Research Article - Pharmaceutical Bioprocessing (2018) Volume 6, Issue 3
Effects of dexamethasone on the EGF mRNA levels and inflammatory factors in rabbits with oral ulcers
- *Corresponding Author:
- Daxu Li
Department of Stomatology
The First Affiliated Hospital
Medical School of Xi’an Jiaotong University
Xi’an,Shaanxi,PR China 710061
E-mail: warwaeyunzhet@163.com
Abstract
Objective: To explore the therapeutic effects of dexamethasone on the rabbits with oral ulcers and the underlying mechanisms of dexamethasone in the treatment of oral ulcers.
Methods: Four of the forty experimental SPF New Zealand rabbits were randomly selected to be used for the identification of oral ulcer model. The rest were randomly divided into three groups: the control group (Group A), the normal saline group (Group B), and the dexamethasone group (Group C), with 12 in each group. The oral ulcer model was established by applying the 40% glacial acetic acid solution to the oral buccal membranes of the rabbits. The changes of the oral ulcers on the model-establishing day and the 2nd, 4th and 7th day after administration were observed. Besides, the epidermal growth factor (EGF) levels in the oral mucosal tissues were examined by reverse transcription PCR (RT-PCR), and the local histopathological changes in the oral ulcers were observed through hematoxylin-eosin (HE) staining.
Results: The ulcer areas in Group C was significantly reduced on the second, fourth and seventh days after dosage when compared with Group B (P<0.05). The EGF levels in the buccal mucosa of rabbits in Group B and C were significantly higher than those in Group A (P<0.05), with a faster increasing pace in EGF levels in Group C when compared with Group B (P<0.05). Results from HE staining showed that inflammatory cells in Group C were significantly decreased compared with those in Group B, the fibroblast proliferation was more obvious in Group C on the 2nd, 4th, and 7th day after administration. Additionally, the epithelial proliferation was better in Group C.
Conclusion: Dexamethasone can significantly improve the symptoms of oral ulcers in rabbits and promote ulcer healing. Consequently, dexamethasone may help accelerate oral ulcer healing by regulating the EGF levels in the process of oral ulcers.
Keywords
oral ulcer, dexamethasone, epidermal growth factors
Introduction
Oral ulcers are superficial ulcers occurring on the oral mucosa and can vary in size from rice grains to soybeans, which are round or oval in shape. They mainly occur on the lips, cheeks, and tongue edges, together with the experiences of congestion as well as local pain. As known to all, severe oral mucosal pain, unbearable burning pain as well as the long-term recurrent attacks caused by oral ulcers will directly affect the patient’s immune functions, cause metabolic disorders, bringing about bad breath, chronic pharyngitis, constipation and other symptoms [1,2]. With the faster pace of life, the incidence of oral ulcers gradually rises. Besides, oral ulcers may lead to low immunity, and in turn low immunity can make it easy for oral ulcers to recur, which might form a vicious circle because of the recurrent attacks of oral ulcers, consequently, seriously affecting the work and life of the patients [3,4]. At present, the treatment of oral ulcers is mainly based on drugs, and different drug treatments might present different effects [5,6]. Among these drugs, dexamethasone is an adrenocortical hormone drug which has anti-inflammatory, anti-fever, anti-allergic and immunosuppressive effects [7]. Relevant data have shown that dexamethasone has a certain effect in the treatment of oral ulcers [8]. However, the mechanisms in which have not been clear stated. Therefore, in this study, the symptoms and the changes in EGF levels in oral cheek mucosa tissues of the rabbits with oral ulcer after drug intervention were observed. Apart from this, we then try to explore the mechanism of dexamethasone in the treatment of oral ulcers, providing a basis for the clinical use of dexamethasone in the treatment of oral ulcers.
Materials and Methods
Materials
Animals
Forty SPF New Zealand rabbits with half male and half female (weighing from 2900 to 3400 g) were purchased from Chongqing Tengxin Bier Experimental Animal Co., Ltd. The animals were fed with pellet feed. Adequate drinking water was applied during the experiment. The room temperature was kept to be (20 ± 2)°C, the average relative humidity (40~60) %.
