Case Report - International Journal of Clinical Rheumatology (2021) Volume 16, Issue 4
Fever of unknown origin and mesenteric mass: a case of kikuchi fujimoto disease and systemic lupus erythematosus
- *Corresponding Author:
- Sara Sharif
Department of Medicine, SUNY Downstate Medical Center, New York, United states
E-mail: sara.sharif@downstate.edu
Abstract
Background: Kikuchi Fujimoto Disease (KFD) is a rare autoimmune disorder manifesting with prolonged fevers and lymphadenitis. It is proposed to be triggered by infectious agents in genetically susceptible patients. Diagnosis is confirmed with lymph node biopsy. In most patients, symptoms resolve spontaneously but rarely glucocorticoids are required for remission.
Clinical Summary: This is a unique case of KFD with mesenteric lymphadenitis leading to a new diagnosis of Systemic Lupus Erythematosus (SLE) in a 24-year-old African American male presenting with two weeks of epigastric pain and fever. Initial CT abdomen showed a heterogeneous soft tissue mass followed by CT guided core biopsy of mesenteric lymph nodes revealing necrotic lymphadenopathy. He was empirically treated for intraabdominal infection with antibiotics and then discharged home. A week later he was re-admitted for similar complaints. He was febrile and tachycardic. Labs showed neutropenia and elevated inflammatory markers. Infectious work up was negative. Autoimmune panel was positive for an Anti-Nucleic Acid (ANA), Ribonucleoprotein (RNP), Smooth Muscle (SM) Antibodies and Urine Protein: Creatinine Ratio (UPCR) of 0.5 gr and 1.2 gron two separate occasions. He was treated empirically with IV antibiotics followed by filgrastim. Mesenteric lymph node open biopsy demonstrated large areas of necrosis and characteristic absence of neutrophils. Thus, KFD and SLE were diagnosed based on SLICC criterion of SLE diagnosis. Treatment with Solumedrol 40 mg IV Q12 resulted in clinical improvement and discharge from hospital. Hydroxychloroquine 200 BID was added with steroid taper as patient continued to improve during outpatient follow ups. Conclusion: It is the first reported case of KFD with mesenteric lymphadenitis as the initial manifestation in a young African American male. This rare presentation masquerades the diagnosis leading to unnecessary procedures and empiric treatments. It warrants further research on the underlying pathophysiology and the need for a standardized diagnostic criterion and treatment guidelines of the disease.
Keywords
kikuchi fujimoto disease (KFD) • systemic lupus erythematosus (SLE) • mesenteric lymphadenitis • lymphadenopathy • fever
Introduction
Kikuchi Fujimoto Disease (KFD) is a rare disorder presenting with fever and lymphadenitis associated with constitutional symptoms [1]. The pathophysiology is unclear although autoimmune and infectious mechanisms are proposed to play a role [2]. Being a great mimicker of other diseases, KFD requires lymph node biopsy and immunohistochemistry to establish a diagnosis. Usually disease course isbenign, but some patients require glucocorticoid therapy with a prolonged taper to resolve their symptoms [1]. We present a unique case of KFD in a young African American male with mesenteric lymphadenitis leading to a new diagnosis of SLE which is unique both in respect to disease epidemiology and clinical manifestations.
