Research Article - Diabetes Management (2022) Volume 12, Issue 1
Evaluating guidelines for de-escalating care from ophthalmology to optometry in the context of diabetic retinopathy
- Corresponding Author:
- Zyg Chapman
Département Ophthalmology,
University of Melbourne,
Melbourne,
Victoria,
Australia
E-mail: zchapman@student.unimelb.edu.au
Received: 05-Jan-2022, Manuscript No. FMDM-22-52832; Editor assigned: 07-Jan-2022, PreQC No. FMDM-22-52832 (PQ); Reviewed: 21-Jan-2022, QC No. FMDM-22-52832; Revised: 24-Jan-2022, Manuscript No. FMDM-22-52832(R); Published: 31-Jan-2022, DOI: 10.37532/1758-1907.2022.12(1).299-306
Abstract
Diabetic retinopathy remains a significant common cause of preventable vision loss. Guidelines exist for the routine screening of diabetic patients, to identify the development of diabetic retinopathy, and manage the progression of the disease. While screening can be performed by optometrists, treatment of diabetic retinopathy requires management by ophthalmologists. Currently, guidelines prescribe when care should be escalated from optometry, but there is a lack of clear protocols for the de-escalation of patients from ophthalmology back to optometry. In this protocol, novel guidelines have been generated in collaboration with ophthalmologists working at Royal Melbourne Hospital, to standardize de-escalation from their care, to allied optometrists working at University of Melbourne Eyecare Clinic. In this protocol we further propose how these new guidelines might be statistically evaluated based on data generated from patients over a three-year prospective study. We hypothesise that these novel guidelines will reduce the average number of ophthalmology appointments per patient without compromising patient outcomes. These guidelines aim to reduce the clinical burden of ophthalmologists managing diabetic retinopathy, while maintaining an excellent standard of care in preventing vision loss.
Keywords
de-escalation; diabetes; diabetic retinopathy; guideline; macular oedema; microvascular; optometry; ophthalmology; protocol; shared care; vision loss
Introduction
Allied health management for the care of patients with Diabetic Retinopathy (DR) is significant in reducing healthcare burdens and fostering better patient outcomes [1]. DR is a major complication of diabetes mellitus and remains a leading cause of blindness in adult populations [2]. The estimated indirect economic cost of DR in Australia exceeds $600 million annually, in addition to considerable direct healthcare costs [3]. One in three diabetic patients are estimated to have some degree of DR progression [4,5], resulting in a large clinical burden for ophthalmologists. Shared care between ophthalmology and optometry has become increasingly prevalent, to reduce healthcare burden [5-7].
DR is a microvascular disease of the retina caused by hyperglycaemia. Multiple metabolic pathways and enzymes have been indicated to be involved in the complex microvascular changes of the capillaries suppling the retinal cells [8-10]. DR is graded based on progressive severity with proliferative retinopathy being the most severe. Quiescent DR describes a halted process no longer progressing at that point in time [8-13]. Primary healthcare providers such as optometrists are typically the first point of contact for diagnosis and screening of DR [6,9,14,15]. Ophthalmologists treat DR with interventions such as Pan-Retinal Laser Photocoagulation, Intravitreal Injections, and Vitrectomies [8,16]. While ophthalmologists provide appropriate treatment plans, this management comes at a significant healthcare cost [3]. Shared cared between optometrists (screening) and ophthalmologists (treatment) has been shown to reduce the clinical burden to ophthalmologists, without compromising patient outcomes [7, 16-20].
• Research question
This paper aims to propose a methodology to evaluate an amended set of guidelines with novel recommendations for the de-escalation of care of patients with DR. This methodology will focus on exploring the impact of these guidelines on patient’s disease progression, rates of sudden vision loss, and frequency of presentation to ophthalmology, in comparison to practice prior to the novel guideline’s implementation.
