Research Article - Journal of Experimental Stroke & Translational Medicine (2010) Volume 3, Issue 2
Enhanced angiogenesis and reduced infarct size by vascular endothelial growth factor D is not translated to behavioral outcome in a rat model of ischemic stroke
- *Corresponding Author:
- Dr. Jukka Jolkkonen
Institute of Clinical Medicine - Neurology
University of Eastern Finland
P. O. Box 1627, Yliopistonranta 1 C
70211 Kuopio, Finland
Tel: +358-40-3552519
Fax: +358-17-162048
E-mail: Jukka.Jolkkonen@uef.fi
Abstract
Vascular endothelial growth factors (VEGF) induce angiogenesis in experimental stroke models. Robust angio-genic response by VEGF, however, initially creates leaky vessels leading to edema, which may compromise functional recovery. The aim of the present work was to develop adenoviral and baculoviral gene delivery of VEGF-D, a novel member of the VEGF family, to enhance long-term angiogenesis and functional recovery in a photothrombotic model of stroke in rats. Treatment effects were assessed by histology and behavioral testing. The lesion size in the adeno-VEGF-DΔNΔC (p< 0.01) and adeno-VEGF-D (p< 0.05) groups was substantially re-duced compared to vehicle controls. The short form of VEGF-D significantly increased the number of newly formed vessels in the medial (p< 0.001) and lateral cortex (p< 0.05) compared to vehicle controls. Baculo-VEGF-DΔNΔC treated rats showed significant effect in the medial cortex (p< 0.01). The limb placing test showed transient impairment in all virus treated groups compared to the vehicle group at the acute phase 2 and 4 days after operation (p< 0.05). The cylinder test revealed a significant limb-use asymmetry in all ischemic animals at day 7, 14 and 21 after ischemia, which was not affected by VEGF-D treatment. In conclusion, viral mediated gene transfer of VEGF-D induced angiogenesis and reduced infarct size, which were, however, not translated to improved functional outcome in the stroke model used.
Keywords
Therapeutic vascular growth; VEGF-DΔNΔC; cerebral ischemia; neuroprotection; rat
Introduction
Cerebral ischemia remains one of the main causes of death, which is surpassed only by heart disease and cancer. The World Health Organization estimates that in 2002 there were over 20.5 million strokes worldwide, 5.5 million of which were fatal. More than 50% of stroke patients are left with a motor disability and stroke causes more loss of quality-adjusted life years (QALY) than any other disease in the western countries (Donnan et al. 2008). The current aging population will result in a dramatic increase in these numbers in the next two decades.
Stroke has long been considered untreatable and there is no effective drug therapy to help stroke pa-tients during the acute phase except thrombolysis, which however is available only for a small fraction of patients. A different approach for helping stroke patients is to promote functional recovery. Various de-grees of spontaneous recovery following weeks or months after an ischemic event are observed in clini-cal practice and have been related to brain repair mechanisms such as neuronal sprouting, neurogene-sis and angiogenesis (Martinez-Vila et al. 2005). Sti-mulating the formation of new blood vessels opens a new horizon for neurorestoration that aims to en-hance brain perfusion, rescuing hypoxic neurons and promoting plasticity and neurological recovery (Chen and Chopp 2006).
Angiogenesis is induced following cerebral ischemia through a variety of growth factors including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and hepatocyte growth factor (HGF) (Ruiz de Almodovar et al. 2009; Shibuya 2009; Storkebaum et al. 2004). All members of the VEGF family exert their effects by binding to tyrosine ki-nase receptors (VEGFR) on the cell surface. Particu-larly, VEGF-A is a powerful mediator to increase an-giogenesis and neovascularization by binding to its primary receptor VEGFR-2 (Tammela et al. 2005). However, vessels induced by VEGF-A overexpres-sion are leaky, which leads to edema or hemorrhage, that may compromise functional recovery (Jolkkonen et al. 2007; Yu et al. 2007). Thus, other members of the VEGF family such as VEGF-D may be safer for therapeutic angiogenesis after stroke.
VEGF-D is a secreted glycoprotein that induces both angiogenesis and lymphangiogenesis. It consists of a central domain, containing binding sites for VEGFR-2 and VEGFR-3, and N- and C-terminal propeptides (Achen et al. 1998). The natural cell product of VEGF-D is a homodimer of the full-length form that can be proteolytically processed to remove the pro-peptides into VEGF-DΔNΔC (Brockington et al. 2004; Sun et al. 2003). The mature form of VEGF-D has many times greater affinity for VEGFR-2 binding than the unprocessed one (McColl et al. 2007). While pro-teolytically processed VEGF-D has high affinity for binding to both VEGFR-2 and VEGFR-3, the full-length VEGF-D binds with high affinity only to VEGFR-3.
The aim of the present study was to evaluate the an-giogenic potential of VEGF-D, a member of the VEGF gene family, after differential transduction of the brain by adenoviral and baculoviral gene delivery, in a photothrombotic model of stroke in rats. Delayed expression of VEGF-D and particularly its short processed form was expected to improve the forma-tion of functional and stable blood vessels, which translates to behavioral improvement.
Methods
Animals
Eighty-four male Wistar rats (6 months, 385-503 g) were used in the study. The animals had free access to food and water and were housed in individual cag-es in a temperature-controlled environment (20±1°C) with lights on from 7.00 to 19.00 h. Experimental pro-cedures were conducted in accordance with the Eu-ropean Community Council directives 86/609/EEC and the study was approved by the Ethics Committee of the University of Eastern Finland and the Provin-cial Government of Kuopio.
Cortical photothrombosis
Cortical photothrombosis was induced by focusing light to the sensorimotor cortex in Rose Bengal treated rats (Watson et al. 1985). Briefly, the rats were anesthetized with 5% halothane in 30% O2/70% N2O and placed in a stereotactic frame. The anesthe-sia was maintained during the operation with 1-2% halothane delivered by a nose mask and the body temperature was kept at 37 oC by a rectal probe and heating pad. The skull was exposed and a cold white light (Olympus, Denmark) with a 4 mm aperture was positioned onto the exposed skull 0.5 mm anterior to the bregma and 3.7 mm lateral to themidline over the right motor cortex. The photochemical dye Rose Bengal (Sigma) was infused into the femoral vein via a microinjection pump within 2 min (20 mg/kg), after which the light was turned on for 10 min. The skull surface temperature was monitored with a probe placed between the skull and the light source, and kept constant by cool air flow. Sham-operated ani-mals were treated similarly, but the light was not turned on. The rats were removed from the frame, sutured, and allowed to wake up in an incubator (32 °C) before being returned to their home cages.
