Research Article - International Journal of Clinical Rheumatology (2017) Volume 12, Issue 5
Effect of curcuma xanthorrhiza suplementation in vitamin D3 administration towards proteinuria, serum anti-dsDNA and IL-17 levels on systemic lupus erythematosus (sle) patients with hypovitamin D
- *Corresponding Author:
- Zoraida Dwi Wahyuni
Universitas Brawijaya, Indonesia
E-mail: id_zora@yahoo.com
Abstract
Background: Vitamin D is an immunomodulator that plays a role in immunoregulation. Curcumin is also a potential immunomodulator in several autoimmune diseases. Curcumin-vitamin D bonds in VDR have different positions, thus leading to a homologous function with vitamin D and is thought to have a synergic effect if given simultaneously.
Aims: To determine the effect of curcumin supplementation in vitamin D3 adminstration towards proteinuria, serum anti-dsDNA and IL-17 levels in SLE patients with hypovitamin D.
Method: This study is a double blind randomized controlled trial on active SLE patients with hypovitamin D in the Oupatient Clinic of Saiful Anwar General Hospital Malang. This study was held from January 2016-March 2017. Measurement of vitamin D, antidsDNA and IL-17 were carried out using ELISA, while proteinuria was assessed with protein-creatinine ratio (enzymatic-turbidimetric method). Data were analyzed using Spearman correlation test and several comparative tests with a significance of p <0.05; which were Wilcoxon, t-test, Saphiro-Wilk normality test, and Levene homogenity variance test.
Results: Total of 39 female subjects was divided into 2 groups; 19 in the treatment group (vitamin D+curcumin) and 20 in the control group (vitamin D + placebo). We observed a significant increase in vitamin D, decreased anti-dsDNA, protein-creatinine ratio and IL-17 levels in each group (p < 0,05), but no significant difference were found when the treatment and control groups were compared. Median of elevated vitamin D levels is of 8.6 (IQR: -0.4-15.2) vs 11.6 (IQR: 6.32-17.47) (P>0.05). Vitamin D increase have a significant correlation with anti-dsDNA and IL-17 decrease (R= 0,514; P=0,001 and R= -0,510; P=0,001)
Conclusion: Curcumin addition in vitamin D administration have no effect compared with singular vitamin D administration. The increase of vitamin D levels is correlated with anti-dsDNA and IL-17 decrease, which lead to a better improvement of proteinuria.
Keywords
curcumin, hypovitaminosis, pathogenesis
Introduction
One of the manifestations of SLE is lupus nephritis (LN), which usually occurs within 5 years after the diagnosis of SLE. LN patients are at risk to develop end-stage renal failure, so it is a major predictor of morbidity and mortality for SLE patients [1]. Kidney involvement accounts for nearly 60% of SLE population with a higher prevalence in Asians, Hispanics, Native Americans and blacks, and especially in women of reproductive age. LN is characterized by loss of self-tolerance, autoantibody production such as nuclear antigen and immune-mediated injury in the kidney [2]. The main manifestations of LN are proteinuria [3].
Lupus nephritis begins with the presence of anti-dsDNA antibody deposition containing immune complexes in the renal parenchyma. The immune complex is accompanied by complement activation, infiltration of immune cells, release of chemokines, cytokines, proteolytic enzymes and oxidative damage causing inflammation of the kidneys and organ damage. The nefritogenic effect of anti-dsDNA antibodies is caused by gene regulation and protein expression of inflammatory and fibrotic mediators in renal cells. The mechanism of this effect is by direct binding to cross-reactive antigens on the surface of kidney cells or the extracellular matrix components, as well as indirect effects through the nucleosomes that are bound to the glomerular basement membrane [1,4].
IL-17 also plays a role in the pathogenesis of LES and lupus nephritis. This cytokine is produced by Th17 and double negative (DN) T cells [5]. IL-17 and IFN-Υ are major cytokines detected in mice kidneys and cause local inflammation, production of autoantibodies and nephritis [6]. A study by Hsu et al. in 2007 on mice showed that prior to the formation of autoantibodies, IL-17 increased. SLE mice indicate that IL-17 interacts with B cells and trigger the activation and production of autoantibodies [7].
From a study conducted by Puspitasari et al in 2012, 84.1% of SLE patients in Malang experienced hypovitaminosis D [8]. Low vitamin D levels have a significant relationship with disease activity, where a lower vitamin D level causes higher disease activity. This is because the role of vitamin D in the regulation of immune response by lowering the response of Th1 and Th17 cells, suppressing the maturation of dendritic cells, decreasing proliferation and maturation of active B cells, and increasing the number of Treg cells [9]. Priyantoro et al., (2014) conducted a study of vitamin D supplementation softgel 3/cholecalciferol 1200 IU/ day or 30 mg/day for 3 months in SLE patients who experienced hypovitaminosis D, and found a significant increased level of vitamin D at 6.55 ± 0.09 ng / mL and decreased levels of anti-dsDNA and proteinuria at post-supplementation [10].