Drugs and reagents
Hydroxypropyl methylcellulose was purchased from Gomez Chemicals China Ltd., dexamethasone tablets were brought from Zhejiang Xianyi Pharmaceutical Co., Ltd., Twain-70 was obtained from Guangzhou Qiaoling Biotechnology. Technology Co., Ltd., glycerol was provided by Henan Tongshang Imp. & Exp. Co., Ltd., glacial acetic acid was brought from Jinshan Chemical Reagent Co., Ltd., First Strand cDNA Synthesis Kit was purchased from Boster Biological Technology Co., Ltd. The reverse transcription kit was provided by Beijing Norbold Technology Co., Ltd. The RNA extraction kit was brought from Beijing Solelab Technology Co., Ltd., 2×TaqPCR MasterMix was purchased from Beijing Biotek Biotech Co., Ltd., and the DNA Master was provided by Beijing Tianwei Times.
Instruments
The tissue homogenizer was purchased from Shanghai Jinuo Electronics Co., Ltd., the automatic dyeing machine was provided by Shenzhen Yongnian Technology Co., Ltd., and the pathological slicer was purchased from Fuguang Precision Instrument (China) Co., Ltd., the optical microscope was brought from Shanghai Guangmi Instrument Factory, the ultra-low temperature refrigerator was purchased from Hangzhou Shuilian Instruments Co., Ltd., Mini-H3 vertical electrophoresis instrument was provided by the United States Biorad Corporation, UV gel imaging analysis system was provided by Beijing Saipel Technology Co., Ltd., the UV spectrophotometer was purchased from Beijing Huake Instrument Technology Co., Ltd., the high-speed and multi-function refrigerated centrifuge was purchased from Guansen Bio-Shanghai Co., Ltd, the Ultra-low temperature refrigerator (-70°C) was purchased from Shandong Boko Scientific Instrument Co., Ltd. Maxi-MixII tube shaker was purchased from Beijing Minosi Technology Co., Ltd., and the color medical graphic analysis system was obtained from Wuhan Qianping Imaging Technology Co., Ltd.
Methods
Preparation of the drug
Preparation of the drug membrane with dexamethasone: Dexamethasone tablets (600 g), Tween-70 (5.0 mL) and glycerol (20.0 mL) were added into the saturated hydroxypropyl methylcellulose solution to a total volume of 1000 mL, afterwards, the above solutions were fully stirred to form gellike solutions. Then the obtained solutions were trayed evenly after a 24-hour standing. After drying, the samples were cut into several films with a diameter of 1 cm. These films were disinfected by ultraviolet radiation and sealed with polyethylene film.
Preparation of the drug membrane with saline: refined salt (9 g) was added into the saturated hydroxypropyl methylcellulose solution to a total volume of 1000 mL, and other preparing methods were the same as dexamethasone.
Animals grouping
40 SPF New Zealand rabbits were used. All rabbits were numbered according to the body weight. Four rabbits were randomly selected to be used for the identification of oral ulcer model (Model group) by random number table. The rest animals were randomly divided into three groups: the control group (Group A), without modeling and any processing; the normal saline (NS) pellicles group (Group B), in which the oral ulcer model was established but the animals were treated with NS pellicles; the dexamethasone group (Group C), in which the animals were treated with dexamethasone after modeling.
There were 12 animals in each group with half male and half female.
Construction of the oral ulcer model
The rabbits to be modeled were anesthetized with 3% sodium pentobarbital (injection dose: 30 mg/kg). 40% glacial acetic acid was added onto the buccal membrane (2 mm from both angulus oris) for 60 s by using a glass rod with the diameter and length to be 7 mm and 10 cm respectively. The cauterization parts were then rinsed for 1 min to form a white lesion with a diameter of 7 mm. Congestion and red swelling could be observed in the pars buccalis 24 h later. Additionally, yellow or white pseudomembrane could be covered in the treated parts, indicating that the oral ulcer model was successfully established.
Administration
Drugs were applied after the model was established. No treatments were carried out in Group A and the Model Group. NS pellicles were applied in the oral ulcer for the animals in Group B. While dexamethasone pellicles were chosen for the rabbits in Group C. The above pellicles were attached twice a day (at 9:00 and 17:00, respectively), with one film on each side for 5 minutes for constant 7 days. After the drug application, the treated parts were rinsed by warm normal saline each time.
Observation indexes
Changes in the symptoms and signs of the rabbits: The symptoms and signs of the rabbits were visually observed, including their activities, body weight, the defecation and urination conditions, coat color, eating and other changes.