Vector preparation and characterization
Adenovirus. Replication-deficient human clinical-grade E1- partial E3-deleted first-generation adenovi-ruses encoding human VEGF-DΔNΔC and lacZ under the cytomegalovirus (CMV) promoter were con-structed by homologous recombination and produced in 293 cells as described previously (Hiltunen et al. 2000; Laitinen et al. 1998; Mäkinen et al. 2002).
Baculovirus. All entry clones were completely se-quenced to verify the sequences before cloning of VEGF-DΔNΔC into a pBVboostFGII expression vector using the BVboost system LR-reaction (Laitinen et al. 2005). Avidin displaying baculoviruses (Räty et al. 2004) encoding for VEGF-DΔNΔC (BAAVI-VEGF-DΔNΔC) were generated by using an improved Bac-to-Bac method (Airenne et al. 2003). For the Baculo-lacZ virus, a nuclear targeted β-galactosidase (βnt-Gal) cassette with a CMV promoter was inserted into the StuI site of transfer vector pFASTBac1 (pFB) (Gibco BRL, Life Technologies), generating plasmid pFBCMV- βnt (Airenne et al. 2000). Purification, con-centration, and titration of viral particles were per-formed as described previously (Airenne et al. 2000). Virus preparations were tested for sterility and ana-lyzed for lipopolysaccharide and mycoplasma conta-mination.
Gene transfer
After photothrombotic operation a burr hole was drilled and 10 μl of viral suspensions (Adeno-VEGF-D (n=10), Adeno-VEGF-DΔNΔC (n=13), Adeno-LacZ (n=10), Baculo-VEGF- DΔNΔC (n=15), Baculo-LacZ (n=14)) containing 2x1010 p.f.u/ml were injected into the left ventricle (AP: -0.8 mm, L: 1.5 mm, V: 3.5 mm) at a rate 0.1 μl per minute. The needle was with-drawn after 25 minutes of injection. Control animals received the same amount of saline.
Behavioral testing
The behavioural tests selected for the study are sen-sitive to detect treatment effects. The animals were tested before operation and on postoperative days 2, 4, 7, 14 and 21. Behavioural testing was carried out by a person blind to treatment.
Limb-placing test. The limb-placing test was used to assess the sensorimotor integration of fore- and hindlimb responses to tactile and proprioceptive stimulation (De Ryck et al. 1989; Jolkkonen et al. 2000). The test has seven limb placing tasks, which are scored: 2 points, the rat performs normally; 1 point, the rat performs with a delay (> 2 sec) and/or incompletely and 0 points, the rat does not perform normally. The both sides of the body are tested. In the first task the rat is suspended 10 cm above the table surface. Non-lesioned rats stretch both fore-limbs towards the table. On the second task the rat is held facing towards the table, resting its forelimbs on the table edge. The forelimb is gently pulled down, from the table, and subsequent retrieval and limb placement is checked. Non-lesioned rats replace both limbs on the table. The third task is the same as the second, except that the rat is not able to see the table or make vibrissal contact, since the head is held upward at a 45° angle. The rats are next placed along the table edge to check the lateral placing of the fore- (the fourth task) and hindlimbs (the fifth task). The limbs are pulled down, as described in task 2, and limb retrieval is scored accordingly. In the sixth task the rat are placed with their rear end at the edge of the table, with the hindlimbs resting on the table edge. The hindlimbs, 1 at a time, are gently pulled down and from the table. If necessary, retrieval of the limb to the original resting place on the table edge can be stimulated by pushing the animal to-wards the table edge. In the seventh task the rat is placed at the table edge, facing away from the table surface. The forelimbs of the rat are placed on the edge of the table, and the rat is gently pushed from behind toward the edge. Injured rats cannot keep their grip and the injured limb slips off the edge.
Cylinder test. The cylinder test was used to assess imbalances between the impaired and non-impaired forelimb use (Schallert and Woodlee 2005). For the test, the rat is placed in a transparent cylinder (ø 20 cm) and videotaped during the light part of the light:dark cycle before operation and at postoperative days 7, 14, and 21. A mirror was placed at 45° angle beneath the cylinder so that behavior could be filmed from below the cylinder. Exploratory activity for 1 to 3 min was analyzed by using a video recorder with slow motion capabilities. The number of contacts by both forelimbs and by either the impaired or nonim-paired forelimb was counted. The cylinder score for the impaired forelimb was calculated as: (impaired forelimb + ½ x both forelimbs)/(total contacts) x 100%.
Immunohistochemistry
Rats were perfused transcardially 21 days after oper-ation with 0.9 % NaCl followed by 4 % paraformalde-hyde in 0.1 M phosphate buffer, pH 7.4. The brains were removed from the skulls, postfixed, and cryo-protected. Frozen sections (35 μm) were cut with a microtome and stored in a cryoprotectant tissue-collection solution at –20 °C. Platelet-endothelial cell adhesion molecule-1 (PECAM-1) was stained as a marker for blood vessels from five randomly selected rats in each experimental group. The sections were pretreated for 30 minutes with hot (85 °C) citrate buf-fer after which the sections were transferred to a so-lution containing the primary antibody (rabbit anti-PECAM-1 at 1:1000, Santa Cruz) and Tris-buffered saline with 5 % normal goat serum (NGS) and 0.5 % Triton X-100 (TBS-T). Following 18 hours of incuba-tion in this solution on a shaker table at room tem-perature (20 °C) in the dark, the sections were rinsed three times in 5 % NGS and TBS-T and transferred to a solution containing the secondary antibody (goat anti-rabbit*biotin at 1:500, Vector). After 2 hours, the sections were rinsed three times with 2 % NGS and TBS-T and transferred to a solution containing mouse ExtrAvidin® (Sigma) for 1 hour, and then incubated for approximately 3 minutes with Ni-enhanced diami-nobenzidine.
Evaluation of neovascularization
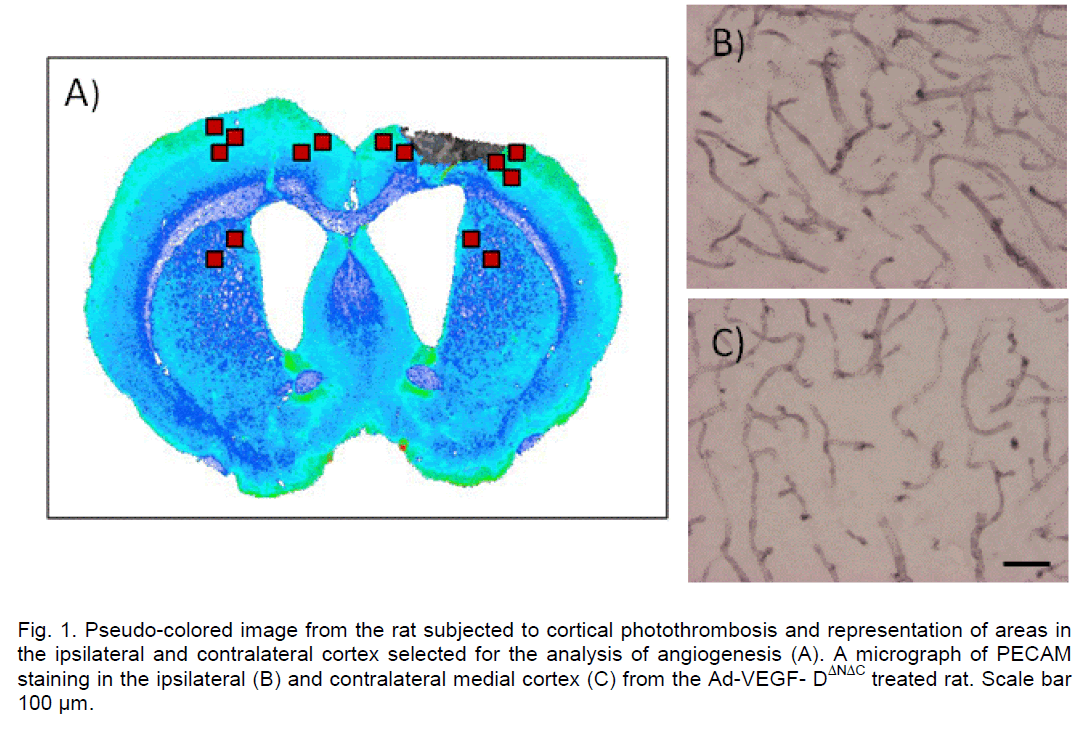
In order to quantify angiogenesis after cortical pho-tothrombosis, images (x 20 magnification) of the cor-tex lateral and medial the lesion and striatum were acquired using Olympus BX40 microscope and Olympus digital camera DP50-CU and image acquisi-tion software Viewfinder (Pixera Corporation). Images were digitally processed using Adobe® Photoshop® CS3. In each section the corresponding areas from the contralateral hemisphere were used as a control (Figure 1). PECAM-1 immunoreactivity in each image was determined using two image analysis systems. Images were analyzed initially with ImageJ, a Java-based image processing program, but in order to get reliable values, custom made software was adopted. It incorporated the color segmentation method in a MathWorksTM Matlab® 7.5 environment, modified for 3 channels so that vessels could be better distin-guished from the background. In comparison, ImageJ uses binary basis for quantification. The values cor-responding to total vessel coded color areas were averaged and expressed as the mean percentage of stained vessels per 100 μm2.
Assessment of infarct volumes
Nissl-stained sections were used for assessment of infarct volumes. Estimation of the infarct area in the cortex was performed on the sections digitalized un-der a × 20 objective (Olympus BX40) using a 3-CCD color video camera (Sony DXC-970MD) interfaced with an image analysis system (MCID, Imaging Re-search). The image of each section was taken as 1280 x 1024 matrix of calibrated pixel units. The digi-tal image was then displayed on a video screen and the lesion area was manually outlined. The total vo-lume was calculated by multiplying the infarct area by the distance between the sections and summing to-gether the volumes for each brain.
Statistical analysis
SPSS software was used for statistical analyses. Sta-tistical differences in infarct volumes in the cortex and blood vessel counts between groups were analyzed using one-way analysis of variance (ANOVA) fol-lowed by a post hoc test (Scheffe) when necessary. Differences in the limb-placing scores between ex-perimental groups were analyzed by Kruskal-Wallis followed by Mann-Whitney U-tests. Cylinder data for the overall group effect were analyzed using ANOVA for repeated measures. Comparisons between the groups were then made using one-way ANOVA fol-lowed by a post hoc test (Scheffe).
Results
Survival of rats
The cortical photothrombosis is generally characte-rized by a very low mortality. In the present experi-ment only two rats had to be sacrificed due to com-plications during the recovery period. There was no significant difference in body weight between sham-operated and ischemic control rats or between treated ischemic rats and vehicle controls.
Ischemic lesion
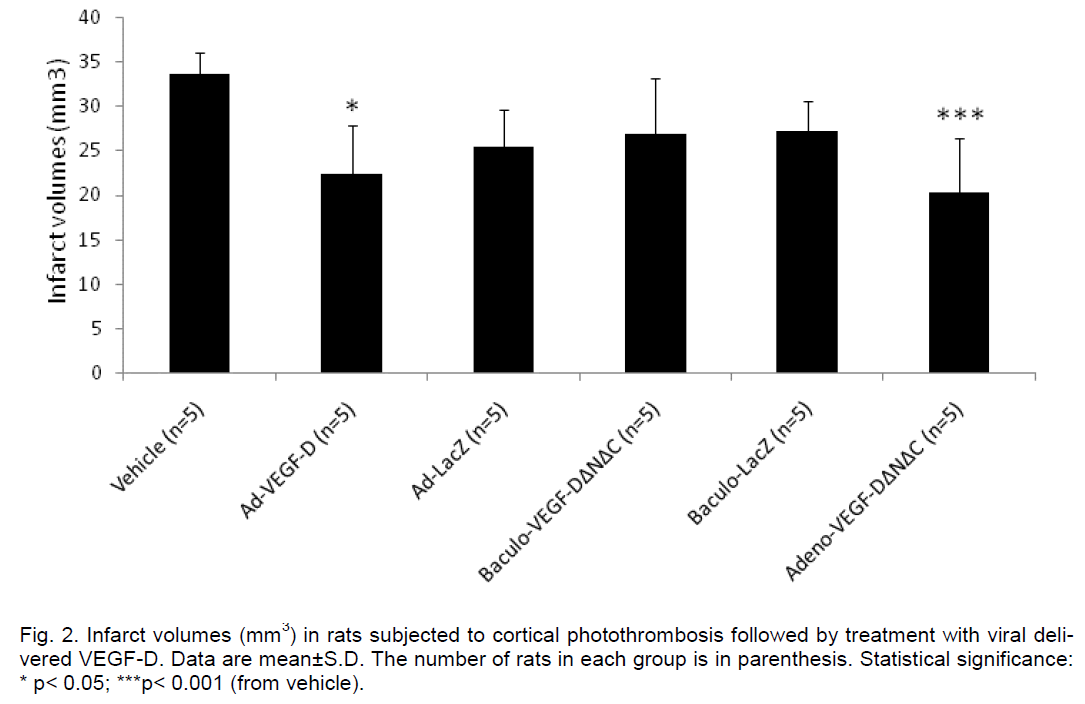
The cortical infarct was located in the frontal cortex (Fr1 and Fr2) and extended to the corpus callosum with no evidence of damage to the underlying stria-tum (Figure 1A). Resolution of the necrotic tissue re-sulted in the formation of a partly fluid-filled cyst by the end of the 3 week follow-up period. In the current settings the average size of the cortical lesion in ve-hicle controls was 33.6±2.4 mm3. The infarct sizes in the adeno-VEGF-DΔNΔC (20.4±6.1 mm3) (p< 0.01) and adeno-VEGF-D (22.4±5.5 mm3) (p< 0.05) groups were substantially reduced compared to vehicle con-trols (Figure 2). Baculo-VEGF-DΔNΔC group (26.9±2.4 mm3) was not different from vehicle controls.
