon angioplasty in most cases where side-branch treatment is required. It is often difficult to assess ostial side branch lesion severity on the basis of angiography alone and the use of FFR has been shown to be of benefit in an animal model [31] and observational studies [32,33]. FFR guided intervention of jailed side branch disease resulted in less frequent restenosis rates [33]. A more recent randomized study published sought to compare angiographically guided provisional side branch stenting and an FFR-guided side branch stenting [34]. Three hundred and twenty patients with coronary bifurcation lesions requiring stenting were randomized to undergo either angiography guided side branch stenting or FFR guided stenting. Both arms had similar one-year composite major adverse cardiac event rates, with no significant differences in target vessel revascularization or stent thrombosis rates in either group, suggesting FFR guided side branch intervention is both feasible and safe.
Acute coronary syndromes
Most studies validating FFR have been in stable patients and little is known about its use in assessing lesions during an acute coronary syndrome (ACS). Microvascular dysfunction is significant in this situation; therefore any pressure gradient measured will be underestimated. Also the presence of residual thrombus, distal embolization and vasoconstriction that often predominates can also have an impact on FFR. During an ST-elevation myocardial infarction (STEMI), the main aim of intervention is to revascularize the target vessel, however there are signals from small randomized trials that patients with multivessel disease show improved clinical outcomes following complete revascularization during their index admission [35,36]. FFR has a potential utility in this cohort of patients, and several observational studies have demonstrated its safety in assessing bystander disease with adequate one-year outcomes [37,38]. In patients with a myocardial infarction greater than or equal to six days previously, De Bruyne et al. found that FFR can be used accurately, as the microvascular resistance in the infarcted territory is inversely proportional to viable myocardium [39]. The recently presented and published FAMOUS-NSTEMI randomized control trial sought to investigate the utility and safety of an FFRguided revascularization approach in patients presenting with a non-ST-elevation myocardial infarction (NSTEMI) [40]. Three hundred and fifty patients diagnosed with an NSTEMI were randomized to receive either FFR guided or angiographically guided revascularization. In the FFR guided group only those lesions with an FFR less than or equal to 0.8 were revascularized. In the angiography-guided group the FFR was also measured but blinded to the operator and research team. The primary outcome was the proportion of patients initially treated by medical therapy. Secondary outcomes included feasibility and safety of routine FFR measurement, major adverse cardiac events (MACE) at 12 months and the relationship between FFR and stenosis severity on angiography. The investigators found that more patients were treated medically in the FFR-guided arm. They also found that the reduced revascularization rates were maintained at 12 months follow-up in the FFR-guided arm. There were no significant differences in MACE between the two groups; however, the study was not powered for this. Interestingly this trial calls into question the current paradigm of the need to revascularize the ‘vulnerable plaque’ to improve outcomes; however, a larger trial is needed to look for any differences in major cardiovascular outcomes.
Microvascular dysfunction
Conditions such a left ventricular hypertrophy, hypertrophic cardiomyopathy, diabetes and hypertension can all lead to microvascular dysfunction. Although each is pathophysiologically distinct, the main causes of coronary microvascular dysfunction include endothelial and smooth muscle dysfunction, vascular remodeling, microvascular atherosclerosis, inflammation, extramural compression and increased sympathetic tone [41]. Often these patients lack the presence of epicardial stenosis, however, do in fact have myocardial ischemia. The challenge is to establish, particularly in those patients who also have concurrent epicardial coronary artery disease, which lesions result in ischemia. FFR, although pitched as being a physiological indice that is independent of microvascular resistance, is in fact influenced by it as described above. Resultant epicardial blood flow is often reduced in patients with microvascular dysfunction, which can lead to an overestimation of the FFR value obtained. This should always be taken into account when performing FFR studies in this subset of patients.
