Case Report - International Journal of Clinical Rheumatology (2022) Volume 17, Issue 3
Dilated cardiomyopathy in a young male with juvenile idiopathic arthritis
Nqoba Tsabedze1*, Mpoti Seboka2, Dineo Mpanya1,Ahmed Solomon2
1Division of Cardiology, Department of Internal Medicine, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
2Department of Rheumatology, Department of Internal Medicine, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
- *Corresponding Author:
- Nqoba Tsabedze
Division of Cardiology, Department of Internal Medicine, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
E-mail: Nqoba.Tsabedze@wits.ac.za
Received: 23-Apr-2020, Manuscript No. FMIJCR-20-9681; Editor assigned: 24-Apr-2020, PreQC No. FMIJCR-20-9681(PQ); Reviewed: 30-Apr-2020, QC No. FMIJCR-20-9681; Revised: 03-Mar-2022, Manuscript No. FMIJCR-20-9681(R); Published: 10-Mar-2022, DOI: 10.37532/1758-4272.2022.17(3).061-065
Abstract
Case Presentation: A 27-year-old male with a chronic history of juvenile idiopathic arthritis presented with acutely decompensated heart failure of unknown origin. He had no other traditional risk factors for cardiovascular disease. However, during the workup for idiopathic dilated cardiomyopathy, he was found to have extensive triple vessel disease on coronary artery angiography. This was subsequently thought to be the most likely aetiology for his dilated cardiomyopathy despite being of young age. The chronic juvenile idiopathic arthritis was identified as the key driver for the ischaemic heart disease.
Conclusion: Cardiovascular diseases remain the highest cause of morbidity and mortality in patients with inflammatory joint disease, irrespective of the presence of traditional cardiovascular risk factors.
Keywords: cardiomyopathy • atherosclerosis • autoimmune • arthritis • ischaemic heart disease
Abbreviations: CT: computed tomography • CVD: cardiovascular disease • DMARDS: disease modifying antirheumatic drugs • ECG: electrocardiogram • ELISA: enzyme-linked immunosorbent assay • EULAR: European League against Rheumatism • JIA: Juvenile idiopathic arthritis • RA: rheumatoid arthritis • SCD: sudden cardiac death • SCORE: Systematic Coronary Risk Evaluation
Introduction
The risk of cardiovascular disease (CVD) in patients with chronic inflammatory joint conditions is substantially increased when compared to the general population [1]. Patients with rheumatoid arthritis (RA) experience an increased cumulative incidence of silent myocardial infarction, heart failure and sudden cardiac death. There is a 2-fold increased risk of CVD events and a standardized cardiovascular mortality rate of 50% in RA [2, 3].
The underlying chronic inflammation may result in accelerated atherosclerosis, myocarditis or pericarditis [4]. The aim of this case report is to emphasis the importance of routine screening for ischaemic heart disease in young patients with chronic inflammatory joint diseases. To the best of our knowledge, this is the first case report describing an unusual presentation of ischaemic heart disease presenting as acutely decompensated dilated cardiomyopathy in a young male with juvenile onset idiopathic arthritis.
Case Report
Case presentation
We present a 27-year-old male with a onemonth history of neck and lower back pain associated with worsening shortness of breath on exertion. On general examination he had conjunctival pallor with grade three pedal oedema and abdominal ascites. His blood pressure measured 110/64 mmHg with a high pulse rate and normal pulse volume. Examination of the cardiovascular system revealed an elevated jugular venous pressure, a myopathic displaced apex beat with a right parasternal heave and an S3 gallop. He was in New York Heart Association functional class III.
On musculoskeletal examination, the patient had loss of cervical lordosis, tender and swollen joint count of eight, a clinical disease activity index score of 34, signifying high disease activity and fixed flexion deformity of the elbows, ankylosis of wrists and proximal interphalangeal joints of both hands. The respiratory examination was normal. The patient had been diagnosed with juvenile idiopathic arthritis at 15 years of age and treated with disease modifying anti-rheumatic drugs (DMARDS) as an outpatient. He had no traditional cardiovascular disease risk factors such as hypertension, diabetes and dyslipidemia. He reported no history of cigarette smoking nor any other form of substance abuse. His dietary intake was predominantly carbohydrate rich.