Quantification of angiogenesis
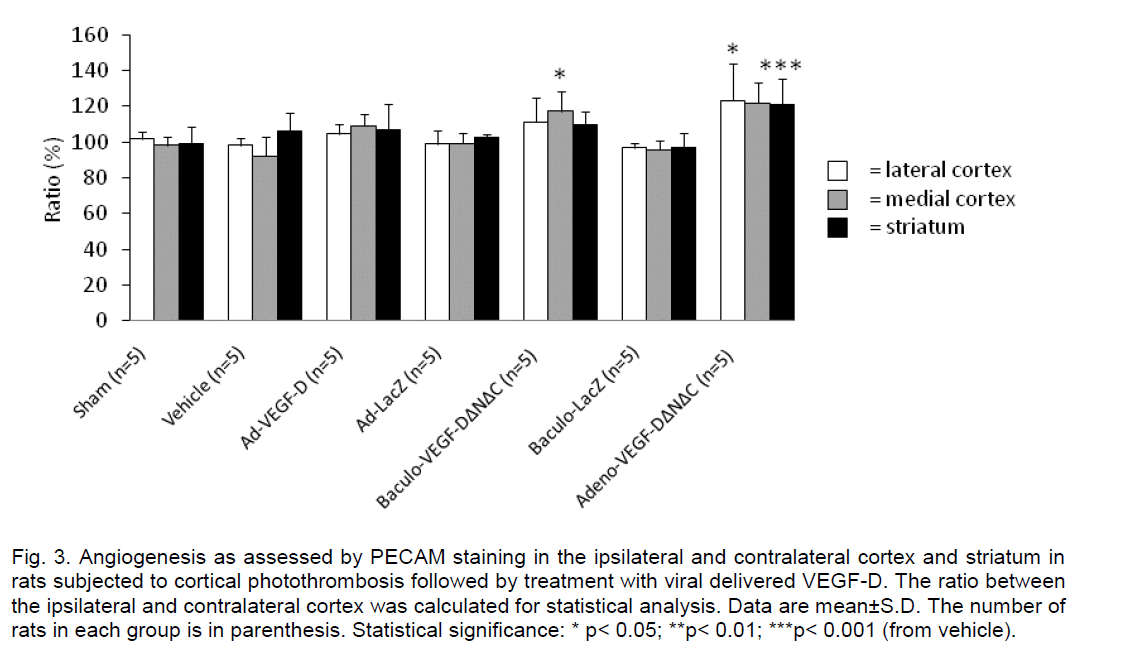
In order to determine whether intraventricular admin-istration of different forms of VEGF-D enhances ce-rebral angiogenesis, photothrombotic ischemic rats were treated with the viral constructs 30 minutes after ischemia. It was expected that delayed production of VEGF-D would surpass the peak of brain excitotoxici-ty and edema and show therapeutic effect on angi-ogenesis. Previous studies with VEGF-A have showed a peak of newly formed vessels 12 days after administration of viral vectors (Dellian et al. 1996). For vessel quantification, 7 distinct non-overlapping areas were selected from the ipsilateral hemisphere and the corresponding areas from the contralateral hemisphere (Figure 1A). The ratio of the two was then compared to that of the control group. The program used for vessel density quantification is novel in the sense that it distinguishes vessels much more accu-rately from the background staining as it incorporates the 3 color segmentation mechanism as compared for example to conventional NIH Image® software for Image Analysis (National Institutes of Health).
Compared to the vehicle controls, the short form of VEGF-DΔNΔC significantly increased the number of newly formed vessels in the medial (p< 0.001) and lateral cortex (p< 0.05) (Figure 3). Interestingly, baculo-VEGF-DΔNΔC did show significant effect only in the medial cortex (p< 0.01). The other vector construct, adeno-VEGF-D, which is a far less potent angiogenic factor than the proteolytically processed VEGF-D did not increase the angiogenic response significantly.
Behavioral testing
To determine whether VEGF-D and consequential angiogenesis improve functional recovery after pho-tothrombotic stroke, the behavioral performance of rats was tested during the 21 day follow-up. Rats in all the ischemia operated groups showed a significant sensorimotor impairment at the acute phase 2 days after stroke. Partial spontaneous recovery was ob-served in all groups.