It has been suggested previously that coronary flow velocity reserve (CFVR) provides more information regarding the state of the microvasculature and suggestion of discordance between CFVR and FFR relate to microvascular dysfunction [42]. A recently published study sought to investigate the impact of discordance of these indices had on clinical outcome and the physiology that it underlines [43]. The investigators studied 157 intermediate coronary stenoses that were evaluated by both CFVR and FFR in which revascularization was deferred. They found discordant CFVR and FFR values occur frequently (between 31 and 37% depending on the FFR cut-off used). They also found that in patients who had a negative FFR but abnormal CFVR had significantly higher rates of major adverse cardiovascular events. They found in patients with equivocal epicardial disease with an abnormal CFVR, basal microvascular resistance was significantly lower with higher levels of hyperemic microvascular resistance, suggesting underlying microvascular dysfunction.
Other physiological indices & methods to measure the functional significance of a coronary artery stenosis iFR
Davies et al. identified a diastolic period where intracoronary resistance at rest appears minimal, which has been coined the ‘wave-free’ period (reference to absence of activity during this phase on wave intensity analysis). iFR is calculated as a ratio between distal coronary pressure and aortic pressure during this wave-free period. The main draw practically to iFR is the fact that this can be measured without the administration of adenosine. The ADVISE study [44] and registry [45] concluded that iFR correlates well with FFR, and that it was independent to changes in heart rate, rhythm and large changes in blood pressure. On closer inspection, while iFR correlated well with FFR at extreme values, at more clinically relevant values (close to the diagnostic threshold) this correlation was in fact not as strong. In contrast to ADVISE, the VERIFY study found little correlation between FFR and iFR measurements [46]. The ADVISE II prospective multicentre study sought to investigate further the diagnostic accuracy of iFR and also sought to investigate an iFR–FFR hybrid approach, whereby FFR use is limited to the intermediate iFR range of values (0.85–0.94) that correlate less strongly with FFR [47]. They found that the percentage of stenoses properly classified by the prespecified hybrid iFR-FFR approach was 94.2%, with a sensitivity of 90.7% and a specificity of 96.2%. This resulted in 65% of patients not requiring adenosine administration. More correlative and prognostic studies are underway but, as with FFR, inherent physiological assumptions underlying iFR should be noted, primarily, the assumption that baseline resistance during this diastolic interval is constant; particularly accounting for the varying physiological and emotional states of patients in the cardiac catheter laboratory.
Hyperemic stenosis resistance (HSR)
The hyperemic stenosis resistance is a physiological indice that takes in account both flow and pressure in its calculation. It is defined as the pressure gradient across a stenosis (mean aortic pressure minus mean distal pressure) divided by the average peak flow velocity (APV) during maximal hyperemia. Tested against single photon emission computed tomography (SPECT) in 151 patients undergoing invasive coronary angiography [48], compared with FFR or CFVR, HSR showed higher sensitivity and specificities with lower false positives. Accuracy was best in those patients that had discordant values of FFR and CFVR. Despite advancing technology following these original studies, measurements of flow velocity with Doppler can be difficult, mainly in acquisition of adequate and reproducible signals. Although this tool has been shown to be superior when compared with FFR and CFVR, it lacks the large outcome data that support the use of FFR, and therefore is currently not used in clinical practice.
CT FFR
CT coronary angiography (CTCA) has been a welcomed tool in the field of cardiac imaging, and has been of particular use in the diagnosis of patients presenting with chest pain who have low and intermediate risk of coronary artery disease. Improving technology leading to better spatial resolution and a reduction in motion artefact have led to this modality’s high negative predictive value [49]. However, like invasive coronary angiography, it results in a purely anatomical assessment of coronary arteries, and has in fact been found to over estimate the severity of a stenosis, particularly as a result of the ability to visualize the vessel wall and quantify plaque burden. Calcification also makes the assessment of coronary lesions on CTCA challenging. Therefore many patients with coronary lesions found on CT are referred for the gold standard of invasive coronary angiography. CT FFR has become an attractive technology, as it allows the noninvasive anatomical and functional assessment of coronary lesions. This technique uses a 3D model of the coronary arterial tree in combination with a mathematical model, which simulates pulsatile flow to calculate an FFR value. The DeFACTO study enrolled 252 patients in 17 centres to undergo CTCA, CT FFR and invasive coronary angiography with FFR [50]. They found improved diagnostic accuracy of CT FFR compared with CTCA alone for the diagnosis of lesions causing ischemia. They also found a high negative predictive value, indicating its value in assessing intermediate stenoses. The prospective multicentre NXT trial also evaluated FFR CT, compared with standard CTCA and also invasive FFR [51]. They also found that FFR CT compared with its anatomic counterpart increased specificity, but to a greater extent compared with results seen in the DeFACTO study. CT FFR can be calculated without extra medications, radiation or techniques compared with a standard CTCA, which makes it an exciting technology, particularly for the assessment of intermediate stenoses. However, it needs further validation in larger randomized trials, and at present remains a research tool due to its complex computational processes.