Apart from vitamin D, some studies also suggest that curcumin has a potential as an immunomodulator in autoimmune diseases. Curcumin is a phenolic compound found in many plants such as ginger, turmeric, and Javanese ginger [11]. Curcumin lowers the percentage of Th1, Th2 and Th17 cells and increase the percentage of Treg cells [12]. Curcumin is also a natural ligand for the vitamin D receptor (VDR), and VDR activation is found to be able to induce Treg and inhibit Th17 activation [13,14]. A study by Pratt et al., (2015) in SLE mice found decreased levels of Th17 and increased Treg cells in an administration concentration of 200 mg / kg / day (equivalent to 20 mg/kg/day in humans) [15].
The synergistic nature of curcumin with vitamin D in regulating the immune cells [16-18] reveals new opportunities for researchers to improve the therapeutic response of vitamin D3, improve clinical symptoms, decrease the progression of disease in SLE patients with hypovitaminosis D by means of combining curcumin supplementation with vitamin D. Curcumin addition in the administration of vitamin D3 is expected to decrease anti-dsDNA autoantibody production and the percentage of Th17 cells, which can be seen from the levels of IL-17 serum. The aim of this study is to determine the effect of curcumin supplementation in vitamin D3 adminstration towards proteinuria, serum anti-dsDNA and IL-17 levels in SLE patients with hypovitamin D.
Methods
This double-blind randomized controlled trial study was conducted in January 2016-March 2017 in Saiful Anwar General Hospital Malang.
Research subjects were active SLE patients. The number of study subjects was 40 people divided into 2 groups; treatment group (curcumin) (n=20) and placebo group (control) (n=20). Sample inclusion criteria in this study were women, aged >18 years, patients diagnosed with SLE based on the criteria of 1997 American College of Rheumatology, low vitamin D levels (<30 ng / mL) and in active state (SLEDAI>3) Sample exclusion criteria were SLE patients who were pregnant, taking supplements containing vitamin D and / or curcumin, unwilling to follow this study, liver dysfunction disorders (SGOT / SGPT levels more than 2.5 times the highest normal limit), severe kidney disease (GFR<25 ml/min or oliguria with urine production<400 cc/day and severe infectious diseases, such as tuberculosis or pneumonia.
The research subject who met the inclusion criteria and approved via informed consent went through venous blood samples extraction of 15 cc. The first 10 cc of venous blood samples were used for complete blood count, calcium, liver function (SGOT / SGPT), kidney function (urea / creatinine), blood levels of vitamin D (25 (OH) D3) and anti-dsDNA. The examination was done in the laboratory of Clinical Pathology of Saiful Anwar General Hospital Malang. The level of vitamin D was assessed by Enzyme Immuno Assay (DIASORIN Inc., Stillwater, MN, USA), while the anti-dsDNA examination were assesed by ELISA (Bioluminescenassay). The remaining 5 cc was used for IL-17 cytokine examination. The IL-17 examination was performed at the Kawi Malang Clinic laboratory, using the Enzyme-Linked Immuno-Sorbent Assay (ELISA) method of BioLegend. Examination of protein-creatinine ratio was done using spot urine. The examination was done in the laboratory of Clinical Pathology of Saiful Anwar
General Hospital Malang by using enzymatic-turbidimetric method with Cobas C311 / 501 (Roche / Hitachi). The examinations were repeated after 3 months of treatment.
Patients continue to receive the usual immunosuppressive drugs (corticosteroids and azathioprine, chloroquine, cyclosporin or cyclophosphamide) and other routine drugs. In the treatment group, each patient received vitamin D3 (Teorol) 400 IU and extract of Curcuma xanthorrhiza 20 mg (taken together, one tablet per meal, 3 tablets per day) for 3 months. In the control group, each patient received vitamin D3 (Teorol) 400 IU and placebo resembling curcumin (taken together, one tablet per meal, 3 tablets a day). All researchers did not know the type of supplementation given to the patient during the course of the study. The physicians who provided capsules containing either curcumin or placebo were physicians in the Rheumatology polyclinic that were not a part of the research team in different examination rooms.
Variant homogeneity was tested by Levene test. Saphiro-Wilk test is used to reveal the normality of data, in which data are assumed as normal if the Saphiro-Wilk test resulted in a p>0.05. After this step, parametric statistical test were done (t-test). As for the variables that are not assumed as normal, these variables were tested by non-parametric statistical approach (Wilcoxon test for paired samples). The results are assumed to be significant when the value of p<0.05. Data analysis was done using Statistical Package for the Social Sciences software version 16.0 (SPSS Inc, Chicago, IL).