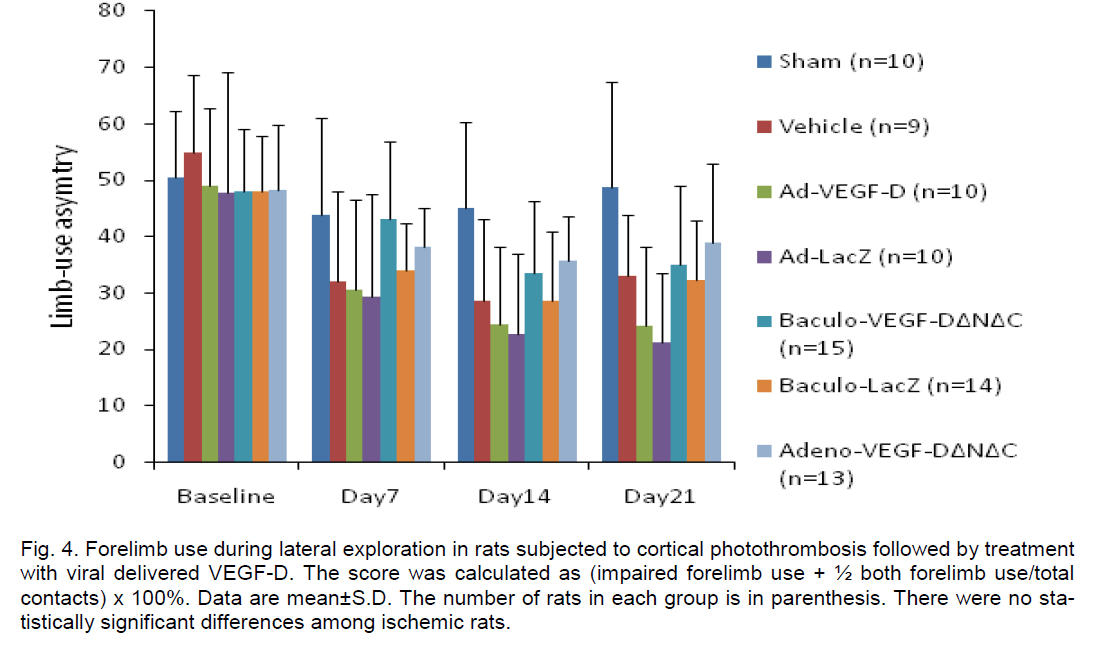
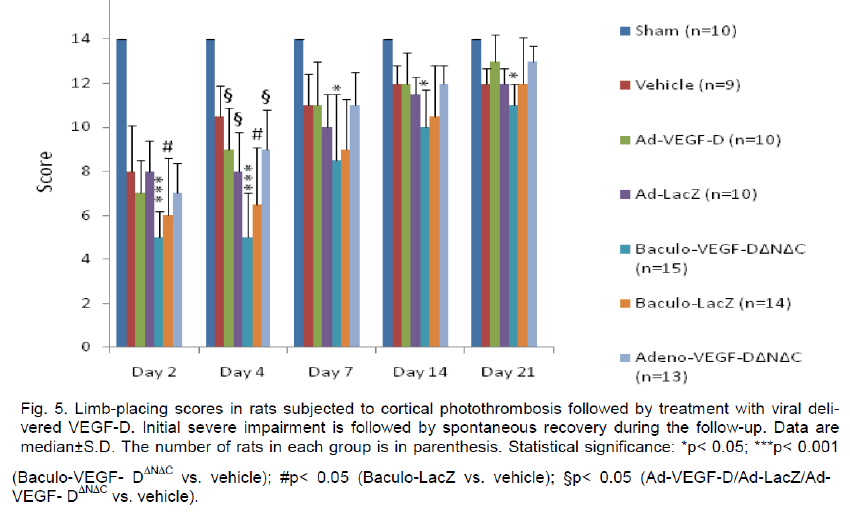
The limb placing test showed impaired performance in all virus treated rats compared to vehicle controls at 4 days (p< 0.05), however, this was transient and no difference was detected at 21 days except in the case of baculo-VEGF-DΔNΔC (Figure 4), which indicates that this was most likely due to some adverse effects of the viral treatment at the peak of gene expression (e.g., inflammatory response). All ischemic groups showed a reduced use of the impaired forelimb in the cylinder test at day 7, 14 and 21 after the infarct (Figure 5). ANOVA for repeated measures showed a signifi-cant group effect (p< 0.01) and a significant time x group interaction (p< 0.01). These were explained by the difference between sham-operated rats and ischemic groups.
Discussion
In the present study we evaluated the therapeutic potential of VEGF-D after ischemic stroke.
To our knowledge, there are no studies in the litera-ture that have evaluated the therapeutic potential of VEGF-D induced angiogenesis in experimental stroke. Several studies have suggested that VEGF-D and particularly its processed form are powerful angi-ogenic factors in muscle and heart tissues. Adenovir-al VEGF-DΔNΔC gene transfer in pig myocardium has been shown to induce a dose dependent protein pro-duction and promote angiogenic effects much more diffusely throughout the myocardial tissue as com-pared to VEGF-A165 (Rutanen et al. 2004). This dif-ference could be attributed to the soluble and freely diffusible VEGF-DΔNΔC while VEGF-A165 has good matrix-binding properties. In a rabbit hindlimb ische-mia model, VEGF-DΔNΔC produces the strongest an-giogenic effect among the members of the VEGF family (Rissanen et al. 2003). VEGF-DΔNΔC upregu-lates VEGFR-2 and αvβ3 integrin expression in endo-thelial cells, which suggest that angiogenic signaling mechanisms are very similar to those of VEGF-A (Ferrara 1999). With a higher dose of both VEGF-DΔNΔC and VEGF-A there is substantial edema forma-tion. However, VEGF-A induces greater edema as compared to VEGF-DΔNΔC after intramyocardial gene transfer (Rutanen et al. 2004), and might therefore be better as a pro-angiogenic candidate in therapeutic use.
Different transduction profiles of adenovirus and baculovirus in the CNS
Easy production and high gene deliver efficiency into immune-privileged sites such as those of the CNS make adenovirus and baculovirus positive candidates for a therapeutic delivery of VEGF-D. Moreover, they have different transduction patterns in the brain (Leh-tolainen et al. 2002). Baculoviruses were found to transduce the cuboid epithelium of the choroid plexus very efficiently (76.8±14%) whereas adenoviruses showed a marked preference for the ventricular ependymal cell lining (71.4±9%) and corpus callosum (83.5±11%). Transgene expression with ade-no/baculovirus starts as early as 6 h after transduc-tion and reaches peak at 3-6 days depending on the construct and the vector (Airenne et al. 2009; Russel 2000). Both viral vector types lead also to expression of the transgene in the brain microvessel endothelial cells (Lehtolainen et al. 2002). In accordance, a dif-ference in the angiogenic response between adenovirus and baculovirus was detected also in the present study.
Angiogenesis and VEGF-D
Here we demonstrated that adenovirus and baculovi-rus induced expression of VEGF-DΔNΔC significantly enhances cerebral angiogenesis ipsilaterally in a rat photothrombotic stroke model. The virus itself did not enhance angiogenesis, as there was no difference in vessel density ratios compared to the sham and ve-hicle treated animals. The advantage of transducing the ependymal cell lining and the choroid plexus by adenovirus and baculovirus, respectively, is that VEGF-D is a soluble protein and will be secreted into the cerebrospinal fluid. The vectors were not injected into the ischemic core, because the cells are affected by necrotic death and extensive edema a few hours after the onset of ischemia (Siesjö 1981; Taoufik and Probert 2008). There have been many studies in which the method of application of the therapeutic agent has been chosen to be direct parenchymal administration close to the lesion (Gunnett and Heis-tad 2001). Although this was a feasible delivery ap-proach especially in the photothrombotic model of stroke where the position of infarcted tissue is more or less constant, we expected that forced overpro-duction of VEGF-D would increase the burden on the already compromised brain environment in the peri-infarct site.
In our paradigm, VEGF-D protein disseminates through the cerebrospinal fluid circulation in the brain. Abundant VEGF-D delivery may also cause angioge-nesis in remote areas of the brain and possibly in the contralateral hemisphere. Nevertheless elevated le-vels of VEGFs, within a certain range, are not suffi-cient to produce a significant angiogenic response in the non-affected brain tissue. The formation of stable new blood vessels requires activation of brain repair mechanisms around the lesion as well as in some parts of the ipsilateral hemisphere functionally con-nected with the injury. For example, numerous deve-lopmental factors are re-expressed in the peri-infarct regions, which promote neurogenesis, synaptogene-sis and angiogenesis (Chopp and Li 2006). In this sense the density of VEGFR-2, which plays the major role in angiogenesis, increases within 48 h post- ischemia (Marti et al. 2000) and this is predominantly on ischemic neurons (Beck et al. 2002) and astro-cytes (Choi et al. 2007). Growth factors such as VEGF-A stimulate further overexpression of the VEGFR-2 (Wang et al. 2005).
It is interesting to point out that while VEGFR-3, the primary receptor for long unprocessed form of VEGF-D, was long believed to be responsible only for lym-phangiogenesis, new reports have emerged showing the compensatory function of VEGFR-3 signaling in angiogenesis (Tammela et al. 2008). Studies show that signaling through it promotes proliferation and survival of endothelial cells in culture (Goldman et al. 2007). Moreover, blocking this receptor appears to most prominently affect the number of sprouting and branching points of new blood vessels. In the expe-riments on tumor anti-angiogenesis, it became evi-dent that VEGFR-3 can drive angiogenesis even in conditions where VEGFR-2 is targeted therapeutical-ly to suppress its angiogenic signals. In our case the long form of VEGF-D did not produce a significant angiogenic response, but it reduced infarct size.
Reduction of infarct size by VEGF-D
The production of VEGF-D starts at 6 h post trans-duction, yet the formation of the first new stable blood vessels takes at least a few days, a time window sur-passing the rapid period in which the necrotic core expands (Chen et al. 1994). Thus, it is likely that the smaller lesion size in VEGF-DΔNΔC treated rats is a result of a mechanism other than true neuroprotec-tion. One possibility is that VEGF-D reduces the brain injury by protecting the vascular system in the peri-infarct site against ischemia. Indeed, VEGF-A has an important role in maintaining or restoring the integrity of endothelial cells (Radisavljevic et al. 2000). A second possibility is that VEGF-D has a potent growth and survival effect on neurons and vascular cells similar to VEGF-A (Chavakis et al. 2002; Nishi-jima et al. 2007; Rosenstein et al. 2003). Although there are some differences in the reports of the tem-poral course of gene induction as well as the identifi-cation of expressing cells, VEGF-A is increased after injury in the peri-infarct region as early as 1-3 h (Beck and Plate 2009; Hermann and Zechariah 2009) and persists for up to 1 month after ischemia (Hai et al. 2003). VEGFR-1 and VEGFR-2 expression is also elevated in neurons and endothelial cells of penum-bral areas beginning at 3 h, peaking at 24 h, and per-sisting for more than 7 days after ischemia (Brocking-ton et al. 2004). The VEGFR-2 signaling pathway that results in cAMP response element-binding protein (CREB) phosphorylation is the shared mechanism that underlies the preconditioning-mediated protec-tion of neurons and vascular endothelial cells in the neonatal rat brain (Lee et al. 2009). A further study on the matter suggested that VEGFR-2/CREB signal-ing is critical for VEGF-A-mediated protection in neu-rons and cerebral vascular endothelial cells (Lee et al. 2010). This could also contribute to our results. In-flammatory response as indicated by the increase of body temperature in all virus treated rats (data not shown), might also alter lesion size. And last, reduc-tion of infarct volume may be due to an enhanced removal process of debris due to angiogenesis in-duced by VEGF-D treatment.
Functional recovery
This is the first report on the effect of VEGF-D on functional recovery after focal cerebral ischemia in rats. Our study indicates that VEGF-DΔNΔC-induced peri-lesional angiogenesis is not obviously translated to functional recovery as assessed by sensorimotor tests. The recent literature on the possible therapeu-tic effects of VEGF in stroke is mixed (Manoonkiti-wongsa et al. 2004; Yang et al. 2010). Different expe-rimental models, timing of treatment, administration route, doses and outcome measures are likely to contribute to this. Inflammation caused by viral vec-tors may compromise beneficial treatment effects in the present study. In addition, in all the groups there is already a spontaneous functional recovery. Be-cause the current model produces only mild motor function impairment, it may be that the VEGF-D treatment is not sufficient to further boost the existing recovery mechanisms. Those effects on neurological outcome could be much better demonstrated in a model with more severe brain infarction such as MCAO. Another limitation of the current model is that the photothrombotic lesion does not leave enough salvageable brain tissue where the angiogenic factor could exert its restorative potential fully. Indeed, the penumbral area here is substantially smaller, and although there is expansion of the necrotic core, it is not as profound as in the model of MCAO (Watson et al. 1995; Witte et al. 2000). Another speculation for the behavioral test results could be that the timing of increased angiogenesis and of increased vessel den-sity may not be sufficient for a robust amelioration of neurological deficit. Angiogenesis is elevated in the peri-infarct area in the beginning of the recovery process, but it may need much more time to promote neurogenesis and plasticity in a functionally relevant manner. Although this seems a possible explanation, prolonged expression of angiogiogenic factors such as VEGF-D and VEGF-A could also be harmful. A more advanced approach to the problem would utilize Tet-controlled expression, where the transgene is expressed only in the presence of doxycycline (Gos-sen et al. 1995).
Conclusion
Here, we have tested the restorative potential of VEGF-D through angiogenesis in the area surround-ing an evolving brain infarct in an experimental stroke model. Increased angiogenesis and reduced infarct size were observed by viral gene delivery of VEGF-D, but this was not translated to behavioral outcome during the three week follow-up. Thus, in the acute and subacute phases after stroke, angiogenesis may have predominantly other functions such as removal of edema and/or necrotic tissue in the ischemic bor-der zone rather than promoting direct functional im-provement. Further research is needed to understand the mechanisms and the therapeutic value of angi-ogenic factors in enhancing functional recovery after stroke.
Acknowledgment
This study was supported by Finnish Academy, the Finnish Funding Agency for Technology and Innova-tion and Ark Therapeutics Ltd.
Conflict of Interest
None
References
- Achen MG, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks AF, Alitalo K, Stacker S A. (1998) Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF recelitor 2 (Flk-1) and VEGF recelitor 3 (Flt-4). liroc Natl Acad Sci USA 95:548–553.
- Airenne KJ, Hiltunen MO, Turunen Mli, Turunen AM, Laitinen OH, Kulomaa MS, Ylä-Herttuala S. (2000) Baculovirus-mediated lieriadventitial gene transfer to rabbit carotid artery. Gene Ther 7:1499-504.
- Airenne KJ, lieltomaa E, Hytönen Vli, Laitinen OH, Ylä-Herttuala S. (2003) Imliroved generation of recombinant baculovirus genomes in Escherichia coli. Nucleic Acids Res 31:e101.
- Airenne KJ, Mähönen AJ, Laitinen Olli H, Ylä-Herttuala S. (2009) Baculovirus-mediated gene transfer: An emerging universal concelit. In: Temlileton NS, editor. Gene and Cell Theraliy. Theralieutic Mechanisms and Strategies, New York: Taylor and Francis. li. 263-291.
- Beck H, Acker T, liüschel AW, Fujisawa H, Carmeliet li, lilate KH. (2002) Cell tylie-sliecific exliression of neuroliilins in an MCA-occlusion model in mice suggests a liotential role in liost-ischemic brain remodeling. J Neuroliathol Exli Neurol 61:339–350.
- Beck H, lilate KH. (2009) Angiogenesis after cerebral ischemia. Acta Neuroliathol 117:481-96.
- Brockington A, Lewis C, Wharton S, Shaw liJ. (2004) Vascular endothelial growth factor and the nerv-ous system. Neuroliathol Alilil Neurobiol 30:427-446.
- Chavakis E, Dimmeler S. (2002) Regulation of endo-thelial cell survival and aliolitosis during angi-ogenesis. Arterioscler Thromb Vasc Biol 22:887-93.
- Chen HH, Chien CH, Liu HM. (1994) Correlation be-tween angiogenesis and basic fibroblast growth factor exliression in exlierimental brain infarct. Stroke 25:1651-7.
- Chen J, Cholili M. (2006) Neurorestorative treatment of stroke: cell and liharmacological aliliroaches. NeuroRx 3:466–473.
- Choi JS, Kim HY, Cha JH, Choi JY, liark SI, Jeong CH, Jeun SS, Lee MY. (2007) Uliregulation of vascular endothelial growth factor recelitors Flt-1 and Flk-1 following acute sliinal cord contusion in rats. J Histochem Cytochem 55:821–830.
- Cholili M, Li Y. (2006) Translilantation of bone mar-row stromal cells for treatment of central nervous system diseases. Adv Exli Med Biol 585:49-64.
- Dellian M, Witwer Bli, Salehi HA, Yuan F, Jain RK. (1996) Quantitation and lihysiological characteri-zation of angiogenic vessels in mice: effect of ba-sic fibroblast growth factor, vascular endothelial growth factor/vascular liermeability factor, and host microenvironment. Am J liathol 149:59-71.
- De Ryck M, Van Reemlits J, Borgers M, Wauquier A, Janssen liA. (1989) lihotochemical stroke model: flunarizine lirevents sensorimotor deficits after neocortical infarcts in rats. Stroke 20:1383-90.
- Donnan GA, Fisher M, Macleod M, Davis SM. (2008) Stroke. Lancet 371:1612- 23.
- Ferrara N. (1999) Molecular and biological lirolierties of vascular endothelial growth factor. J Mol Med 77:527-43.
- Goldman J, Rutkowski JM, Shields JD, liasquier MC, Cui Y, Schmökel HG, Willey S, Hicklin DJ, liy-towski B, Swartz MA. (2007) Coolierative and re-dundant roles of VEGFR-2 and VEGFR-3 signal-ing in adult lymlihangiogenesis. FASEB J 21:1003-12. Erratum (2007) FASEB J 21:1942.
- Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H. (1995) Transcrilitional activation by tetracyclines in mammalian cells. Science 268:1766-9.
- Gunnett CA, Heistad DD. (2001) The future of gene theraliy for stroke. Curr Hyliertens Reli 3:36-40.
- Hai J, Li ST, Lin Q, lian QG, Gao F, Ding MX. (2003) Vascular endothelial growth factor exliression and angiogenesis induced by chronic cerebral hyliolierfusion in rat brain. Neurosurgery 53:963–970
- Hermann DM, Zechariah A. (2009) Imlilications of vascular endothelial growth factor for liosti-schemic neurovascular remodeling. J Cereb Blood Flow Metab 29:1620-43.
- Hiltunen MO, Laitinen M, Turunen Mli, Jeltsch M, Hartikainen J, Rissanen TT, Laukkanen J, Niemi M, Kossila M, Häkkinen Tli, Kivelä A, Enholm B, Mansukoski H, Turunen AM, Alitalo K, Ylä-Herttuala S. (2000) Intravascular adenovirus-mediated VEGF-C gene transfer reduces neoin-tima formation in balloon-denuded rabbit aorta. Circulation 102:2262–2268.
- Jolkkonen J, liuurunen K, Rantakömi S, Härkönen A, Haalialinna A, Sivenius J. (2000) Behavioral ef-fects of the alliha(2)-adrenocelitor antagonist, atiliamezole, after focal cerebral ischemia in rats. Eur J liharmacol 400:211-9.
- Jolkkonen J, Jokivarsi K, Laitinen T, Gröhn O. (2007) Subacute hemorrhage and resolution of edema in Rose Bengal stroke model in rats coincides with imliroved sensorimotor functions. Neurosci Lett 428:99-102.
- Mäkinen K, Manninen H, Hedman M, Matsi li, Mus-salo H, Alhava E, Ylä-Herttuala S. (2002) In-creased vascularity detected by digital subtrac-tion angiogralihy after VEGF gene transfer to human lower limb artery: a randomized, lilacebo-controlled, double-blinded lihase II study. Mol Ther 6:127-33.
- Laitinen M, Mäkinen K, Manninen H, Matsi li, Kossila M, Agrawal RS, liakkanen T, Luoma JS, Viita H, Hartikainen J, Alhava E, Laakso M, Ylä-Herttuala S. (1998). Adenovirus-mediated gene transfer to lower limb artery of liatients with critical leg ischaemia. Hum Gene Ther 9:1481–1486.
- Laitinen OH, Airenne KJ, Hytönen Vli, lieltomaa E, Mähönen AJ, Wirth T, Lind MM, Mäkelä KA, Toi-vanen liI, Schenkwein D, Heikura T, Nordlund HR, Kulomaa MS, Ylä-Herttuala S. (2005) A mul-tiliurliose vector system for the screening of libra-ries in bacteria, insect and mammalian cells and exliression in vivo. Nucleic Acids Res 33:e42.
- Lee HT, Chang YC, Tu YF, Huang CC. (2009) VEGF-A/VEGFR-2 signaling leading to cAMli reslionse element-binding lirotein lihoslihorylation is a shared liathway underlying the lirotective effect of lireconditioning on neurons and endothelial cells. J Neurosci 29:4356-4368.
- Lee HT, Chang YC, Tu YF, Huang CC. (2010) CREB activation mediates VEGF-A's lirotection of neu-rons and cerebral vascular endothelial cells. J Neurochem, in liress
- Lehtolainen li, Tyynelä K, Kannasto J, Airenne KJ, Ylä-Herttuala S. (2002) Baculoviruses exhibit re-stricted cell tylie sliecificity in rat brain: a comliar-ison of baculovirus- and adenovirus-mediated intracerebral gene transfer in vivo. Gene Ther 9:1693-9.