Clinical summary
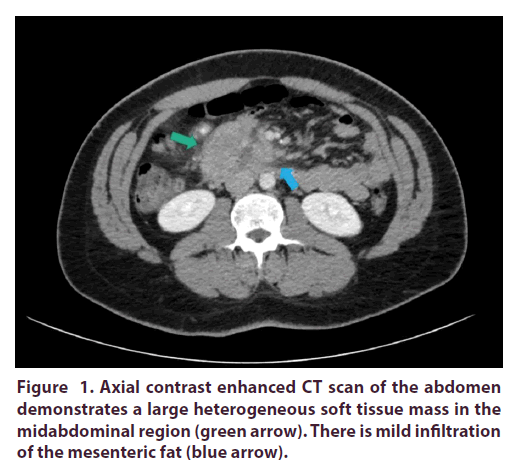
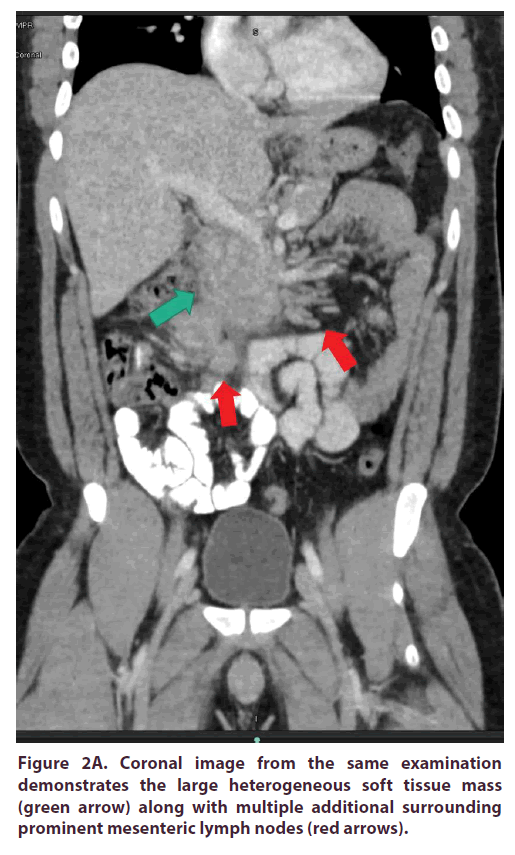
Our patient is a 24-year-old male of African American ethnicity who presented with two weeks of epigastric pain associated with non-bilious non-bloody vomiting and fever. On admission he had a temperature of 101°F and epigastric tenderness. A CT abdomen with IV and PO contrast showed a heterogeneous soft tissue mass within the mid abdomen measuring 2.8 x 5.2 cm in largest axial dimensions with surrounding reactive lymphadenopathy (Figure 1, Figures 2A and 2B).
Complete blood count and comprehensive metabolic panel were unremarkable. A CT guided core biopsy of the mesenteric mass/lymph nodes was performed demonstrating necrotic lymphadenopathy. Differential diagnosis included autoimmune diseases, infections, or malignancy. The patient was empirically treated for intraabdominal infection with ceftriaxone and metronidazole for 6 d. His symptoms improved by the 6th day of hospitalization and he was discharged home.
A week later the patient was re-admitted for recurrence of nausea, vomiting and weight loss. Vital signs were remarkable for fever of 101.3°F and heart rate of 114. Further workup as remarkable for WBC count 1.84 K/μL with absolute neutrophil count 0.8 K/μL. Hemoglobin 12.1 g/dL. Ferritin 598.4 ng/ml, LDH 391 U/L, and Haptoglobin 250 mg/dL. The rest of infectious workup including blood cultures, fungal cultures, Hepatitis B, C, HIV, viral respiratory panel, EBV antibodies was negative.
Autoimmune panel was positive for an ANA of 1:40, RNP of 2.6, SM Antibodies of 4.3. UPCR of 0.5 gr and 1.2 gron two separate occasions.
The patient was treated empirically with IV vancomycin and Piperacillin/Tazobactam for neutropenic fevers. But his clinical status remained unchanged. Hence, filgrastim was administered. An open biopsy was performed due to suspicion of malignancy. A bone marrow biopsy was also performed to rule out lymphoma or leukemia in setting of neutropenia and lymphadenopathy.
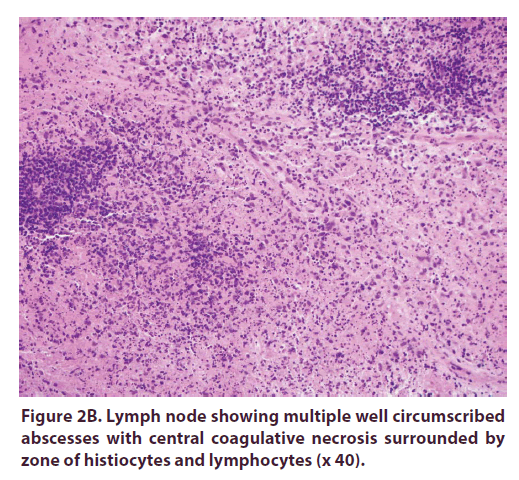
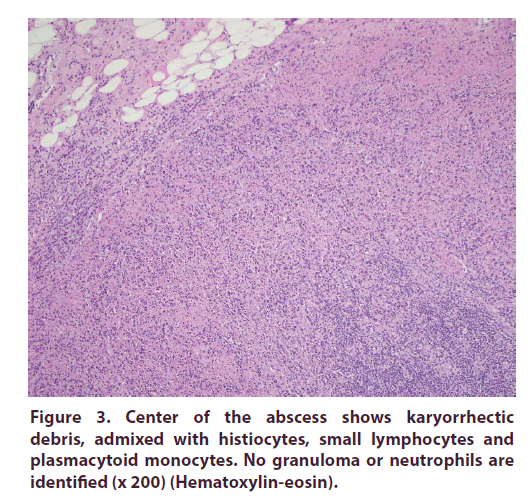
Mesenteric lymph node open biopsy showed multiple lymph nodes having large areas of necrosis with apoptotic debris (without neutrophils). Most of the small lymphocytes around the necrosis were T cells (CD3+). Reactive germinal centers in the periphery showed B cells (CD20+). No granulomas or viral inclusions were present. Lymph node flow cytometry showed no evidence of neoplastic B or T cells (Figure 3).
On bone marrow biopsy, the core biopsy was mostly bone with small foci of marrow sampled. The clot sections were blood only and the aspirates were without spicules. The myeloid: erythroid cell (M:E) ratio was approximately 3:1 with maturing myeloid cells. Megakaryocytes showed some effete and hypo-lobulated forms. Few plasma cells were noted. Acid Fast Bacilli and Geimsa stains were negative. The most likely differential per pathology was KFD vs SLE.
Once the autoimmune panel resulted, we believed that SLE with concomitant KFD was probable. The patient fulfilled SLICC criteria for the diagnosis of SLE: Positive anti-Smith Ab, positive ANA, leukopenia, lymphopenia, and renal disorder. Thrombocytopenia and anemia were transient and resolved prior to treatment with steroids.
Considering the patient’s worsening clinical status treatment for presumed SLE and KFD was started with Solumedrol 40 mg IV Q12. In the subsequent dhis fever resolved, and clinical status improved. Six days later he was discharged from the hospital on a tapering regimen of prednisone.
To reduce the progression of proteinuria patient was started on lisinopril, with resulting decrease in UPCR to 0.2 after several weeks. An attempted kidney biopsy failed to include any glomeruli. With the addition of hydroxychloroquine 200 mg BID the patient continued to improve clinically and steroid taper was continued.
Discussion
KFD is one of the etiologies for pyrexia of unknown origin first described in Japan [1]. It has a female preponderance in the adult population and male predominance in pediatric population [3-5]. There is a lack of large-scale studies on KFD in this population. Small studies show a mean age of onset of 30.5 years (range 5y to 59 y) and a female: male ratio of 3:1 [5,6]. Our case is atypical both in terms of gender and age at presentation even when compared to similar population.
Fever and lymphadenitis are the most common clinical characteristics of KFD. Other symptoms include skin lesions, arthralgias, myalgia, weight loss, night sweats, fatigue and headache similar to many other infectious and inflammatory etiologies [1,3]. Cervical lymphadenopathy and prolonged fevers are the main presenting complaints [7]. Involved lymph nodes are enlarged and tender. Cervical lymphadenopathy is most common and involvement of mesenteric, mediastinal, iliac and retroperitoneal nodes is rare [1,8,9]. Mesenteric lymphadenitis in our patient exemplifies an uncommon manifestation of this rare disease. According to our literature search on PUBMED, only 5 cases of KFD presenting with mesenteric lymphadenopathy have been reported since 1999 [10-14]. Our case presents the first report of KFD with fever and mesenteric lymphadenitis as the initial presenting complaint in a young African American male (Table 1).
S.NO |
Authors | Year of Puublication | Age | Ethnicity | Gender | Symptoms | Diagnosis | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | Van Rij& Wright | 2010 | 10 | Chinese | M | Right Lower Quadrant (RLQ) abdominal pain, loss of appetite, diarrhea | Excisional biopsy via laparotomy | Surgical excision of necrotic lymph node and appendix |
| 2 | Vijayaraghavan and Co | 2011 | teenage | unknown | M | Abdominal pain, odynophagia, reduced appetite, intermittent fever | Laparoscopic lymph node biopsy | Surgical excision of appendix, lymph nodes and wedge resection of liver, low dose steroids |
| 3 | Shrestha and Co | 2013 | 26 | Caucasian | M | RLQ abdominal pain and low-grade fever | Excisional biopsy via laparotomy | Right hemicolectomy |
| 5 | Patel and Co | 2014 | 29 | Japanese | M | RLQ abdominal pain, fever, diarrhea | Excisional biopsy (technique | Excision biopsy |
| unknown) | ||||||||
| 4 | Pandey and Co | 2017 | 30 | unknown | F | Abdominal pain, vomiting, fever | Excisional biopsy via laparotomy | Surgical excision of appendix and mesenteric lymph node |
Table 1. Comparison between published cases of KFD with mesenteric lymphadenitis.