Results
Characteristics of Research Subjects
Data on the characteristics of the study include: age, duration of illness, anti-dsDNA, IL-17, PCR, Hb, TLC, urea / creatinine, SGOT, SGPT, calcium, SLEDAI, and previous medications. Characteristics of SLE patients involved in the study are in (Table 1), where there was no significant difference between the control group and the treatment group.
| Characteristics | Vitamin D + Curcumin | Vitamin D | p-value |
|---|---|---|---|
| Age (years) | 27.89 ± 7.94 | 30.3 ± 10.09 | 0.415 |
| Duration of illness (tahun) | 2.63 ± 2.09 | 2.68 ± 1.96 | 0.937 |
| Vitamin D (ng/ml) | 14.29 ± 6.5 | 14.93 ± 7.47 | 0.779 |
| SLEDAI | 14.79 ± 7.92 | 15.05 ± 7.6 | 0.917 |
| Anti-dsDNA (IU/ml) | 140.97 ± 122.44 | 110.21 ± 97.29 | 0.683 |
| Calcium (mmol/L) | 8.84 ± 0.44 | 8.93 ± 0.66 | 0.63 |
| Urea (mg/dl) | 26.91 ± 10.67 | 28.36 ± 22.55 | 0.491 |
| Creatinine (mg/dl) | 0.64 ± 0.19 | 0.76 ± 0.46 | 0.623 |
| Hb (g/dL) | 11.98 ± 1.26 | 11.25 ± 1.61 | 0.122 |
| TLC (.../mm3) | 1027.32 ± 384.43 | 1321.48 ± 608.22 | 0.081 |
| SGOT (U/L) | 27.32 ± 3.31 | 25.45 ± 3.65 | 0.641 |
| SGPT (U/L) | 25.21 ± 3.61 | 25.45 ± 4.59 | 0.426 |
| Early Manifestation | |||
| - Mucocutaneous | 13 (33.3%) | 17 (43.6%) | 0.219 |
| - Arthritis | 7 (17.9%) | 9 (23.1%) | 0.605 |
| - Nephritis | 8 (20.5%) | 4 (10.3%) | 0.135 |
| - Hematology | 3 (7.7%) | 2 (5.1%) | 0.589 |
| - Vasculitis | 1 (2.6%) | 1 (2.6%) | 0.97 |
| - Serositis | 1 (2.6%) | 1 (2.6%) | 0.97 |
| - Neuropsychiatric | 4 (10.3%) | 3 (7.7%) | 0.622 |
| Immunosuppressant | |||
| - Chloroquine | 7 (17.9%) | 10 (25.6%) | 0.408 |
| - Cyclosporine | 0 (0%) | 2 (5.1%) | 0.157 |
| - Cyclophosphamide | 5 (12.8%) | 1 (2.6%) | 0.065 |
| - Azathioprine | 14 (35.9%) | 10 (25.6%) | 0.129 |
| PCR (mg/g) * | 1243.68 ± 438.31 | 976.50 ± 187.02 | 0.076 |
| IL-17 (pg/ml) | 5.24 ± 0.81 | 4.36 ± 0.77 | 0.801 |
Table 1. Basic Characteristics of Research Subjects
Comparison of Anti-dsDNA, Vitamin D, Protein-Creatinine Ratio, and IL-17 Pre-Post levels in both groups
In (Table 2) and (Table 3), there were significant differences in the results of vitamin D, anti-dsDNA, IL-17 and protein-creatinine ratios before and after treatment in both treatment and control groups. But from (Table 4), there was no difference in the delta of vitamin D increased or decreased antidsDNA, IL-17 and proteinuria in both groups.