Investigations
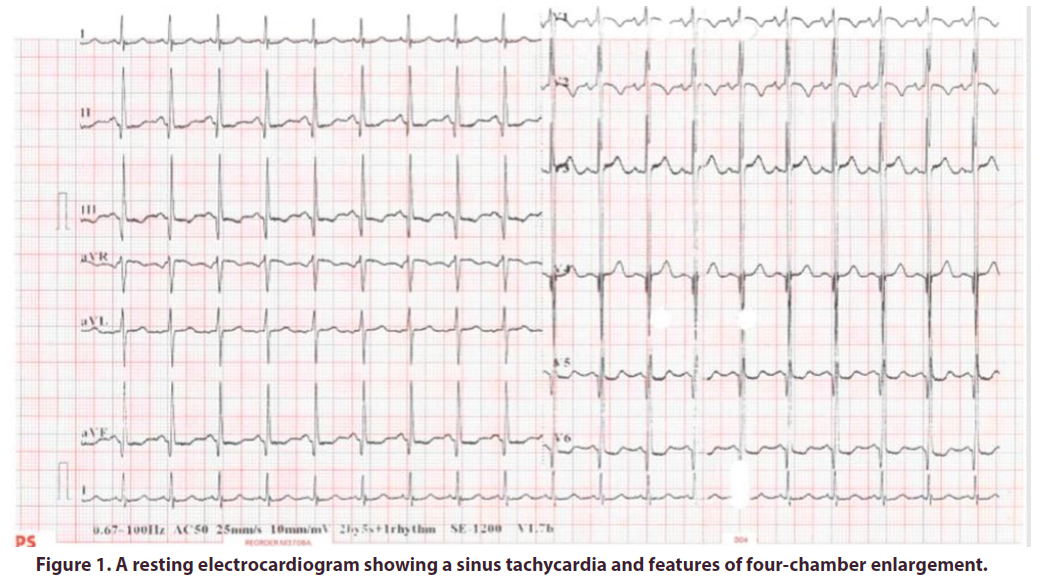
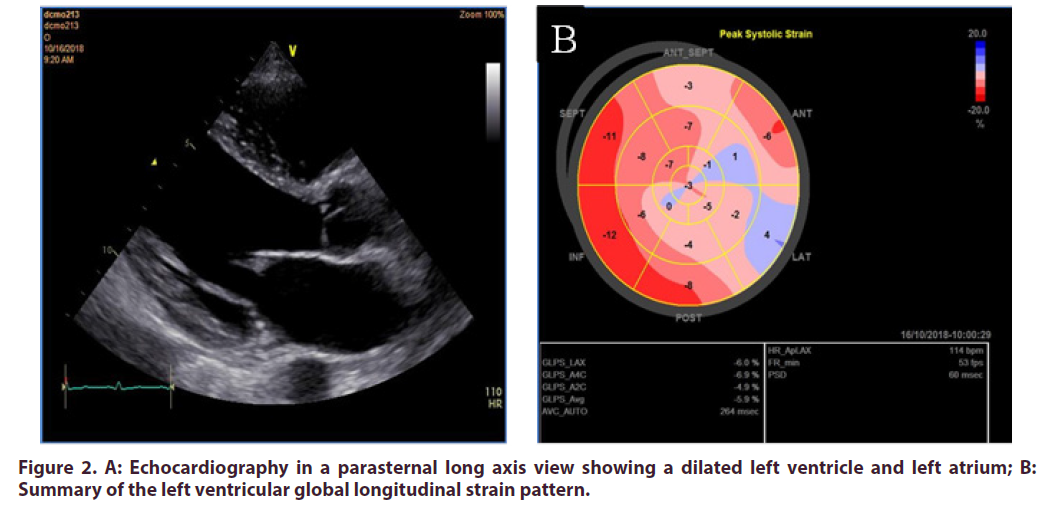
On routine biochemical investigations, all inflammatory markers were elevated (Table 1) however, the rheumatologic serological studies were unremarkable (Table 2). The Human Immunodeficiency Virus ELISA screen was negative. A resting electrocardiogram (ECG) showed a narrow QRS complex sinus tachycardia (Figure 1). Echocardiography demonstrated four chamber enlargement with global hypokinesis of the left ventricle and a left ventricular ejection fraction of 20- 25% (Figure 2). The patient also had mild functional mitral and tricuspid regurgitation. To investigate for the presence of vasculitis, a computed tomography (CT) aortogram of the chest was done which demonstrated a normal ascending aorta, aortic arch, descending thoracic and abdominal aorta and with no filling defects, no strictures and no aneurysmal dilatation.