Conclusion
FFR is an important invasive physiological tool that has altered how patients are managed in the catheter laboratory globally. Despite its simple technical acquisition, its underlying physiological derivation is complex, and its practical application is not without its limitations. Understanding its physiological basis, assumptions and limitations allows greater accuracy in acquisition and also interpretation. Although it has been clinically validated and has good outcome data for single and multi-vessel coronary artery disease from several large randomised trials, further studies are awaited in other clinical conditions such as LV dysfunction, left main stem disease and sequential coronary artery disease to validate its use in these situations. The field of CT FFR has exciting promise, and may in future change the way low and intermediate risk patients with intermediate stenoses are managed.
Future perspective
Although there is strong prognostic data supporting the use of FFR to guide revascularization, real world use remains to be low. There are several reasons behind this, including the perceived increase in cost and the potential increase in procedural time. However, in centers that perform FFR routinely procedural times are not impacted significantly and the reduction of unnecessary coronary intervention on nonischemic lesions in fact reduces the cost overall. We believe it should be adopted as part of the standard diagnostic angiogram, enabling all patients to undergo a one stop anatomical and physiological assessment. However, this may mean that diagnostic angiography becomes a tool only for the interventional cardiologist, which may have implications on the wider cardiology community for a variety of reasons.
We are, however, seeing FFR used more frequently in complex coronary disease including left main stem and bifurcation disease, and are likely to see it play a larger role in the management of both culprit and nonculprit lesions in acute coronary syndromes. Larger randomized controlled trials are needed in these more complex clinical scenarios to fully validate this physiological assessment tool. Although there are other physiological indices in both the research and clinical arena, none have yet been validated to the extent to that which FFR has, but we await the results of future randomized clinical trials. CFVR can provide important information regarding the state of the microvasculature, however, the impact of baseline hemodynamics and the lack of expertise in its acquisition means that its adoption into routine clinical use is unrealistic at this point in time. However, the upcoming DEFINEFLOW trial (NCT02328820), which will evaluate the prognostic value and therapeutic potential of combined coronary pressure and flow measurements in assessing and managing coronary stenosis (with deferral of PCI in patients with preserved CFVR but reduced FFR) is eagerly awaited. Finally, noninvasive FFR assessment with CT is an exciting new technology, and may revolutionize the management of low-to-intermediate risk patients.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
• The functional assessment of coronary artery stenoses with fractional flow reserve (FFR) when performed accurately with the understanding of its limitations is a powerful tool and has been validated in several large randomized trials.
• One of the main assumptions made when calculating FFR is that pressure is a direct surrogate of flow, which is incorrect. This relationship is in fact curvilinear.
• Microvascular resistance in the calculation of FFR is assumed to be constant and minimal, with the ratio of resistance in a normal artery to a stenosed artery assumed to be one. However, this relies on inducing maximal vasodilatation, which is not always achieved and can impact on the FFR result obtained.
• Venous pressure is often felt to be negligible and therefore is ignored in the current calculation of FFR. However, there are situations, such as in LV dysfunction, where ignoring the venous pressure can lead to errors in FFR measurement.
• There is an ‘FFR gray-zone’ between 0.75 and 0.8, as a result of the earlier validation studies using an FFR cut off of 0.75 and the latter prognostic studies using a cut off of 0.8. What is important to note is that physiology consists of continuous variables, and it is often difficult as a result to impose a dichotomous value to guide management. However, the prognostic data that exist in the current clinical arena are based on a cut off value of 0.8, and therefore at present this should be used to guide revascularization.