| Vitamin D+ Curcumin Group | |||
|---|---|---|---|
| Variable | Before | After | p-value |
| Anti-dsDNA (IU/ml) | 97.4 (IQR: 15.6-223.5) | 34.20 (IQR: 21.6-184.6) | 0.007* |
| Vitamin D (ng/ml) | 15.2 (IQR: 7.7-20.3) | 24.4 (IQR: 18-28) | 0.001* |
| Ratio Protein- Creatinine (mg/g) | 400 (IQR: 240-1040) | 200 (IQR: 100-390) | 0.000* |
| Kadar IL-17 (pg/ml) | 43.0 (IQR: 30-75) | 20 (IQR: 13-29) | 0.001* |
Table 2. Comparison of Anti-dsDNA, Vitamin D, Protein-Creatinine, and IL-17 Pre-Post Ratios in the Vitamin D+ Curcumin Treatment Group
| Vitamin D+ Curcumin Group | |||
|---|---|---|---|
| Variable | Before | After | p-value |
| Anti-dsDNA (IU/ml) | 68.10 (IQR: 28.92-210.08) | 23.45 (IQR: 15.72-75.65) | 0.001* |
| Vitamin D (ng/ml) | 14.3 (IQR: 7.4-22.3) | 26 (IQR: 25-28) | 0.000* |
| Ratio Protein- Creatinine (mg/g) | 655 (IQR: 197.5-1637.5) | 210 (IQR: 110-885) | 0.000* |
| Kadar IL-17 (pg/ml) | 27 (IQR: 20-65) | 16 (IQR: 10-29) | 0.001* |
Table 3. Comparison of Anti-dsDNA, Vitamin D, Protein-Creatinine, and IL-17 Pre-Post Ratios in Vitamin D Placebo Group
| Vitamin D+ Curcumin Group | |||
|---|---|---|---|
| Variable | Before | After | p-value |
| Anti-dsDNA (IU/ml) | 34.2 (IQR: 21.6-184.6) | 23.45 (IQR: 15.72-75.65) | 0.757 |
| Vitamin D (ng/ml) | 8.6 (IQR: -0.4-15.2) | 11.6 (IQR: 6.32-17.47) | 0.211 |
| Ratio Protein- Creatinine (mg/g) | 200 (IQR:100-390) | 210 (IQR: 110-885) | 0.482 |
| Kadar IL-17 (pg/ml) | 1.9 (IQR: 0.1-5.2) | 1.5 (IQR: 0.05-3.62) | 0.474 |
Table 4. Comparison of Delta Levels of Anti-dsDNA, Vitamin D, Protein-Creatinine Ratio, and IL-17 among the Vitamin D + Curcumin treatment group with Vitamin D Plasebo group
Relationship of Increased Vitamin D Levels with Anti-dsDNA and IL-17 levels in SLE Patients
The relationship between research variables can be tested by using Rank Spearman correlation test. The results were significant (correlation) significant (p<0.05), namely as follows:
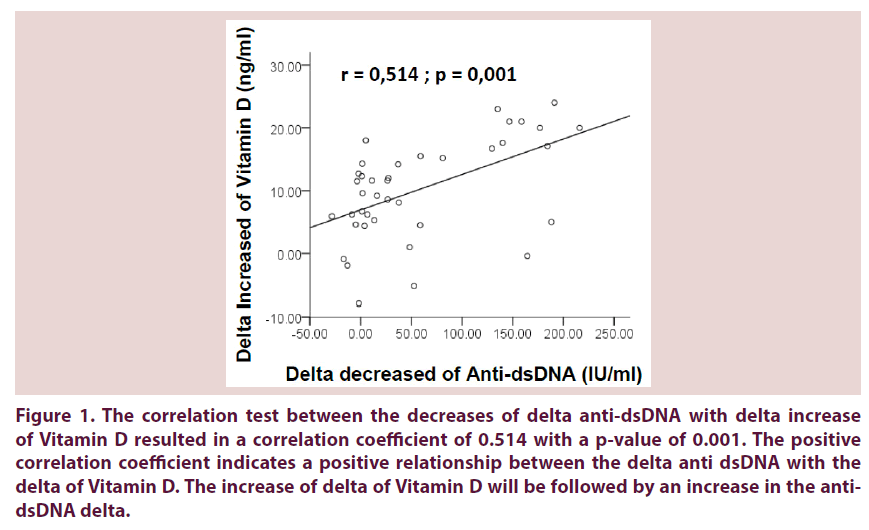
• The correlation test between the decreases of delta anti-dsDNA with delta increase of Vitamin D resulted in a correlation coefficient of 0.514 with a p-value of 0.001. The positive correlation coefficient indicates a positive relationship between the delta anti dsDNA with the delta of Vitamin D. The increase of delta of Vitamin D will be followed by an increase in the anti-dsDNA delta (Figure 1).
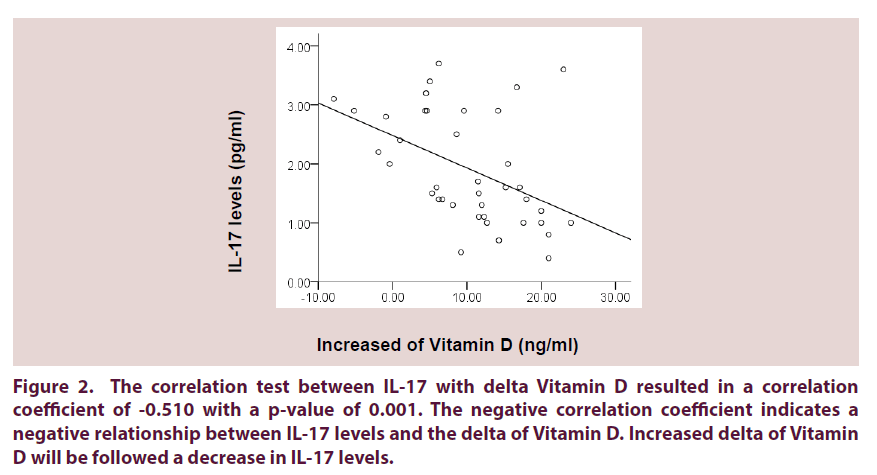
• The correlation test between IL-17 with delta Vitamin D resulted in a correlation coefficient of -0.510 with a p-value of 0.001. The negative correlation coefficient indicates a negative relationship between IL-17 levels and the delta of Vitamin D. Increased delta of Vitamin D will be followed a decrease in IL-17 levels (Figure 2).