Morphological changes and the measurement of ulcer areas: Rabbits used for ulcer model identification were sacrificed on the day that the animals were successfully modeled (0 d); Four rabbits in Group A, B and C were sacrificed according to the random number table on the 2nd, 4th and 7th day after medication. The morphology of the oral ulcer was observed, such as the conditions of congestion, the color of oral mucosal, whether pseudomembrane coverage appeared and so on. The oral cavity of the sacrificed rabbits was fully exposed. Then the oral mucosa was selected (the tissues at the junction of ulcer and normal mucosa was appropriate) [3]. The ulcer size was measured with a vernier caliper, and the ulcer area was calculated with Image Converter software.
Determination of EGF levels in the oral mucosa of the rabbits: The right side of the pathological specimens of the extracted ulcers were fixed in 10% formaldehyde solution and embedded in paraffin. However, the left side was quickly frozen into the liquid nitrogen to prevent the mRNA degradation, after which these tissues were then stored in a -70°C refrigerator for use. The mRNA level of EGF was examined by using RT-PCR method. Quantity One software was applied to calculate the relative ratios of the optical density obtained from the bands of the RT-PCR products and β-actin.
Local histopathological changes in the ulcers: the paraffin-embedded specimens were stained with HE. The pathological changes in the ulcer tissues in each animal were observed (the corresponding tissue in Group A was selected according to the position of the ulcer in other groups).
Statistical analysis
SPSS 21.0 statistical software was used for data analysis. The measurement data were expressed as ͞x ± s . T-test was used for the analysis between two groups and one-way ANOVA for the comparison among many groups. Besides, the multiple comparison was conducted with Duncan method, P<0.05 was considered statistically significant.
Results
Comparison of the changes in symptoms and signs of rabbits in each group
Before modeling, rabbits in Group A had dry lips, normal activities, flexible responses, good appetite, smooth shiny coat, with light yellow urine, and normal defecation. After the model was stimulated, their lips were wet with occasional drooling. The activities of the animals have decreased, and their spirit was poor, with food intake, water intake as well as body weight decreased. The hair color was lighter and rougher than before, urine was yellow and less, and the defecation frequency was decreased as well. The animals treated with dexamethasone in Group C were gradually recovered, with an increase in food intake, water intake as well as body weight. Besides, the lips were dryer than before, the hair color was gradually becoming smooth, the activities were gradually increased, and the defecation and urination conditions become normal. The recovery rate in Group B was slower than that of Group C.
Comparison of the morphology of ulcer and ulcer areas of the rabbits in Group B and C
The oral mucosa was light, reddish and shiny in Group A. After burning for 24 hours with glacial acetic acid, a round or oval ulcer was formed on the oral cheeks of the rabbits, with a diameter of about 7 mm. The edges of the ulcer were neat, with red swelling and congestion surrounding the ulcer area, the surface of which was covered with yellow or gray pseudomembrane. With the time going on, the congestion and edema of the oral mucosa were gradually improved and the luster were gradually recovered. The recovery conditions in Group C were better than those in Group B. The ulcer areas in Group C were significantly smaller than those in group B on the 2nd, 4th and 7th day after administration (P<0.05). See Table 1.
| Time point | Group B | Group C |
|---|---|---|
| The 2nd day after medication | 46.43 ± 2.68 | 39.27 ± 2.51a |
| The 4th day after medication | 35.14 ± 1.95 | 26.91 ± 0.76a |
| The 7th day after medication | 26.55 ± 2.14 | 15.86 ± 1.42a |
Note: aP<0.05 when compared with Group B
Table 1. Comparison of the ulcer areas at different time points in Group B and C (͞x ± s, mm2)
Comparison of EGF levels in the oral buccal mucosa tissues in each group
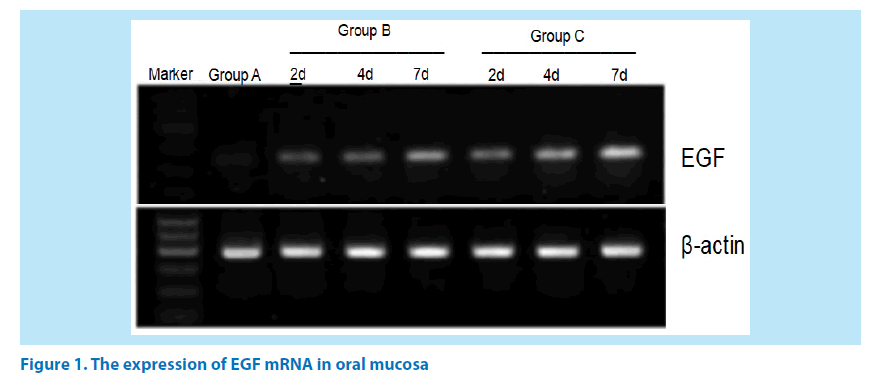
The expression of EGF mRNA in Group B and C were increased gradually on the 2nd, 4th and 7th day after administration, showing a statistically significant difference between the groups (P<0.05). See Table 2 and Figure 1.