• Although not fully validated in pathologies such as left main stem disease, sequential/diffuse coronary disease, bifurcation lesions, acute coronary syndromes and microvascular dysfunction, FFR is being used increasingly in these situations. Certain techniques should be adopted, (such as the use of pull-back) and the understanding of the role of the microvasculature in certain conditions should be remembered when interpreting the FFR value obtained. Further randomized studies investigating the role of FFR in these varying clinical situations are welcomed.
• The impact of newer invasive physiological indices and the role of noninvasive FFR through the means of CT is promising; however, further research is required to validate these methods in order to translate them into routine clinical practice.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- Van de Hoef TP, Nolte F, Rolandi MC et al. Coronary pressure-flow relations as basis for the understanding of coronary physiology. J. Mol. Cell. Cardiol.52(4), 786–793 (2012).
- Spaan JAE, Piek JJ, Hoffman JIE, Siebes M. Physiological basis of clinically used coronary hemodynamic indices. Circulation 113(3), 446–455 (2006).
- Pijls NH, van Son J a, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation 87(4), 1354–1367 (1993).
- Pijls NH, De Bruyne B, Peels K et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N. Engl. J. Med.334(26), 1703–1708 (1996).
- De Bruyne B, Baudhuin T, Melin J a et al. Coronary flow reserve calculated from pressure measurements in humans. Validation with positron emission tomography. Circulation89(3),1013–1022 (1994).
- De Bruyne B, Pijls NHJ, Kalesan B et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med.367(11), 991–1001 (2012)
- Bech GJ, De Bruyne B, Pijls NH et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation 103(24), 2928–2934 (2001).
- Pijls NHJ, van Schaardenburgh P, Manoharan G et al. Percutaneous coronary intervention of functionally nonsignificant stenosis. 5-Year follow-up of the DEFER study. J. Am. Coll. Cardiol.49(21), 2105–2111 (2007).
- Tonino PA, De Bruyne B, Pijls NH et al. Fractional flow reserve versus angiography for guiding percuatenous coronary intervention. N. Engl. J. Med. 360(3), 213-224 (2009).
- Nascimento BR, Belfort AFL, Macedo FAC et al. Meta-analysis of deferral versus performance of coronary intervention based on coronary pressure-derived fractional flow reserve. Am. J. Cardiol.115(3), 385–391 (2015).
- Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/ AHA/SCAI guideline for percutaneous coronary intervention a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 124(23), 574–651 (2011).
- Windecker S, Kolh P, Alfonso F et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 35(37), 2541–619 (2014).
- Van de Hoef TP, Meuwissen M, Escaned J et al. Fractional flow reserve as a surrogate for inducible myocardial ischaemia. Nat. Rev. Cardiol.10(8), 439–452 (2013).
- Vranckx P, Cutlip DE, McFadden EP, Kern MJ, Mehran R, Muller O. Coronary pressure-derived fractional flow reserve measurements: recommendations for standardization, recording, and reporting as a core laboratory technique. Proposals for integration in clinical trials. Circ. Cardiovasc. Interv. 5(2), 312–317 (2012).
- Matsumoto H, Nakatsuma K, Shimada T et al. Effect of caffeine on intravenous adenosine-induced hyperemia in fractional flow reserve measurement. J. Invasive Cardiol.26(11), 580–585 (2014).
- Perera D, Biggart S, Postema P et al. Right atrial pressure: can it be ignored when calculating fractional flow reserve and collateral flow index. J. Am. Coll. Cardiol.44(10), 2089–2091 (2004).
- Layland J, Wilson AM, Whitbourn RJ et al. Impact of right atrial pressure on decision-making using fractional flow reserve (FFR) in elective percutaneous intervention. Int. J. Cardiol.167(3), 951–953 (2013).
- Kumar G. Letter to the editor: the influence of right atrial pressure on fractional flow reserve. J. Invasive Cardiol.24(10), A43–A44 (2012).
- Leonardi RA, Townsend JC, Patel CA et al. Left ventricular end-diastolic pressure affects measurement of fractional flow reserve. Cardiovasc. Revasc. Med.14(4), 218–222 (2013).
- De Bruyne B, Bartunek J, Stanislas HGS. Relation between myocardial fractional flow reserve calculated from coronary pressure measurements and exercise-induced myocardial ischaemia. Circulation92, 39–46 (1995).
- Pijls NHJ, Van Gelder B, Van de Voort P, Peels K, Bracke F, Hans GMB. Fractional flow reserve. a useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation 92, 3181–3191 (1995).
- Petraco R, Sen S, Nijjer S et al. Fractional flow reserve-guided revascularization: practical implications of a diagnostic gray zone and measurement variability on clinical decisions. JACC Cardiovasc. Interv. 6(3), 222–225 (2013).
- Johnson NP, Tóth GG, Lai D et al. Prognostic value of fractional flow reserve. J. Am. Coll. Cardiol.64(16), 1641–1654 (2014).
- Serruys P, Morice M-C, Kappetien P et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med.360(10), 961–972 (2005).
- Park S-J, Kim Y-H, Park D-W et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N. Engl. J. Med.364(18), 1718–1727 (2011).
- Bech GJ, Droste H, Pijls NH et al. Value of fractional flow reserve in making decisions about bypass surgery for equivocal left main coronary artery disease. Heart 86(5), 547–552 (2001).
- Lindstaedt M, Yazar A, Germing A et al. Clinical outcome in patients with intermediate or equivocal left main coronary artery disease after deferral of surgical revascularization on the basis of fractional flow reserve measurements. Am. Heart J. 152(1), 156.e1–9 (2006).
- Hamilos M, Muller O, Cuisset T et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation120, 1505–1512 (2009).
- Mallidi J, Atreya AR, Cook J et al. Original studies long-term outcomes following fractional flow reserve-guided treatment of angiographically ambiguous left main coronary artery disease: a meta-analysis of prospective cohort studies. Catheter. Cardiovasc. Interv. 86(1), 12–18 (2015).
- De Bruyne B, Pijls NH, Heyndrickx GR, Hodeige D, Kirkeeide R, Gould KL. Pressure-derived fractional flow reserve to assess serial epicardial stenoses: theoretical basis and animal validation. Circulation 101, 1840–1847 (2000).
- Yong ASC, Daniels D, Bruyne B De et al. Fractional flow reserve assessment of left main stenosis in the presence of downstream coronary stenoses. Circ. Cardiovasc. Interv.6, 161–165 (2013).
- Ahn JM, Lee JY, Kang SJ et al. Functional assessment of jailed side branches in coronary bifurcation lesions using fractional flow reserve. JACC Cardiovasc. Interv.5(2), 155–161 (2012).
- Koo B-K. Physiologic evaluation of bifurcation lesions using fractional flow reserve. J. Interv. Cardiol.22(2), 110–113 (2009).
- Chen S-L, Ye F, Zhang J-J et al. Randomized comparison of FFR-guided and angiography-guided provisional stenting of true coronary bifurcation lesions: the DKCRUSH-VI Trial (Double Kissing Crush Versus Provisional Stenting Technique for Treatment of Coronary Bifurcation Lesions VI). JACC Cardiovasc. Interv.8(4), 536–546 (2015).
- Gershlick AH, Khan JN, Kelly DJ et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease. J. Am. Coll. Cardiol.65(10), 963–972 (2015).
- Wald DS, Morris JK, Wald NJ et al. Randomized trial of preventive angioplasty in myocardial infarction. N. Engl. J. Med.369(12), 1115–1123 (2013).
- Fischer JJ, Wang XQ, Samady H et al. Outcome of patients with acute coronary syndromes and moderate coronary lesions undergoing deferral of revascularization based on fractional flow reserve assessment. Catheter. Cardiovasc. Interv.68(4), 544–548 (2006).
- López-Palop R, Carrillo P, Frutos A et al. Usefulness of the fractional flow reserve derived by intracoronary pressure wire for evaluating angiographically intermediate lesions in acute coronary syndrome. Rev. Esp. Cardiol.63(6), 686–694 (2010).