A higher frequency of haplotype HLA-DPA1 and HLA-DPB1 in patients with KFD indicate a genetic predisposition to the disease [15]. It has a strong association with SLE, evidenced by identification of similar tubuloreticular structures in cells of KFD and SLE patients [2]. Other autoimmune associations include Wegener granulomatosis, Sjogren syndrome, Graves’ disease and adult onset Still’s disease [16-19]. Associated infectious agents include herpes simplex virus, varicella zoster, paramyxovirus, parainfluenza, rubella, hepatitis B, human immunodeficiency virus, human T-lymphotropic virus type 1, dengue virus, CMV, EBV, HHV-6, HHV-7, Parvovirus B19, and mycobacteria [9,20,21].
It is proposed that viral infections in genetically predisposed individuals trigger an inflammatory reaction which can stimulate autoimmunity. Although acute infections were ruled out in our patient, any previous infection or KFD itself may have activated his autoimmunity or vice versa. The interplay between infectious triggers, autoimmunity and KFD is still unclear. But evidence is strong enough to warrant a through infectious and autoimmune workup, particularly for SLE.
Based on the SLICC criterion, SLE diagnosis requires at least 4 of the following features including at least one clinical and one immunological finding. The clinical features include manifestations of skin involvement seen as acute and/or chronic cutaneous lupus including, oral ulcers, synovitis involving two or more joints, pleural or pericardial serositis, renal involvement indicated by urine protein/creatinine ratio of >0.5 gr, neurological manifestations without any other known primary cause, hemolytic anemia, leucopenia <4000/mm3 or lymphopenia <1000 cells/mm3 and thrombocytopenia of <100,000/mm3 without any known primary cause. Immunological criterion includes positive ANA, ds- DNA (two readings), anti-smith, anti-phospholipid antibody, low complements and positive direct coombs test [22]. Our patient met the SLICC criterion based on his WBC count 1.84 K/μL with absolute neutrophil count 0.8 K/μL, UPCR value of 0.5gr and 1.2 gr of two separate occasions and positive ANA and Anti Smith antibodies. This makes our case the first reported instance of mesenteric KFD leading to a new diagnosis SLE.
KFD is a diagnosis of exclusion confirmed by lymph node biopsy. The fever pattern for KFD is irregular which differentiates it from fevers of tuberculosis, malaria, and Non-Hodgkin’s Lymphoma’s (NHL) [23]. Biopsy also distinguishes it from infections and malignancies. Like our case, many patients undergo various imaging studies e.g. ultrasounds, CT scans and PET scans as well as procedures like imaging guided FNA, core biopsy or even excision biopsy. Given his rare presentation of a rare disorder, our patient underwent excision biopsy as CT guided core biopsy and bone marrow biopsies were non yielding.
There is no standardized criterion for diagnosis of KFD. The American Society of Hematology (ASH), declared the presence of karyorrhexis and proliferative plasmacytoid dendritic cells pathognomonic for KFD [24]. Therefore, KFD is a diagnosis of exclusion confirmed by the presence of abovementioned features on lymph node biopsy. In a review by Darcie and co, clinical features (localized lymphadenopathy, fever, fatigue, headache), lab studies (leucopenia, elevated CRP and ESR) and histopathology comprised the proposed diagnostic criterion. However, only histopathological findings of crescent shaped histiocytes and plasmacytoid monocytes with karyorrhexis was deemed necessary for diagnosis [25].
The distinct morphological features of KFD distinguish it from mimicking granulomatous conditionse.g.Tb, cat scratch disease, yersinia and Lupus lymphadenitis. In KFD granulomas, neutrophils are characteristically absent despite active proliferation of immune cells [9,26]. KFD can be classified into 3 types i.e. proliferative, necrotizing and xanthomatous. In proliferative type, Lymph nodes show paracortical expansion caused by increased proliferation of histiocytes and dendritic cells creating a dense meshwork to entrap karyorrhectic nuclear debris. It can then advance into necrotizing type as seen in form of foci of coagulative necrosis in our patient’s core biopsy. Some histiocytes may convert into foam cells, called xanthamatous type which has been reported as a distinct non resolving variant of KFD [27].
KFD can lead to fatal complications like Macrophage Activation Syndrome (MAS)and Disseminated Intravascular Coagulation (DIC). Delay in diagnosis of rare diseases increases the risk of these complications which can prolong hospitalizations, worsen outcomes, and cause death. Our patients bone marrow biopsy and coagulation studies were negative for hemophagocytosis and blood dyscrasias but the prolonged work up that resulted from his rare presentation put him at risk for fatal complications.
KFD often self resolves in 6 months and treatment is for symptomatic relief only e.g. anti-pyretic and NSAIDs [1,9]. Patients may receive unnecessary empiric antibiotics, anti-tuberculous medications and chemotherapy for infections and neutropenia. Our patient’s presentation potentiated this error by masking his diagnosis. It eludes to the importance of standardized diagnostic guidelines to prevent unwanted side effects and undue inconvenience to patients with rare diseases, particularly those with unique manifestations of these disorders.
Glucocorticoid treatments have shown good response, especially in case of concomitant SLE [28]. Most patients are given high dose glucocorticoids with a slow taper and continuous monitoring of symptoms.Less common treatments include hydroxychloroquine with low dose steroids [29], immunoglobulins, ciprofloxacin and minocycline [1]. Our patient responded well to tapering glucocorticoid therapy followed by the addition of hydroxychloroquine as a maintenance drug. There are no comparative studies on steroid dosing, administration, and steroid vs antimalarials in these patients. Therefore, the choice, dosing and duration of therapy is at the physicians’ discretion.
Conclusion
KFD is a rare benign disease mostly seen in Asian women. There is limited understanding of its pathophysiology and association with infections and autoimmune diseases like SLE. Diagnosis can be delayed due to nonspecific features overlapping with other entities, especially in cases of atypical presentation. It is based on clinical features and lymph node biopsy requiring skilled pathologists to differentiate it from mimicking conditions. Further research is warranted to create a more structured classification system based on etiology for a less invasive diagnostic algorithm.
While most patients improve without dedicated treatment, many undergo empiric therapies prior to definitive diagnosis. KFD with SLE has shown good response to glucocorticoid therapy. Further studies can determine best practices in terms of dosing, treatment duration and length of steroid taper, as well as the role of other pharmacotherapies like hydroxychloroquine.
References
- F Pepe, S Disma, C Teodoro et al. Kikuchi-Fujimoto disease: a clinicopathologic update. Pathologica. 108(3), 120–129 (2016).
- Diego F Baenas, Fernando A Diehl, María J Haye Salinaset al. Kikuchi–Fujimoto disease and systemic lupus erythematosus. Int. Med. Case. Rep. J. 9, 163 (2016).
- In Young Jung, Hea Won Ann, Jung Ju Kim et al. The incidence and clinical characteristics by gender differences in patients with Kikuchi-Fujimoto disease. Medicine (Baltimore).96(11), e6332 (2017).
- Tae Yeun Kim, Kee-Soo Ha, Yunkyung Kim et al. Characteristics of Kikuchi-Fujimoto disease in children compared with adults. Eur. J. Pediatr. 173(1), 111–116 (2014).
- Florence Moinet, Vincent Molinié, Guillaume Béraud et al. Epidemiology and Characteristics of Kikuchi-Fujimoto Disease in the African-Descent Population of Martinique, French West Indies. Arthritis. Care. Res (Hoboken). 68(12), 1883–1887 (2016).
- RaoV, F Bauer, JJVredenburgh et al. Kikuchi-Fujimoto Disease in aYoung African American Male: Are we Seeing a Paradigm Shift in Disease Epidemiology? A Case Report. Conn. Med. 80(7), 409–412 (2016).
- Ps Rakesh, Reginald G Alex, George M Varghese et al. Kikuchi-Fujimoto disease: clinical and laboratory characteristics and outcome. J. Glob. Infect. Dis. 6(4), 47 (2014).
- Haruaki Hino, Takashi Nishimura, Jun-Ichi Nitadori et al. An uncommon presentation of Kikuchi-Fujimoto disease as mediastinal lymphadenopathy. J. Thorac. Dis. 8(5), E330 (2016).
- Perry AM, SM Choi. Kikuchi-Fujimoto Disease: A Review. Arch. Pathol. Lab. Med. 142(11), 1341–1346 (2018).