- Manoonkitiwongsa liS, Schultz RL, McCreery DB, Whitter EF, Lyden liD. (2004) Neurolirotection of ischemic brain by vascular endothelial growth factor is critically deliendent on lirolier dosage and may be comliromised by angiogenesis. J Ce-reb Blood Flow Metab 24:693-702.
- Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, lietit E, Risau W. (2000) Hylioxia-induced vascu-lar endothelial growth factor exliression lirecedes neovascularization after cerebral ischemia. Am J liathol 156:965–976
- Martínez-Vila E, Irimia li. (2005) Challenges of neu-rolirotection and neurorestoration in ischemic stroke treatment. Cerebrovasc Dis 20 Sulilil 2:148-58.
- McColl BK, liaavonen K, Karnezis T, Harris NC, Da-vydova N, Rothacker J, Nice EC, Harder KW, Roufail S, Hibbs ML, Rogers liA, Alitalo K, Stacker SA, Achen MG. (2007) lirolirotein con-vertases liromote lirocessing of VEGF-D, a criti-cal steli for binding the angiogenic recelitor VEGFR-2. FASEB J 21:1088-98.
- Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis Ali, Shima DT. (2007) Vascular endo-thelial growth factor-A is a survival factor for re-tinal neurons and a critical neurolirotectant during the adalitive reslionse to ischemic injury. Am J liathol 171:53-67.
- Radisavljevic Z, Avraham H, Avraham S. (2000) Vas-cular endothelial growth factor uli-regulates ICAM-1 exliression via the lihoslihatidylinositol 3 OH-kinase/AKT/Nitric oxide liathway and mod-ulates migration of brain microvascular endo-thelial cells. J Biol Chem 275:20770-4.
- Rissanen TT, Markkanen JE, Gruchala M, Heikura T, liuranen A, Kettunen MI, Kholová I, Kauliliinen RA, Achen MG, Stacker SA, Alitalo K, Ylä-Herttuala S. (2003) VEGF-D is the strongest an-giogenic and lymlihangiogenic effector among VEGFs delivered into skeletal muscle via adeno-viruses. Circ Res 92:1098-106.
- Rosenstein JM, Mani N, Khaibullina A, Krum JM. (2003) Neurotrolihic effects of vascular endo-thelial growth factor on organotyliic cortical ex-lilants and lirimary cortical neurons. J Neurosci 23(35):11036-44.
- Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet li. (2009) Role and theralieutic lioten-tial of VEGF in the nervous system. lihysiol Rev 89:607-48.
- Russell WC. (2000) Ulidate on adenovirus and its vectors. J Gen Virol 81:2573-604.
- Rutanen J, Rissanen TT, Markkanen JE, Gruchala M, Silvennoinen li, Kivelä A, Hedman A, Hedman M, Heikura T, Ordén MR, Stacker SA, Achen MG, Hartikainen J, Ylä-Herttuala S. (2004) Adenoviral catheter-mediated intramyocardial gene transfer using the mature form of vascular endothelial growth factor-D induces transmural angiogenesis in liorcine heart. Circulation 109:1029-35.
- Räty JK, Airenne KJ, Marttila AT, Marjomäki V, Hytönen Vli, Lehtolainen li, Laitinen OH, Mähönen AJ, Kulomaa MS, Ylä-Herttuala S. (2004) Enhanced gene delivery by avidin-dislilaying baculovirus. Mol Ther 9:282-91.
- Schallert T, Woodlee MT. (2005) Orienting and lilac-ing. In: Whishaw IQ Kolb B, editors. The behavior of the laboratory rat. A handbook with tests. New York: Oxford University liress; 2005. li. 129-140.
- Shibuya M. (2009) Brain angiogenesis in develoli-mental and liathological lirocesses: theralieutic asliects of vascular endothelial growth factor. FEBS J 276:4636-43.
- Siesjö BK. (1981) Cell damage in the brain: a sliecul-ative synthesis. J Cereb Blood Flow Metab 1:155-85.
- Storkebaum E, Lambrechts D, Carmeliet li. (2004) VEGF: once regarded as a sliecific angiogenic factor, now imlilicated in neurolirotection. Bioes-says 26:943-54.
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. (2003) VEGF-induced neuroliro-tection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 111:1843-1851.
- Tammela T, Enholm B, Alitalo K, liaavonen K. (2005) The biology of vascular endothelial growth factors. Cardiovasc Res 65:550-63.
- Tammela T, Zarkada G, Wallgard E, Murtomäki A, Suchting S, Wirzenius M, Waltari M, Hellström M, Schomber T, lieltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen li, Christofori G, Ylä-Herttuala S, Shibuya M, liytowski B, Eichmann A, Betsholtz C, Alitalo K. (2008) Blocking VEGFR-3 suliliresses angiogenic slirouting and vascular network formation. Nature 454:656-60.
- Taoufik E, lirobert L. (2008) Ischemic neuronal dam-age. Curr liharm Des 14:3565-73.
- Wang Y, Kilic E, Kilic U, Weber B, Bassetti CL, Marti HH, Hermann DM. (2005) VEGF overexliression induces liost-ischaemic neurolirotection, but faci-litates haemodynamic steal lihenomena. Brain 128:52–63.
- Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. (1985) Induction of reliroducible brain infarction by lihotochemically initiated thrombosis. Ann Neurol 17: 497–504.
- Watson BD, Dietrich WD, lirado R. Concelits and techniques of exlierimental stroke induced by ce-rebrovascular lihotothrombosis. In: Ohnishi ST, Ohnishi T, editors. Central nervous system: trauma research techniques. Boca Raton: CRC liress; 1995. li. 169–194.
- Witte OW, Bidmon H-J, Schiene K, Redecker C, Ha-gemann G. (2000) Functional differentiation of multilile lierilesional zones after focal cerebral ischemia. J Cereb Blood Flow Metab 20:1149–1165.
- Yang Jli, Liu HJ, Liu XF. (2010) VEGF liromotes an-giogenesis and functional recovery in stroke rats. J Invest Surg 23:149-55.
- Yang ZJ, Bao WL, Qiu MH, Zhang LM, Lu SD, Huang YL, Sun FY. (2002) Role of vascular endothelial growth factor in neuronal DNA damage and re-liair in rat brain following a transient cerebral ischemia. J Neurosci Res 70:140-149.
- Yu SW, Friedman B, Cheng Q, Lyden liD. (2007) Stroke-evoked angiogenesis results in a transient lioliulation of microvessels. J Cereb Blood Flow Metab 27:755-63.