- De Bruyne B, Pijls NH, Bartunek J et al. Fractional flow reserve in patients with prior myocardial infarction. Circulation104(2), 157–162 (2001).
- Layland J, Oldroyd KG, Curzen N et al. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST- segment elevation myocardial infarction: the British Heart Foundation FAMOUS – NSTEMI randomized trial. Eur. Heart J. 36(2), 100–111 (2015)
- Crea F, Camici PG, Merz CNB. Coronary microvascular dysfunction: an update. Eur. Heart J. 35(17), 1101–1111 (2014).
- Meuwissen M, Chamuleau SA, Siebes M et al. Role of variability in microvascular resistance on fractional flow reserve and coronary blood flow velocity reserve in intermediate coronary lesions. Circulation103(2), 184–187 (2001).
- Hoef TP Van De, Lavieren MA Van, Damman P et al. Physiological basis and long-term clinical and coronary flow velocity reserve in coronary stenoses. Circ. Cardiovasc. Interv. 7, 301 (2014).
- Sen S, Escaned J, Malik IS et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary waveintensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J. Am. Coll. Cardiol.59(15), 1392–1402 (2012).
- Petraco R, Escaned J, Sen S et al. Classification performance of instantaneous wave-free ratio (iFR) and fractional flow reserve in a clinical population of intermediate coronary stenoses: results of the ADVISE registry. EuroIntervention 9(1), 91–101 (2013).
- Berry C, Van ‘T Veer M, Witt N et al. VERIFY (VERification of instantaneous wave-free ratio and fractional flow reserve for the assessment of coronary artery stenosis severity in everyday practice), a multicenter study in consecutive patients. J. Am. Coll. Cardiol. 61(13), 1421–1427 (2013).
- Escaned J, Echavarría-Pinto M, Garcia-Garcia HM et al. Prospective assessment of the diagnostic accuracy ofinstantaneous wave-free ratio to assess coronary stenosis relevance. JACC Cardiovasc. Interv.8(6), 824–833 (2015).
- Meuwissen M, Siebes M, Chamuleau SAJ et al. Hyperemic stenosis resistance index for evaluation of functional coronary lesion severity. Circulation 106(4), 441–446 (2002).
- Loewe C, Stadler A. Computed tomography assessment of hemodynamic significance of coronary artery disease: CT perfusion, contrast gradients by coronary CTA, and fractional flow reserve review. J. Thorac. Imaging 29(3), 163–172 (2014).
- Nakazato R, Park H-B, Berman DS et al. Noninvasive fractional flow reserve derived from computed tomography angiography for coronary lesions of intermediate stenosis severity: results from the DeFACTO study. Circ. Cardiovasc. Imaging 6(6), 881–889 (2013).
- NØrgaard BL, Leipsic J, Gaur S et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (analysis of coronary blood flow using CT angiography: next steps). J. Am. Coll. Cardiol. 63(12), 1145–1155 (2014).
• Comprehensive review of the physiological basis of clinically used coronary haemodynamic indices.
•• One of two landmark studies on fractional flow reserve (FFR; DEFER and FAME II), validating its utility and providing strong outcome data. See also reference 9.
•• One of two landmark studies on FFR (DEFER and FAME II), validating its utility and providing strong outcome data. See also reference 6.
• One of the first studies evaluating FFR in equivocal left main stem lesions showing that deferring PCI in those patients with a left main stem lesion with a negative FFR is safe.
• One of the first randomized studies evaluating FFR guided versus angiography-guided stenting of jailed side branches following bifurcation stenting.
•• Interesting study evaluating coronary flow velocity reserve (CFVR) and its discordance with FFR in certain cases. They found that patients with an abnormal CFVR (those with microvascular dysfunction) with a negative FFR result do worse than those with a normal CFVR.
•• One of the first prospective studies looking at the new pressure derived hemodynamic indice instantaneous wavefree ratio.
• One of the first studies evaluating CT FFR, showing it’s particular value in those patients with intermediate stenoses.