| Time point | Group A | Group B | Group C |
|---|---|---|---|
| The 2nd day after medication | 0.26 ± 0.05 | 0.68 ± 0.03a | 0.92 ± 0.04ab |
| The 4th day after medication | 0.18 ± 0.09 | 0.79 ± 0.04a | 1.51 ± 0.12ab |
| The 7th day after medication | 0.22 ± 0.02 | 1.26 ± 0.10a | 1.76 ± 0.11ab |
Note: aP<0.05 when compared with Group A; bP<0.05 when compared with Group B
Table 2. Comparison of EGF Levels in the oral buccal mucosa tissues in each group (͞x ± s).
The pathological changes of the buccal mucosa by using light microscope
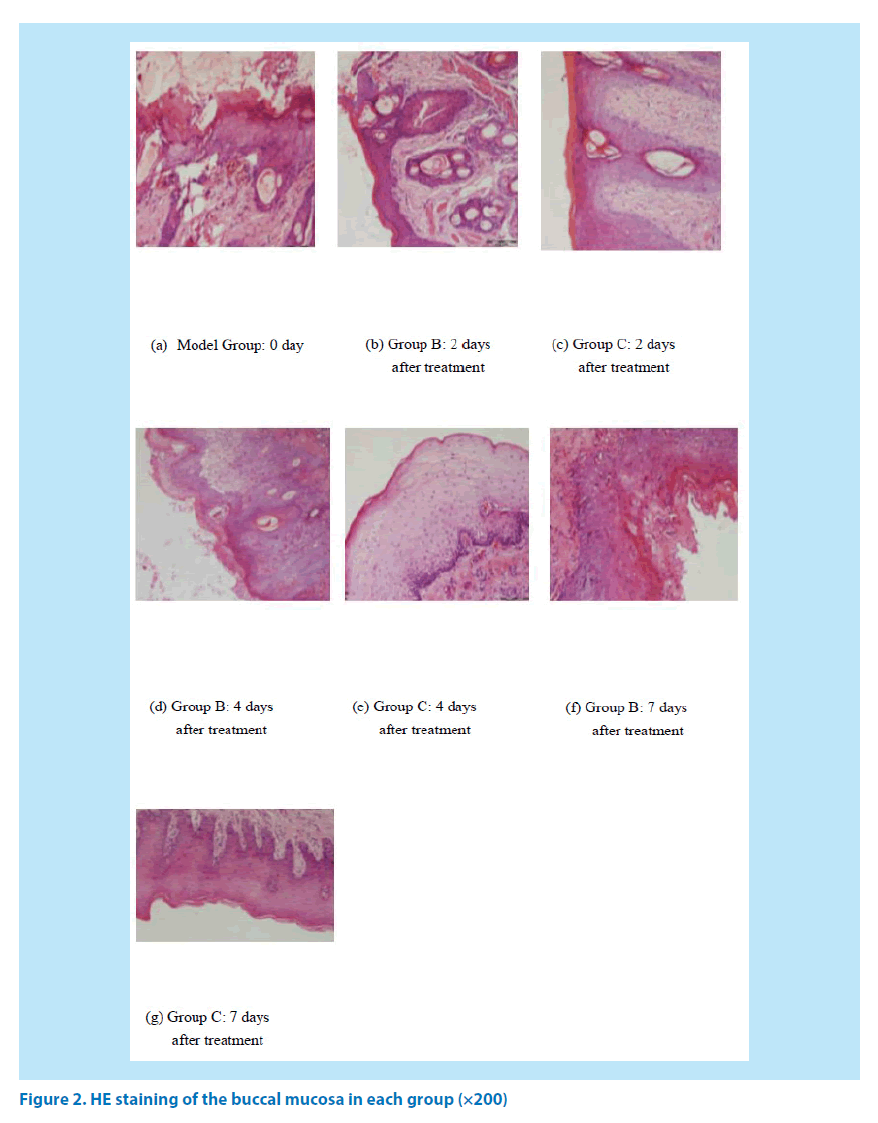
The nucleus was blue and the cytoplasm appeared to be pale red after HE staining. In Group A, the oral mucosal epithelium was completely covered and the underlying layer was the lamina propria which was composed of the connective tissues, besides, no obvious inflammatory cell infiltration was observed. The mucosal epithelial cells were necrotic and detached after 2 days of treatment in Goup B and Group C. There were more inflammatory cells and almost no new fibroblasts could be found in Group B. The number of inflammatory cells was significantly reduced, and a few new born fibroblasts could be observed in Group B. After 4 days of treatment, the number of inflammatory cells in Group B began to decrease; Besides, the number of inflammatory cells in Group C was decreased continuously, and many new fibroblasts could be detected. After 7 days of medication, the number of inflammatory cells in Group B was significantly reduced, and many new fibroblasts were appeared; Meanwhile, the ulcer surface of Group C was almost covered by mucosal epithelium, and the ulcer tended to be healed (Figure 2).
Discussion
Oral ulcer is a kind of oral mucosal diseases which can be frequently experienced, as we know, oral ulcer can be self-healed and recurrent. There was a certain periodicity for recurrent aphthous ulcers, and they are often occurred in the tongue, buccal mucosa, dorsum of tongue, inside of the lips and other parts [9,10].
The pathogenesis of oral ulcers has still not been fully illustrated. The potential predisposing factors include genetic, viral and bacterial infections, food allergies, vitamin or trace element deficiency, systemic diseases (such as celiac disease, Crohn’s disease, ulcerative colitis, and AIDS), enhanced oxidative stress responses, local trauma, stress and endocrine disorders and so on [11,12]. A large number of leukocyte infiltration in the lesion areas in oral ulcers could be observed by pathological examinations. Epithelial fistula could be formed because of the intracellular and intercellular edemas, which was mainly infiltrated by the dense lymphocytes and monocytes. As the pathological process changes, the polymorphonuclear leukocytes and plasma cells infiltration could be occurred, and the epithelial tissues would be detached, thus forming the ulcers [13]. At present, no treatments for oral ulcers are available, and local treatments are mainly used to reduce the inflammatory responses, relieve the pain and prevent infection. As for those serious patients, systemic therapy is needed [14].
Dexamethasone is a glucocorticoid, which has anti-inflammatory, anti-endotoxin, and immunosuppressive effects. It can reduce the leakage of inflammatory substances, improve the edema conditions of the tissues, and also reduce the synthesis and release of inflammatory chemicals (histamine and so on) by reducing the permeability of cells and capillaries, thus promoting the local metabolism activities and reducing the inflammatory responses and thereby accelerating the healing of the ulcer [15]. Studies have shown that [16], xi lei powder plus Sanqi powder combined with dexamethasone could be used in the treatment of oral ulcers, this combination therapy could show anti-inflammatory, analgetic effects and heat-clearing and detoxifying effects as well as the activity of eliminating necrotic tissues and promoting granulation. Besides, it could show immunoregulation abilities and thus making the immune functions to turn to be normal. As a result, this combination treatment has won excellent clinical effects. Other researches have also shown that [17] the effects of dexamethasone acetate tablets on the treatment of recurrent oral ulcers are certain, indicating that they can be used in the treatment of recurrent oral ulcers.
The animal experiments showed that dexamethasone can effectively improve the symptoms of oral ulcers in rabbits, relieve the inflammatory reactions of the local tissues, reduce the exudation of tissue fluid, promote the rapid recovery of ulcers, and shorten the lasting time of ulcers. EGF has the effects of inducing cell growth and proliferation. Studies have shown [18] that EGF could play an important role as the protective barrier of oral mucosa, and provide a basis for the growth, differentiation and proliferation of oral mucosal epithelial cells, and are especially relevant with the proliferation of mucosal basal cells. Patients with oral ulcers often have a shortage of EGF, which causes the delayed healing of oral ulcers. The results of this study showed that there was a significant difference in the expression of EGF mRNA between Group B and Group C on the 2nd, 4th and 7th day after administration (P<0.05), suggesting that dexamethasone can enhance the expression of EGF mRNA in oral mucosa, promote the secretion of EGF. With time going on, the expression of EGF mRNA was gradually increased, suggesting that EGF was involved in the process of oral ulcer healing. Studies have shown [19] that the reduction of EGF secretion in the oral mucosa of the patients with ulcer could hinder the recovery of oral ulcers. Therefore, it can be considered that dexamethasone could improve the self-repair ability of the oral ulcers by promoting the secretion of EGF in the oral mucosa, and thus promoting the healing of ulcers.
In summary, dexamethasone can significantly improve the symptoms of oral ulcers in rabbits and promote the ulcer healing process. Dexamethasone may enhance the self-repair ability of the oral ulcers by promoting the secretion of EGF in the oral mucosa.
References
- Santarelli A, Mascitti M, Galeazzi R et al. Oral ulcer by Sphingomonas paucimobilis: first report. Int. J. Oral. Maxillofacial. Surg. 45(10), 1280-1282 (2016).
- Dubrocq G, Needles M, Jantausch B. Recurrent fever and mouth ulcers in a healthy child. J. Ped. Infect. Dis. Soc. 6(3), e155-e157 (2017).
- Ramírezamador VA, Espinosa E, Gonzálezramírez I et al. Identification of oral candidosis, hairy leukoplakia and recurrent oral ulcers as distinct cases of immune reconstitution inflammatory syndrome. Int. J. STD. Aids. 20(4), 259-261 (2009).
- Yu Z, Jin C, Xin M et al. Effect of Aloe vera polysaccharides on immunity and antioxidant activities in oral ulcer animal models. Carb. Poly. 75(2), 307-311 (2009).
- Barrons RW. Treatment strategies for recurrent oral aphthous ulcers. Am. J. Health. Sys. Pharm. 58(1), 41-50 (2001).
- Lim YS, Kwon SK, Park JH et al. Enhanced mucosal healing with curcumin in animal oral ulcer model. Laryngoscope. 126(2), E68-E73 (2016).
- Alamoudi NM, El Ashiry EA, Farsi NM et al. Treatment of oral ulcers in dogs using adipose tissue-derived mesenchymal stem cells. J. Clin. Ped. Dent. 38(3), 215-222 (2014).
- Schürkämper M, Medele R, Zausinger S, et al. Dexamethasone in the treatment of subarachnoid hemorrhage revisited: a comparative analysis of the effect of the total dose on complications and outcome. J. Clin. Neurosci. 11(1), 20-24 (2004).
- Patten SF, Tomecki KJ. Wegener's granulomatosis: cutaneous and oral mucosal disease. J. Am. Acad. Dermatol. 28(1), 710-718 (1993).
- Leão JC, Gomes VB, Porter S. Ulcerative lesions of the mouth: an update for the general medical practitioner. Clinics. 62(6), 769-780 (2007).
- Han J, He Z, Li K et al. Microarray analysis of potential genes in the pathogenesis of recurrent oral ulcer. Int. J. Clin. Exp. Pathol. 8(10), 12419-12427 (2015).
- Birek C, Grandhi R, Mcneill K et al. Detection of helicobacter pylori in oral aphthous ulcers. J. Oral. Pathol. Med. 28(5), 197-203 (2010).
- Loots M A, Lamme E N, Zeegelaar J et al. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J. Invest. Dermatol. 111(5), 850-857 (1998).
- Karacayli U, Mumcu G, Simsek I et al. The close association between dental and periodontal treatments and oral ulcer course in behcet’s disease: a prospective clinical study. J. Oral. Pathol. Med. 38(5), 410-415 (2009).
- Funato H, Kobayashi A, Watanabe Y. Differential effects of antidepressants on dexamethasone-induced nuclear translocation and expression of glucocorticoid receptor. Brain. Res. 1117(1), 125-134 (2006).
- Farshi FS, Ozer AY, Ercan MT et al. In-vivo studies in the treatment of oral ulcers with liposomal dexamethasone sodium phosphate. J. Microencapsulation. 13(5), 537-544 (1996).
- Wang QQ, Chen HL, Zong F et al. Observation of the clinical efficacy of treating recurrent oral ulcer with He-Ne laser and acupoint-injection. Laser. J. 123, 123-132 (2008).
- Maynard AA, Dvorak K, Khailova L et al. Epidermal growth factor reduces autophagy in intestinal epithelium and in the rat model of necrotizing enterocolitis. Am. J. Physiol. Gastrointestinal. Liver. Physiol. 299(3), G614-G622 (2002).
- Konturek SJ, Brzozowski T, Dembinski A et al. Comparison of solcoseryl and epidermal growth factors (EGF) in healing of chronic gastroduodenal ulcerations and mucosal growth in rats. Hepato-gastroenterol. 35(1), 25-29 (1988).