| Laboratory study | Value | Reference Range |
|---|---|---|
| Sodium | 138 | 136 – 145 mmol/L |
| Potassium | 4.3 | 3.5 – 5.1 mmol/L |
| Chloride | 97 | 98 – 107 mmol/L |
| Bicarbonate | 20 | 23 – 29 mmol/L |
| Anion gap | 25 | 9 – 16 mmol/L |
| Urea | 7.4 | 2.1 – 7.1 mmol/L |
| Creatinine | 77 | 64 – 104 mmol/L |
| Estimated glomerular filtration rate | 135.4 | > 60 mL/min |
| Calcium | 2.25 | 2.15 – 2.50 mmol/L |
| Magnesium | 0.78 | 0.63 – 1.05 mmol/L |
| Liver Function Tests | ||
| Total protein | 83 | 60 – 78 g/L |
| Aspartate transaminase | 144 | 13 – 35 U/L |
| Alanine transaminase | 62 | 7 -35 U/L |
| Alkaline phosphatase | 172 | 42 – 98 U/L |
| Gamma-glutamyl transferase | 142 | < 40 U/L |
| Total bilirubin | 6 | 5 – 21 mmol/L |
| Albumin | 33 | 35 – 52 g/L |
| Lipid Profile | ||
| Total cholesterol | 2.83 | < 4.0 mmol/L |
| Triglyceride | 0.72 | < 1.7 mmol/L |
| LDL | 1.94 | < 1.8 mmol/L |
| HDL | 0.41 | > 1.0 mmol/L |
| Complete Blood Count | ||
| Haemoglobin | 9.3 | 11.6 – 16.4 g/dL |
| Platelets | 475 | 186 – 454 x109/L |
| White blood cells | 8.42 | 3.90 – 12.60 x 109/L |
| Red blood cells | 4.16 | 3.93 – 5.40 1012/L |
| Mean corpuscular volume | 76.2 | 78.9 – 98.5 fL |
| Cardiac enzymes | ||
| Creatine kinase | 31 | 20 – 180 U/L |
| CK-MB | 1.21 | 0.00 – 6.22 mg/L |
| Troponin T | 73 | £14 ng/L |
| Inflammatory Markers | ||
| Erythrocyte sedimentation rate | 120 | 0 – 10 mm/hr |
| C-reactive protein | 132 | < 10 mg/L |
| Iron Studies | ||
| Ferritin | 174 | 13 – 150 mg/L |
| Iron | 3.1 | 9.0 – 30.4 mmol/L |
| Transferrin | 1.92 | 2.0 – 3.6 g/L |
| Percent saturation | 6 | 15 – 50 % |
| Vitamin B12 | 570 | 145 – 569 pmol/L |
| Thyroid stimulating hormone | 3.65 | 0.27 – 4.20 mIU/L |
| CK-MB = creatine kinase -MB, HDL= high-density lipoprotein, LDL=low-density lipoprotein | ||
Table1. Basic laboratory studies.
| Laboratory study | Value | Reference Range |
|---|---|---|
| Anti-nuclear antibodies | Negative | |
| Anti-cyclic citrullinated peptide antibody | 2.0 | < 20 U/mL |
| Thyroglobulin | 42.6 | 3.5 – 77.0 mg/L |
| Anti-thyroglobulin antibody | 12 | < 115 U/mL |
| Anti-proteinase 3 antibody | 1.0 | 0 – 0.9 U/mL |
| Anti-myeloperoxidase antibody | 1.0 | 0 – 0.9 U/mL |
| Direct Coombs (typing) | ||
| IgG | positive | |
| C3d | negative | |
| Coagulation | ||
| INR | 1.29 | 2.0 – 3.0 |
| Prothrombin time | 18.2 | 14.0 sec (control) |
| INR = international normalized ratio | ||
Table 2. Rheumatologic Serologic Studies and Hypercoagulability Studies.
Figure 2. A: Echocardiography in a parasternal long axis view showing a dilated left ventricle and left atrium; B: Summary of the left ventricular global longitudinal strain pattern.