Figure 1. The correlation test between the decreases of delta anti-dsDNA with delta increase of Vitamin D resulted in a correlation coefficient of 0.514 with a p-value of 0.001. The positive correlation coefficient indicates a positive relationship between the delta anti dsDNA with the delta of Vitamin D. The increase of delta of Vitamin D will be followed by an increase in the anti-dsDNA delta.
Figure 2. The correlation test between IL-17 with delta Vitamin D resulted in a correlation coefficient of -0.510 with a p-value of 0.001. The negative correlation coefficient indicates a negative relationship between IL-17 levels and the delta of Vitamin D. Increased delta of Vitamin D will be followed a decrease in IL-17 levels.
Discussion
Increased Vitamin D levels
In this study, a significant increase of vitamin D levels after supplementation of vitamin D3 (cholecalciferol) at a dose of 1200 IU per day for 3 months, in both treatment and control groups. In the treatment group that received vitamin D3 and curcumin for 3 months, the mean levels of vitamin D increased from 14.29 ± 6.5 ng/ml to 22.71 ± 5.35, with a median 15.2 (IQR: 7.7- 20.3) to 24.4 (IQR: 18-28) ( P<0.05). Whereas in the control group that received vitamin D3 and placebo, the mean levels of vitamin D increased from 7:47 ± 14.93 ng/ml to 26.85 ± 3.71 ng/ml, with a median 14.3 (IQR: 7.4-22.3) to 26 (IQR: 25-28) (P<0.05).
The dosage of 1200 IU/day of vitamin D3 were based on a study by Irastorza (2010), which recommends vitamin D3 with doses higher than 800 IU/day to prevent vitamin D insufficiency [19]. This is also supported also by a study by Priyantoro et al (2015) which uses a dose of 1200 IU of vitamin D3 per day for 3 months, which showed an increase in levels of vitamin D from 3:08 ± 23.89 ng / ml to 30.44 ± 3.16 ng / Ml [10]. However, from that study the initial vitamin D levels were already low. Similar to that study, the mean initial vitamin D levels in the present study indicates a deficiency in both groups. It is possible that this cause a persisting condition of vitamin D insufficiency.
A few other studies using larger doses include Anna Abou Raya (2012) at a dose of vitamin D3 at 2000 IU per day for 12 months, Terrier (2012) with a dose of 100,000 IU per week for 4 weeks followed 100,000 IU per month for 1 month, and Petri (2012) at a dose of 50,000 IU per week for 12 weeks. In all three studies, vitamin D levels after treatment was normal. [20-22] Kamen recommends vitamin D3 at a dose of 1000 to 2000 IU per day with a minimum target for adequate vitamin D levels in SLE patients with LES of 30 ng/ml. To maintain vitamin D levels (25 (OH) D), the dose depends on the initial level of 25 (OH) D. It is assumed that 100 IU of vitamin D3 may increase the levels of 25 (OH) D by 1 ng/ml. To evaluate the treatment results, follow up after 3 months of supplementation is needed because the levels of 25 (OH) D levels reach a steady state after 3 months of dosing [23].
Clinical studies are ongoing in 2015 by the National Institute of Allergy and Infectious Diseases (NIAID) comparing vitamin D3 in SLE patients LES with doses of 800, 2000 and 4000 IU per day, where the phase 1 test showed that administration of cholecalciferol 4000 IU Day is safe and tolerable in African-American SLE patients. From these results, the administration of vitamin D should be individualized depending on baseline 25 (OH) D levels, with doses of at least 2000 IU per day in SLE patients with vitamin D deficiency, and 1000-1500 IU per day in SLE patients with vitamin D insufficiency.
Supplementation with Vitamin D Decreased anti-dsDNA levels
In this study, there was a significant anti-dsDNA decrease after vitamin D supplementation, either in the curcumin or placebo group, with a median of 97.4 (IQR: 15.6-223.5) IU / ml before supplementation and 34.20 ( IQR: 21.6-184.6) IU / ml (p<0.05) after supplementation. In the vitamin D placebo group, the median was 68.10 (IQR: 28.92-210.08) IU / ml to 23.45 (IQR: 15.72-75.65) IU / ml (p<0.05). There was also a positive correlation between increased delta levels of vitamin D with decreased delta levels of anti-dsDNA (p<0.05).
A study conducted by Attar (2013) in SLE patients with hypovitaminosis D showed that there was a negative correlation between vitamin D levels and anti-dsDNA levels, in which lower vitamin D levels will have a higher antidsDNA level [24]. These results are supported by previous studies by Thud et al., Kusworini et al., Terrier et al. and Anna A Kingdom, which found similar results [20,21,25,26]. This is according to a study conducted by Linker (2001), which indicates that there are B cells that express VDR. PBMC incubation in SLE patients with 1,25OH 2 D3 can reduce the proliferation and production of anti-dsDNA derived from B-lymphoblasts and induces apoptosis in active B-lymphoblasts [27]. A study conducted by Terrier (2012) in SLE patients with hypovitaminosis D who received 100,000 IU cholecalciferol per week for 4 weeks followed by 100,000 IU per month for 6 months showed a significant increase in vitamin D levels and decreased levels of anti- dsDNA. Treg was increase in both the percentage and absolute count on resting and activated Treg memory. The increase in Treg is associated with increased expression of molecules such as CTLA4, GITR and LAP [21].
A significant decrease in Th17 is also present, thus indirectly reduce autoantibodies through reductions in B-cell activating factor (BAFF). In the administration of vitamin D, a decrease of IgD - CD27+ and IgD-CD27- B lymphocytes (memory B cells) and anti-dsDNA are also present. This is in accordance with in vitro studies, that 1,25 (OH) 2 vitamin D3 decreases cell B proliferation, plasma cell differentiation and decreased IgG secretion.
Supplementation with Vitamin D Decreased Proteinuria
In this study we found significant decreased proteinuria in each treatment group. In the vitamin D + curcumin group the results were 400 (IQR: 240-1040) mg / g to 200 (IQR: 100- 390) mg / g (p<0.05). While in the vitamin D group placebo the results were 655 mg/g (IQR: 197.5-1637.5) to 210 mg / g (IQR:110-885) (p<0.05).
One of the manifestations of SLE is lupus nephritis (LN). This kidney involvement accounts for nearly 60% of the SLE population with more prevalence in Asians, Hispanics, Native Americans and blacks, and especially in women of reproductive age. LN characterized by loss of self-tolerance, autoantibody production, like nuclear antigen and immune-mediated injury in the kidney. The main manifestation of NL is proteinuria. This is according to a study by Petri who followed 1006 SLE patients with hypovitaminosis D for 128 weeks who received supplementation of Vitamin D 2 50,000 IU per week with Ca / D3 2×200 IU per day, which showed an increase of 20 ng/ml concentration Vitamin D will lead to a decrease in protein-creatinine ratio of 4% and the likelihood of proteinuria greater than 0.5 grams per 24 hours decreased by 15% [22]. In a study using curcumin by Khajehdehi et al. curcumin was found to have a homologous function of vitamin D, suggesting that in SLE patients with lupus nephritis who relapsed or in a refractory period, administration of curcumin 3×22 mg for 3 months can improve protenuria, hypertension and hematuria without side effects [28].
Vitamin D 3 adminstration may lower levels of anti-dsDNA through a reduction in the proliferation and production of anti-dsDNA derived from B-lymphoblasts and induces apoptosis in active B-lymphoblasts, resulting in decreased proteinuria. In addition, increased levels of vitamin D also decrease proteinuria through a decrease effect of Th17 [27].
Supplementation with Vitamin D Decreased IL-17
In this study, IL-17 levels decreased significantly in both treatment groups. In the vitamin D + curcumin group, the levels were 43.0 (IQR: 30- 75) pg/ml to 20 (IQR: 13-29) (p<0.05). In the placebo vitamin D group, the levels were (IQR: 20-65) pg/Ml to 16 (IQR: 10-29) (IQR: 25- 28) pg/ml (p<0.05). However, when compared between the two groups there is no statistical difference.
Immunomodulatory function of vitamin D is shown from studies in mice by Chang (2010), where the administration of 1,25-dihydroxyvitamin D3 (1,25D3) showed improvement in autoimmune encephalomyelitis, which is followed by a decrease in the expression of IL-17 and IL-17F. In vitro, the administration of 1,25D3 on CD4+ T cells inhibits cytokine production by Th17. Administration of 1,25D3 induces the expression of C / EBP homologous protein (CHOP), a molecule that inhibits translation and involved in endoplasmic reticulum stress. Excessive CHOP expression can suppress the production of pro-inflammatory cytokines produced by Th17 [14]. A study by Denise in patients with multiple sclerosis showed that administration of 1,25-dihydroxyvitamin D 3 in vitro may decrease Th17 and its produced cytokines, one of which is IL-17 [29]. Data from the study by Terrier also showed a significant decrease in Th17 after administration of vitamin D.
Curcumin supplementation effect on the administration of Vitamin D3
Despite significant results in elevated levels of vitamin D, decreased anti-dsDNA, protein-creatinine and IL-17 ratios in both groups, both treatment and placebo, no difference in vitamin D levels was found when the two groups were compared. The median results of the increase (delta) in the treatment group were 8.6 (IQR: -0.4-15.2), compared to 11.6 (IQR: 6.32- 17.47) in the control group (p> 0.05). In the control group, vitamin D levels were higher than the treatment group although no significant differences were obtained.
Curcumin is a ligand of VDR (Vitamin D receptor) and has a functional homology with vitamin D. In the administration of curcumin alone without combination with vitamin D, good results are obtained. A study conducted by Lee in mice of lupus nephritis showed that supplementation of curcumin can decrease proteinuria and anti-dsDNA levels of antibodies [30]. This is supported by a study by Khajehdehi et al. in patients with lupus nephritis relapsing or in refractory which indicates that curcumin administration of 3×22 mg for 3 months can improve proteinuria, hypertension and hematuria without side effects.
In a study conducted by Liu, administration of vitamin D and curcumin is synergistic in inhibiting the proliferation of HL-60 cells in promyelocytic leukemia [16]. Mizwicki et al. also showed that simultaneous vitamin D and curcumin adminsitration can repair a defect in phagocytic macrophages in β amyloid in Alzheimer’s disease. Vtamin D and curcumin have different pathways in stabilizing the VDR helix associated with gene transcription regulation [17]. Menegaz stated that the binding site of curcumin is different from vitamin D, in which its site is in an alternative-pocket whereas the active form of vitamin D is in the genomic pocket.
This study showed similar results to the study conducted by Bartik, which indicates that in real-time PCR, administration of curcumin with 1,25OH 2 D3 is less effective than vitamin D alone in increasing levels of VDR mRNA that can initiate transcription. With western blot method it is also known that administration of curcumin with vitamin D does not increase levels of VDR, and even cause lower VDR compared to a single vitamin D adminstration. In vitro studies using radiolabeled also found that curcumin competed more than 50% by 1,25OH 2 D3 on VDR compared to other ligands. Therefore it is said by Bartik et al (2010) that administration of curcumin does not potentiate the action of vitamin D [13].
Another study supporting the results of this study was conducted by Guo (2013), from in vitro results showed that combination of vitamin D with curcumin was not better than vitamin D alone in cathelicidin antimicrobial peptide (CAMP) expression [31].
No side effects of hypercalcemia were found in this study. Serum calcium levels are within normal limits after 3 months of treatment. In the treatment group, the levels were 8.84 ± 0.44 to 8.90 ± 0.39 mg/dl; and in the control group the levels were 8.93 ± 0.66 to 8.93 ± 0.8 mg/dl.
Conclusion
The addition of curcumin in vitamin D is not better than vitamin D alone. Increased vitamin D levels is correlated with decreased anti-dsDNA and IL-17 antibodies, and provide good results of improvement of proteinuria.
Limitation
The Curcuma xanthorrhiza used in this study had low bioavailability. No examination of curcuma xanthorrhiza levels in the blood after obtaining curcuma.
References
- Yung S, Chang TM. Mechanisms of kidney injury in lupus nephritis- the role of anti-dsDNA antibodies. Front. Immunol. 6, 475 (2015).
- http://emedicine.medscape.com/article/330369-overview
- Cameron JS. Lupus Nephritis. J. Am. Soc. Nephrol. 10, 413– 424 (1999).
- Fenton K, Fismen S, Hedberg A et al. Anti ds-DNA Antibodies Promote Initiation, and Acquired Loss of Renal Dnase1 Promotes Progression of Lupus Nephritis in Autoimmune (NZB×NZW) F1 mice. PLoS One. 4(12), e8474 (2009).
- Chunfang Gu, Ling Wu, Xiaoxia Li. IL-17 family: cytokines, receptors and signaling. Cytokine. 64(2), (2013).
- Apostolidis SA, Crispı´n JC, Tsokos GC. IL-17-producing T cells in lupus nephritis. Lupus. 20, 120–124 (2011).
- Hsu HC, Yang P, Wang J,et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat. Immunol. 9, 166–175 (2008).
- Puspitasari L, Kalim H, Wahono S. Correlation of Vitamin D Serum with Systemic Lupus Erythematosus Disease Activity. Arthritis. Res. Ther. (2012).
- Kamen DL, Cooper GS, Bouali H et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmune. Rev. 5, 114–117 (2006).
- Priyantoro ST, Kalim H, Wahono S et al. Effects of Vitamin D3 on Anti-dsDNA Levels and Proteinuria Degrees in Patients with Systemic Lupus Erythematosus (SLE) with Hypovitamin D. (2014).
- Kurien BT, D’Souza A, Scofield RH. Heat-solubilized curry spice curcumin inhibits antibody-antigen interaction in invitro studies: A possible therapy to alleviate autoimmune disorders. Mol. Nutr. Food. Res. 54(8), 1202–1209 (2010).
- Han SS, Keum YS, Seo HJ et al. Curcumin supresses activation of NF-κB and AP-1 induced by phorbol ester in cultured human promyelocytic leukemia cells. J. Biochem. Mol. Bio. 35, 337–342 (2012).
- Bartik L, Whitfield GK, Kaczmarska M et al. Curcumin: a novel nutritionally-derived ligand of the vitamin D receptor with implications for colon cancer chemoprevention. J. Nutr. Biochem. 23(12), 1153–1161 (2010).
- Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J. Bio. Chem. 285(50), 38751–38755 (2010).
- Pratama MZ, Kusworini, Endharti AT. Effect of Curcumin Supplementation to T Helper 17 and T Regulator In CD4+ T Cell Culture Systemic Lupus Erythematosus. (2015).
- Liu Y, Chang RL, Cui XX et al. Synergistic effects of curcumin on all-trans retinoic acid- and 1 alpha,25-dihydroxyvitamin D3-induced differentiation in human promyelocytic leukemia HL-60 cells. Oncol. Res. 9(2), 99 (1997).
- Mizwicki MT, Menegaz D, Zhang J et al. Genomic and nongenomic signaling induced by 1α,25(OH)2-vitamin D3 promotes the recovery of amyloid-β phagocytosis by Alzheimer's disease macrophages. J. Alzheimer Dis. 29(1), 51–62 (2012).
- Youssef DA, Peiris AN, Kelley JL et al.The possible roles of vitamin D and curcumin in treating gonorrhea. Clin. Immunol. 99(1), 82–93 (2013).
- Irastorza GI, Gordo S, Olivares N. Changes in Vitamin D Levels in Patients With Systemic Lupus Erythematosus: Effects on Fatigue, Disease Activity, and Damage. Arthritis. Care. Res. 62(8), 1160–1165 (2010).
- Abou-Raya A, Abou-Raya S, Helmii M. The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trial. J. Rheumatol. 40(3), 265–72 (2013).
- Terrier B, Derian N, Schoindre Y, et al. Restoration of Regulatory and Effector T cell balance and B cell Homeostasis in Systemic Lupus Erythematosus Patient through Vitamin D supplementation. Arthritis. Res. Ther. 14, 221 (2012).
- Petri M, Bello KJ, Fang H, et al. Vitamin D in Systemic Lupus Erythematosus modest association with disease activity and the Urine protein to creatinine ratio. Arthritis & Rheumatism. 65(7), 1865–1871 (2013).
- Singh A, Kamen DL. Potential benefits of vitamin D for patients with systemic lupus erythematosus. Dermato-Endocrinology. 4(2), 146–151 (2012).
- Attar SM, Siddiqui AM. Vitamin D Deficiency in Patients with Systemic Lupus Erythematosus. Oman. Med. J. 28(1), 42–47 (2013).
- Thud A, Yin S, Wandstrat AE et al. Vitamin D levels and disease status in Texas patient with Lupus Erythematosus. Am. J. Med. Sci. 335, 99–104 (2008).
- Kusworini H, Agus A, Singgih Wahono et al. Serum level of Vitamin D and Autoantibodies Level in Systemic Lupus Erythematosus (SLE) Patients. J. Pharm. Biol. Sci. 3(4), 16–20 (2012).
- Linker-Israeli M, Elstner E, Klinenberg JR et al. Vitamin D3 and its synthetic analogs inhibit the spontaneous in vitro immunoglobulin production by SLE-derived PBMC. Clin. Immunol. 99, 82–92 (2001).
- Khajehdehi P, Zanjaninejad B, Aflaki B. Oral Supplementation of Turmeric Decreases Proteinuria, Hematuria, and Systolic Blood Pressure in Patients Suffering From Relapsing or Refractory Lupus Nephritis: A Randomized and Placebo-controlled Study. J. Renal. Nutr. 22, 50–57 (2012).
- Denise SM, Medrado da Costa, Fereira TB et al. Vitamin D modulates different IL-17-secreting T cell subsets in multiple sclerosis patients. J. Neuroimmunology. 299, 8–18 (2016).
- Lee H, Kim H, Lee G et al. Curcumin attenuates lupus nephritis upon interaction with regulatory T cells in New Zealand Black/White mice. British J. Nutr. 110, 69–76 (2013).
- Guo C, Rosoha E, Lowry MB et al. Curcumin induces human cathelicidin antimicrobial peptide gene expression through a vitamin D receptor-independent pathway. J Nutr. Biochem. 24(5), 754–759 (2013).