- Van RijS, DM Wright. Kikuchi-Fujimoto's disease mimicking acute appendicitis. ANZ. J. Surg.80(10), 760–761 (2010).
- R Vijayaraghavan, R Chandrashekar, Saraswathi A et al. Kikuchi-Fujimoto's disease involving mesenteric nodes: a report and review of literature. BMJ. Case. Rep (2011).
- Anne Shrestha, Katie Newton, Emyr Benbowet al. Kikuchi- Fujimoto disease of mesenteric lymph nodes mimicking acute appendicitis. JNMA. J. Nepal. Med. Assoc. 52(192), 627–630 (2013).
- Nirav Patel, Dahlia Philips, Masayuki Nigo et al. Kikuchi-Fujimoto disease and acute appendicitis. BMJ. Case. Rep. 2014, (2014).
- Vinita Pandey, Yasmeen Khatib, Rahul Pandey et al. Kikuchi-Fujimoto Disease Masquerading as Acute Appendicitis. J. Clin. Diagn. Res. 11(6), ED26 (2017).
- T Tanaka, M Ohmori, S Yasunaga et al. DNA typing of HLA class II genes (HLA‐DR,‐DQ and‐DP) in Japanese patients with histiocytic necrotizing lymphadenitis (Kikuchi’s disease). Tissue. Antigens. 54(3), 246–253 (1999).
- Eun Joo Lee, Hae Sang Lee, Jun Eun Park et al. Association Kikuchi disease with Hashimoto thyroiditis: a case report and literature review. Ann. Pediatr. Endocrinol. Metab.23(2), 99 (2018).
- Al-Allaf AW, YYahia. Kikuchi-Fujimoto Disease Associated with Sjögren’s Syndrome: A Case Report. Eur. J. Case. Rep. Intern. Med. 5(5), (2018).
- W Sondermann, U Hillen, AC Reiset al. Kikuchi-Fujimoto's disease and adult-onset Still's disease. A rare co-occurence. Hautarzt. 66(12), 940–943 (2015).
- DeFilipp Z. Cutaneous manifestation of Kikuchi-Fujimoto disease in the setting of granulomatosis with polyangiitis (Wegener's). J. Gen. Intern. Med. 27(9), 1220–1222 (2012).
- Zhe Xu, Ying Liu, Haijing Li et al. Detection of mycobacterial and viral DNA in Kikuchi-Fujimoto disease: an analysis of 153 Chinese pediatric cases. Sci. China. Life. Sci. 60, 775–777 (2017).
- S David Hudnall, Tiansheng Chen, Samir Amr et al. Detection of human herpesvirus DNA in Kikuchi-Fujimoto disease and reactive lymphoid hyperplasia. Int. J. Clin. Exp. Pathol. 1(4), 362 (2008).
- Michelle Petri, Ana-Maria Orbai, Graciela S Alarcón et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis. Rheum. 64(8), 2677–2686 (2012).
- Xu S, WSun, J Liuet al. Kikuchi-Fujimoto disease: a case report and the evaluation of diagnostic procedures. BMC. Oral. Health. 19(1), 223 (2019).
- CuglievanB, RN Miranda. Kikuchi-Fujimoto disease. Blood. 129(7), 917 (2017).
- Darcie Deaver, Pedro Horna, Hernani Cualinget al. Pathogenesis, diagnosis, and management of Kikuchi-Fujimoto disease. Cancer. Control. 21(4), 313–321 (2014).
- Narittee Sukswai, Hye Ra Jung, Samir S Amr et al. Immunopathology of Kikuchi-Fujimoto Disease: A reappraisal using novel immunohistochemistry combinations. Histopathology. 77(2), 262–274 (2020).
- KuoTT, SKLo. Significance of histological subtypes of Kikuchi's disease: comparative immunohistochemical and apoptotic studies. Pathol. Int. 54(4), 237–240 (2004).
- Mihaela Găman, Ana-Maria Vlădăreanu, Camelia Dobreaet al. A challenging case of Kikuchi-Fujimoto disease associated with systemic lupus erythematosus and review of the literature. Case. Rep. Hematol. 2018,(2018).
- Fumika Honda, Hiroto Tsuboi, Hirofumi Toko et al. Recurrent Kikuchi-Fujimoto disease successfully treated by the concomitant use of hydroxychloroquine and corticosteroids. Intern. Med. 56(24), 3373–3377 (2017).