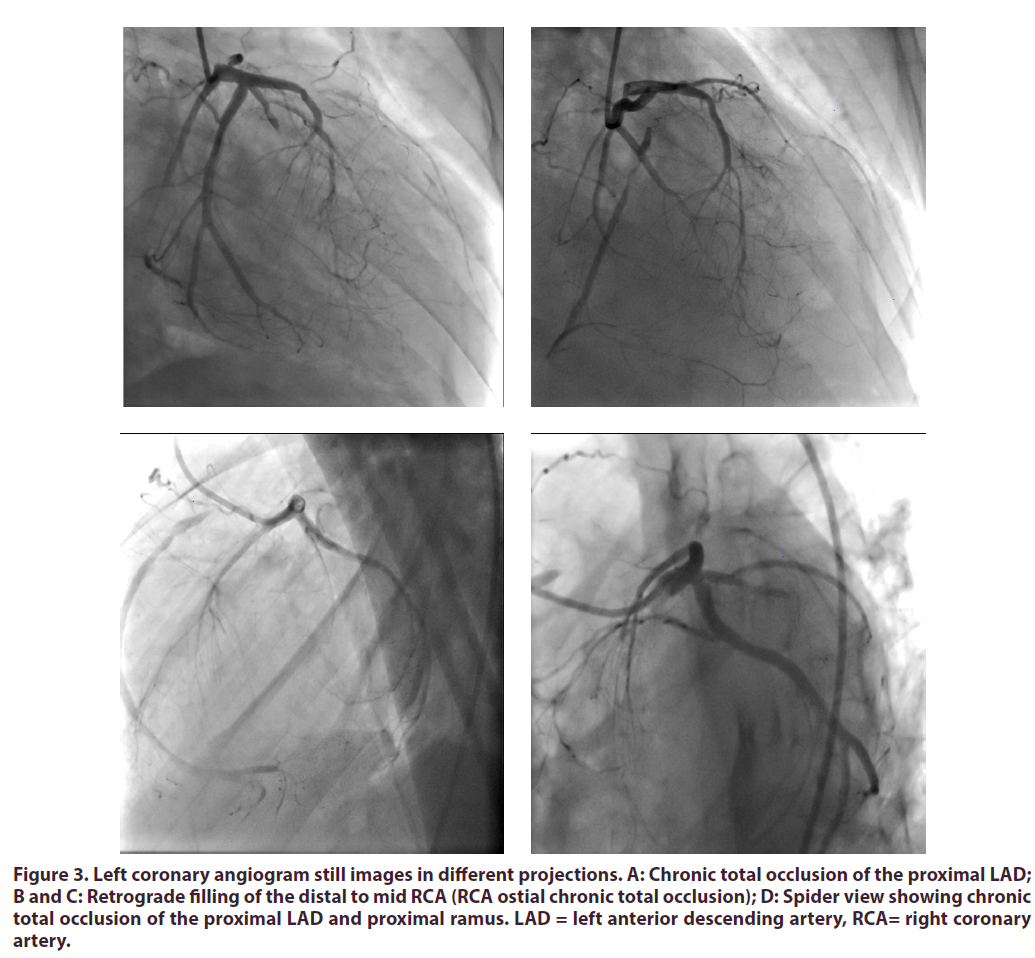
The diagnostic coronary angiogram revealed triple vessel disease (Figure 3) with a syntax score I of 36.5. There was no improvement in the caliber of the coronary arteries after administering intracoronary nitroglycerin, thus excluding coronary artery vasospasm as a possible aetiology for the coronary artery disease. Furthermore, the patient gave no prior history in keeping with typical angina pectoris.
Figure 3. Left coronary angiogram still images in different projections. A: Chronic total occlusion of the proximal LAD; B and C: Retrograde filling of the distal to mid RCA (RCA ostial chronic total occlusion); D: Spider view showing chronic total occlusion of the proximal LAD and proximal ramus. LAD = left anterior descending artery, RCA= right coronary artery.
Management
The patients’ rheumatic symptoms were treated with pulsed solumedrol 1g for 3 days followed by prednisone at 0.5mg/kg. Thereafter, two cycles of cyclophosphamide were administered two weeks apart using the Euro- Lupus regimen. As part of the heart failure therapy, the patient was acutely initiated on furosemide and low dose carvedilol, an angiotensin converting enzyme inhibitor and spironolactone to which he responded well. The patient was also treated with simvastatin, baclofen, chloroquine, methotrexate, folic acid and carbamazepine. The patient’s rheumatic symptoms settled once DMARDS were initiated. The institutional heart team decision was to plan for an elective coronary artery bypass grafting once the inflammatory markers had settled. Unfortunately, three weeks later, the patient experienced a sudden cardiac arrest while at home. He was found unconscious on a couch. A post-mortem examination was not requested.