• Amended guidelines
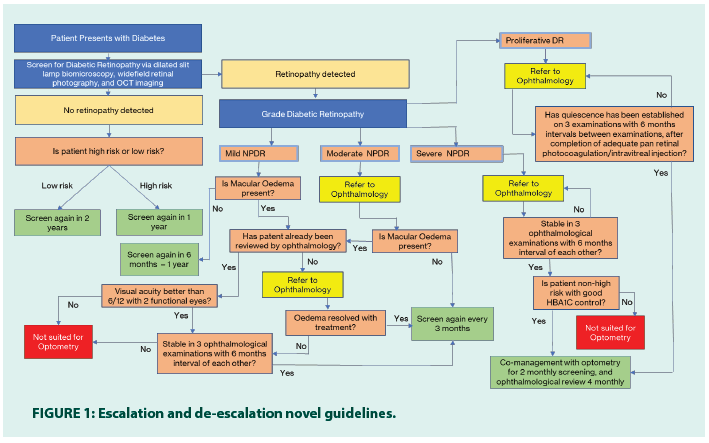
Below is a novel set of guidelines primarily based on existing guidelines aggregated from the NHMRC, The Royal College of Ophthalmologists, The NICE guidelines, Optometry Australia, and RANZCO [9,10,17- 20]. Novel features of these guidelines have been italicized. These guidelines are also represented graphically in Figure 1.
• Mild NPDR
• If macular oedema is absent: On-going screening by optometrist every 6 months- annually.
• If macular oedema is present, refer to ophthalmology for further monitoring.
• After initial assessment by ophthalmology, initiate treatment until the resolution of macular oedema, then refer back to optometry for 3 monthly screening.
• Re-escalate to ophthalmology if macular oedema reoccurs in patient with one eye or poor visual acuity (<6/12).
• Macular oedema, not requiring any further treatment, with good visual acuity (>6/12) can be deescalated to optometry for 3 monthly screening if proven to be stable by 3 ophthalmological examinations with 6 months interval of each other, after discussion with patient to understand the importance of on-going follow up.
• Moderate NPDR
1. If macular oedema is absent, monitor every 3-4 months or referral to ophthalmology if at high risk*of progression.
• Refer to ophthalmology. After initial assessment by ophthalmology, initiate management under ophthalmological care or refer back to optometry if no additional treatment required for 3 monthly screening.
2. If macular oedema is present refer to ophthalmology for further monitoring.
• After initial assessment by ophthalmology, initiate treatment until the resolution of macular oedema, then refer back to optometry for 3 monthly screening.
• Re-escalate to ophthalmology if macular oedema reoccurs in patient with one eye or poor visual acuity (<6/12).
• Macular oedema, not requiring any further treatment, with good visual acuity (>6/12) can be deescalated to optometry for 3 monthly screening if proven to be stable by 3 ophthalmological examinations with 6 months interval of each other, after discussion with patient to understand the importance of on-going follow up.
• Severe NPDR (Pre-proliferative diabetic retinopathy)
• Not suitable for optometry care.
• Refer to ophthalmologist within 4 weeks of diagnosis
• Initiate treatment. At a time point where no changes have been observed on 3 examinations with 6 months intervals between examinations, stable non-high risk* severe NPDR with good HBA1C control is suitable for co-management with optometry for 2 monthly screening, and ophthalmological review 4 monthly.
• Re-escalate to ophthalmology if macular oedema or any neo-vascularisation is seen on slit lamp or OCT
• Proliferative diabetic retinopathy
• Not suitable for optometry care.
• Urgent ophthalmology referral within 1 week
• Initiate treatment (pan-retinal photocoagulation and/or intravitreal injection). Once quiescence has been established on 3 examinations with 6 months intervals between examinations, after completion of adequate pan retinal photocoagulation, refer for co- management with optometry for 2 monthly screening and ophthalmological review 4 monthly.
• Re-escalate to ophthalmology if macular oedema, pre-retinal/vitreous haemorrhage, or any neo-vascularisation is seen on slit lamp or OCT. *See Appendix A for factors that attribute to higher risk diabetic retinopathy.
• Study design
This study is an exploration of the non-inferiority of novel protocols for shared care referral pathways. The study will collect qualitative and quantitative data generated on electronic health care records for patients, grouped based on care pathway. Stratified sampling will be used to identify existing patients whose treatment history have reasonably aligned or differed to the proposed guidelines to assess a baseline level of care. Subsequently systematic sampling will be used to follow all patients undergoing shared treatment between Royal Melbourne Hospital (RMH) and University of Melbourne Eyecare Clinic (MEC) for diabetic retinopathy management. Eligible participants will be selected over a one-year period then followed up over a two-year period. Patient co-morbidities and treatment outcomes will be collected from health care records to establish the non- inferiority of outcomes for patients following the novel guidelines, and to establish statistically significant factors that may impact patient outcomes or necessitate amendment to the guidelines. After the completion of the study, the GRADE system [21] will be used to re-evaluate the guidelines and propose an empirically validated set of guidelines that may be suitable for adoption by other institutes in high resource nations.
• Guideline scope
Existing shared care guidelines for DR already clearly indicate when care should be escalated from primary care to ophthalmology. These novel components of these guidelines focus on the de-escalation of care from tertiary settings, back to primary care. The guidelines included in this paper are components most relevant to ophthalmology care, with some components relevant solely to primary care being partially omitted. This has been done for clarity, as novel protocols have only been generated for ophthalmology relevant referrals. The additional guidelines that have not been included can easily be reincorporated in the future, to create a more comprehensive guideline.
• Setting
The research will be conducted at RMH and allied MEC. These sites have the advantage of shared electronic medical records, which will be beneficial for the completion of data collection for patients following a shared health care plan. Data obtained from EMR will be manually input onto REDcap for statistical analysis. Additional responsibilities for statistical analysis may be further outsourced to external statisticians based on availability. The data collection period may be extended beyond the indicated timeline if initial statistical analysis justifies the need for additional participants.
• Study population
Eligible participants will be type 1 or type 2 diabetics diagnosed with quiescent mild – severe NPDR or PDR, who have been de-escalated from RMH ophthalmology to MEC over a one-year period, then subsequently been followed up over a two-year period. Additionally, retrospective analysis of patient files from the years 2018-2020 will be employed to create a statistical baseline for comparison of patient outcomes. Participants will be de-identified, stratified based on comorbidities, and outcomes assessed. Any patients lost to follow up will be excluded from analysis.
• Comorbidities
Multiple comorbidities have been associated with increased risk of progression of diabetic retinopathy [12,13,21]. To investigate if these variables warrant alternative referral pathways, patient outcomes will be mapped to several factors to identify significant correlations. These patient factors will be:
1. Diabetes Type 1 or type 2
2. Age
3. Gender
4. Aboriginal and Torres Strait Islander patients
5. Years since diagnosis of diabetes
6. HBA1c greater than 6.5% at time of de- escalation
7. Systolic blood pressure>140 mmHg at time of de-escalation
8. Smoking status
9. Non-healing ulcers, and or nephropathy
10. Macular oedema+/-
11. Visual acuity at time of de-escalation
12. PRP or intravitreal injection
• Outcomes
These guidelines aim to decrease the patient burden to ophthalmologists without compromising patient outcomes. The guidelines aim to achieve this goal by reducing the per patient follow up appointments to tertiary care ophthalmology clinics, for patients not requiring intervention. To this end, the outcomes assessed by the study will be: total number of visits per patient to ophthalmology, progression of diabetic retinopathy, visual acuity, and rate of sudden vision loss (visual acuity <6/60). Visual acuity will be assessed via ETDRS acuity testing at baseline and at the two-year mark. Retinopathy progression and rates of sudden vision loss will be compared to practice prior to the novel guideline’s implementation. Success of these guidelines will be demonstrated by decreasing the number of ophthalmological visits, thus decreasing clinical ophthalmology burden, without a significant increase to rates of progression, reduced visual acuity, or vision loss. Secondary outcomes will aim to identify statistically significant patient subgroups that may benefit from unique referral pathways. The demographics that will be analysed for this purpose have been listed under the Comorbidities section of this paper. Statistical analysis will identify correlations between comorbidities and changes in primary outcomes.
• Timeline
Ethics approval submission will commence in early 2022. Retrospective analysis of existing electronic records will be completed once ethic approval has been granted. Eligible participants will be identified throughout the year of 2022. Data collection will commence in January of 2023 for an interim analysis to identify trends in outcomes and assess the safety of continuing the study. If there is no evidence of poorer outcomes compared to baseline, data collection will conclude in January of 2024 to assess the outcomes of participants over a two-year period from initial de-escalation from ophthalmology. Data collection and statistical analysis will be complete by March 2024. Write up of findings will be completed by April 2024 and submitted for publication.
• Statistical analysis
Data is to be analyzed using IBM SPSS statistics. Differences between groups will analysed by Independent T-tests and Mann-Whitney tests for ordinal/continuous variables and Chi-Square for categorical variables. Binary logistic regressions will be performed to determine the association between groups in accordance with guidelines and groups not-accordance to guidelines and outcomes. Post hoc analysis will be used to determine a suitable number of participants to include for modelling and may justify an extension of the data collection period. Models first will be performed unadjusted, and then adjusted for potential confounders. Statistical significance is set as p<0.05 a priori. At the one-year mark of this study, an interim analysis will be performed (as described above) to assess the general trends of patient outcomes in comparison to baseline. This analysis will be conducted to compare patient outcomes, assess the safety of the new guidelines, and consider early withdrawal of the study if outcomes are indicated to be significantly inferior. We hypothesise that implementation of these new guidelines will cause a statistically significance reduction in ophthalmological consults per patient in comparison to baseline. We additionally hypothesise that no statistical significance will be identified between the mean change in patient’s visual acuity or rates of sudden vision loss over a two-year period, in comparison to baseline.
• Ethics considerations
Ethics approval will be submitted to The Royal Melbourne Hospital Human Research Ethics Committee (HREC), as a low-risk study. This study poses minimal risk to participant’s risks to participants. Participant’s data will be deidentified and encoded on secure networks leaving little privacy risk. In addition, the data collected will mostly be of a non-sensitive nature further mitigating this risk. If in the extremely unlikely circumstance that the rates of sudden vision loss, or disease progression, in participants following the new guidelines are found to be significantly increased compared to baseline outcomes, the study will be immediately halted to undergo early analysis.
• Limitations
A significant logistical limitation of this protocol is the three-year duration required to assess the impact of the novel guidelines. Despite the long duration, this period may still be too short to accurately map the progression of patients through the shared care system.
Due to the impact of the Covid-19 pandemic, in the year 2020 and onwards, access to healthcare may be more restricted. As a baseline for comparison is made from analysis of the years 2018-2020, the estimated impact of the new guidelines may be exaggerated due to external factors.
Although the allied care between RMH and MEC supports patient care, this system may be a poor representation of external healthcare providers due to the high competency of the sites. These sites have a significant history of cooperation, and collaboration that may not be representative of other healthcare providers’ relationships. This could potentially be a cause for poor replicability at other sites compared to what is observed in this study.
Methodology
RMH ophthalmology and MEC are uniquely poised by their integrated EMR system, and shared care practices for an extended follow up of patients. Shared care referrals are already common practice; however, de-escalation guidelines are not available in the literature.
Risk of sudden vision loss for untreated patients in the lowest risk group of DR remains as high as 3.6% over a two-year period [10], thus a two- year follow up has been deemed sufficient to demonstrate trends of outcomes within the initial stages of treatment following the novel elements of the guidelines. Additional investigation may still be insightful in the future, to demonstrate the longer-term impact of these guidelines on patients.
Although the Covid-19 pandemic may impact patient access to healthcare, patients were still permitted to attend medical appointments. In the analysis of the data collected for this study, trends should be viewed in the context of the years in which they occurred. The influence of the pandemic on the results is difficult to estimate, so it will be important that the data when analysed is considered contextually.
The comorbidities indicated above are known risk factors for the progression of diabetic retinopathy, thus may demonstrate requirements for more intensive supervision by ophthalmology. Accounting for these variables may identify certain populations that may require alternate referral pathways to those recommended more generally. The methodology above aims to identify these groups and allows for the flexibility to modify the guidelines to support their outcomes.
This study is a low-cost model for evaluating the efficacy of clear guidelines for shared care between ophthalmology and optometry. The novel guidelines, existing in tandem with existing guidelines, would further standardize the progression of care for patients with DR. The results of this study will serve to better guide professionals to share care and save healthcare resources in a patient safe manner.
Results
Guidelines for the screening of DR in diabetic patients are well prescribed [9,10,17-20], and are mainly performed by optometrists [5,14]. DR can be categorized by The International Clinical Diabetic Retinopathy and Diabetic Macula Edema Disease Severity Scale [17], as represented in Table 1. The National Health and Medical Research Council (NHMRC) guidelines [9] recommend routine screening of DR via dilated fundus examination and bestcorrected visual acuity at time of diagnosis of diabetes, with additional future screenings if no DR is detected [9,15]. Existing guidelines prompt ophthalmology referral based on the grade of DR detected [9,10,17-20]. Screening and referral guidelines have been represented in Table 2.
| Grading |
|
|---|---|
| None | No apparent retinopathy |
| Mild NPDR | The presence of a few micro aneurysms |
| Moderate NPDR | Micro aneurysms, intra retinal haemorrhages or venous beading that do not reach the severity of severe NPDR |
| Severe NPDR | Haemorrhages are present in all 4 quadrants, 2 quadrants or more have venous beading, or one or more quadrant has intra retinal microvascular abnormalities. |
| PDR | Neovascularization of the disc, retina, iris, or angle. Or vitreous haemorrhage or tractional retinal detachment |
| Oedema | Presence and severity of diabetic macula oedema is classified separately and is typically described as present or absent |
Table 1: The international clinical diabetic retinopathy and diabetic macula edema disease severity scale. Assessment is made with some combination of slit lamp bio microscopy, grading stereoscopic macular photographs, and optical coherence tomography.
| Stage of diabetic retinopathy | Recommended guidelines |
|---|---|
| Screening |
|
| Non-detectable retinopathy |
|
| Mild |
|
| Moderate |
|
| Severe |
|
| Proliferative diabetic retinopathy |
|
Table 2: Aggregated guideline from the NHMRC, the royal college of ophthalmologists, the nice guidelines, and optometry Australia, for diabetic retinopathy screening and referral indications.
Referral from ophthalmology to optometry for stable non-proliferative diabetic retinopathy or treated, quiescent proliferative DR, is common practice to reduce clinical burden. To this author’s knowledge, few guidelines exist to structure the de-escalation pathways of patients from ophthalmology, back to primary care. Such guidelines would likely be useful in standardizing clear recommendations for patient management.
Discussion and Conclusion
Shared care of patients between ophthalmology and primary healthcare providers, for diabetic retinopathy, is well supported by existing literature. However, a significant gap still exists for empirically supported prescriptions for the de-escalation of care from ophthalmology back to primary providers. Each individual ophthalmologist uses their own judgment when deciding which patients are fit to be de-escalated from their care. The novel additions to existing guidelines recommended by this protocol aim to rectify and standardize this procedure. This protocol is posed to critically assess the efficacy of these guidelines in a clinical setting, to demonstrate a non-inferiority to standard care, with fewer tertiary healthcare visits. Demonstrated efficacy of these recommendations would provide clear clinical guidelines to facilitate fewer patient ophthalmology visits with no increased progression of retinopathy or blindness.
Acknowledgement
I would like to thank "The Royal Melbourne Hospital and University of Melbourne for their fostering of their research".
Funding
There are no external funding arrangements
References
- Wallick CJ, Hansen RN, Campbell J, et al. Comorbidity and Health Care Resource Use Among Commercially Insured Non-Elderly Patients with Diabetic Macular Edema. Ophthalmic Surg Lasers Imaging Retina. 46(7):744-51 (2015).
- Pandova M. Diabetic Retinopathy and Blindness: An Epidemiological Overview. IntechOpen, London, UK (2019).
- Deloitte Access Economics Pty Ltd. The Economic Impact of Diabetic Macular Oedema in Australia. Bayer Australia Ltd, Pymble, Australia (2015).
- Ruta LM, Magliano DJ, Lemesurier R, et al. Prevalence of Diabetic Retinopathy in Type 2 Diabetes in Developing and Developed Countries. Diabet Med. 30(4):387-98 (2013).
- Vujosevic S, Aldington SJ, Silva P, et al. Screening for Diabetic Retinopathy: New Perspectives and Challenges. Lancet Diabetes Endocrinol. 8(4):337-347 (2020).
- Maatouk CM, Hentati F, Urbano CA, et al. Effects of Quality Improvement Education in Diabetic Retinopathy on Routine Clinical Practice Patterns of Optometrists. Optom Vis Sci. 97(11):936-943 (2020).
- O'Connor PM, Harper CA, Brunton CL, et al. Shared Care for Chronic Eye Diseases: Perspectives of Ophthalmologists, Optometrists and Patients. Med J Aust. 196(10): 646-50 (2012).
- Wang W, Lo ACY. Diabetic Retinopathy: Pathophysiology and Treatments. Int J Mol Sci. 19(6):1816 (2018).
- Mitchell P. Guidelines for the Management of Diabetic Retinopathy. National Health and Medical Research Council. Canberra, Australia (2008).
- Ghanchi F. Diabetic Retinopathy Guidelines. The Royal College of Ophthalmologists, London, UK (2012).
- Spooner KL, Guinan G, Koller S, et al. Burden of Treatment Among Patients Undergoing Intravitreal Injections For Diabetic Macular Oedema In Australia. Diabetes Metab Syndr Obes. 12:1913-1921 (2019).
- Harris Nwanyanwu K, Talwar N, Gardner TW, et al. Predicting development of proliferative diabetic retinopathy. Diabetes Care. 36(6):1562-1568 (2013).
- Liu Y, Yang J, Tao L, et al. Risk Factors of Diabetic Retinopathy and Sight-threatening Diabetic Retinopathy: A Cross-sectional Study of 13 473 Patients with Type 2 Diabetes Mellitus in Mainland China. BMJ Open. 7(9):e016280 (2017).
- Ting D, Ng J, Morlet N, et al. Diabetic Retinopathy-screening and Management by Australian GPs. Aust Fam Physician. 40(4):233-238 (2011). CrossRef
- Hanna S. Optometry Australia Diabetes Guidelines Working Group. Optometry Australia-Guidelines on the examination and management of patients with diabetes. Clin Exp Optom. 99(2):120-126 (2016).
- Liew G, Mitchell P, Wong TY. Systemic Management of Diabetic Retinopathy. BMJ. 338:b441(2009).
- Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of Diabetic Retinopathy and Diabetic Macular Edema. World J Diabetes. 4(6):290-294 (2013).
- Royal Australian and New Zealand College of Ophthalmologists. RANZCO: Patient Screening and Referral Pathway Guidelines for Diabetic Retinopathy (Including Diabetic Maculopathy). RANZCO, Australia (2019).
- Optometry Australia. Examination and Management of Patients with Diabetes South Melbourne, Australia (2018).
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ. 336(7650):924-926 (2008).
- Klein R, Klein BE. Blood Pressure Control and Diabetic Retinopathy. Br J Ophthalmol. 86(4):365-367 (2002).