Differential Diagnosis
The differential diagnoses were dilated cardiomyopathy secondary to accelerated atherosclerosis and vasculopathy of the coronary arteries as a sequela of the juvenile idiopathic arthritis. Idiopathic dilated cardiomyopathy was also considered, being a common condition seen in young African men with no cardiovascular risk factors. Considering the patient’s age, we explored the possibility of coronary artery vasospasm as the cause of the coronary artery disease. However, the patient did not report a history of angina-like chest pains nor did he report a history of recreational substance abuse.
Follow-up
Our patient eventually died at home soon after discharge. This was most likely due to a lethal arrhythmia such as ventricular tachycardia. It is widely accepted that acutely decompensated severe heart failure patients die in the acute setting from pump failure and complicate with multi organ dysfunction. Those patients who survive the acute setting usually complicate later with a sudden cardiac death (SCD) event. A life vest would have been an ideal therapy to prevent SCD [5]. However, in our local clinical setting these therapies are not readily available due to cost.
Discussion
Juvenile idiopathic arthritis (JIA) refers to a group of conditions characterized by inflammatory joint disease of unknown aetiology, occurring before the age of 16 years and lasting more than 6 weeks [6]. This is one of the common rheumatologic disabling conditions of childhood.
Patients with RA experience high-grade inflammation driven by augmented cytokine production, which associates with metabolic risk including insulin resistance and reduced HDL cholesterol levels. Furthermore, inflammatory molecules can also directly impair endothelial function. There is also a trend for a higher prevalence of traditional cardiovascular risk factors in patients with rheumatoid arthritis. In light of these findings, the European League against Rheumatism (EULAR) task force formulated guidelines on screening, risk-stratification and management of CVD in patients with inflammatory joint disease (IJD) [4].
Numerous risk scoring tools are available for estimating the 10-year risk for CVD, however in a population of patients with underlying IJD, some of the risk scoring tools have been found to under-estimate the risk of CVD. Arts et al. evaluated the predictive ability of four cardiovascular risk models, namely the Framingham risk score, Systematic Coronary Risk Evaluation (SCORE) risk chart, QRisk II and Reynolds risk score in a cohort of European patients with rheumatoid arthritis. The QRisk II overestimated cardiovascular risk, while the rest of the models underestimated CV risk [7]. The EULAR task force advocated for the use of a 1.5 multiplication factor on the calculated score for these risk prediction models when certain features of RA disease were present [8]. The Framingham risk score, SCORE risk chart and the Reynold’s risk score were not useful in our patient, since the patient was younger than 30 years of age.
Conclusion
Clinicians treating patients with rheumatological conditions should actively screen for cardiovascular disease, despite the absence of traditional cardiovascular risk factors.
Availability of data and materials
Data sharing is not applicable to this article since no datasets were analysed.
References
- Solomon DH, Peters MJ, Nurmohamed MT et al. Unresolved questions in rheumatology: motion for debate: the data support evidence-based management recommendations for cardiovascular disease in rheumatoid arthritis. Arthritis Rheum. 65(7),1675-83 (2013).
- Charles-Schoeman C. Cardiovascular disease and rheumatoid arthritis: an update. Curr Rheumatol Rep. 14(5),455-62 (2012).
- Avina-Zubieta JA, Choi HK, Sadatsafavi M et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 59(12),1690-7 (2008).
- Agca R, Heslinga SC, Rollefstad S et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 76(1),17-28 (2017).
- Theuns DA, Smith T, Hunink MG et al. Effectiveness of prophylactic implantation of cardioverter-defibrillators without cardiac resynchronization therapy in patients with ischaemic or non-ischaemic heart disease: a systematic review and meta-analysis. Europace. 12(11),1564-70 (2010).
- Petty RE, Southwood TR, Manners P et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 31(2),390-2 (2004).
- Arts EE, Popa C, Den Broeder AA et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 74(4),668-74 (2015).
- Peters MJ, Symmons DP, McCarey D et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 69(2),325-31 (2010).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref