Case Report - International Journal of Clinical Rheumatology (2021) Volume 16, Issue 4
Coronavirus disease 2019 (COVID-19) an emerging trigger for primary fibromyalgia syndrome: A tale of three cases post-COVID-19
- *Corresponding Author:
- Tamer A Gheita
Rheumatology and Clinical Immunology, Faculty of Medicine, Cairo University, Egypt
E-mail: gheitamer@hotmail.com
Abstract
Background: Corona virus (COVID-19) is an emerging worldwide infectious disease that may be followed in some patients by post COVID-19 syndrome; patients who experience symptom of disease long time after their recovery. Among the frequent post-COVID-19 symptoms are fatigue, headache, sleep disorder, anxiety and musculoskeletal pain which coincide with features of Fibromyalgia Syndrome (FMS). This study reports out of the ordinary scenarios and outcomes for three cases who developed primary FMS post COVID-19.
Method: Three females, not known to have previous FMS or any other rheumatic disease, post COVID-19 were referred for rheumatology consultation due to the prolonged persistence of symptoms after recovery from COVID-19.
Results: The cases presented with anxiety and depression, headache, generalized musculoskeletal pain, paresthesia and non-restorative sleep. General examination and various laboratory investigation including autoimmune profile as well as radiological investigation were all normal. Tender points on examination lead to the diagnosis of FMS. Non-pharmacological and pharmacological management with antidepressant therapy were prescribed with improvement.
Conclusions: We are presenting the first three cases of COVID-19 infection as an emerging trigger for FMS. This association should be considered during management of post COVID-19 syndrome to improve pain care and prevent worsening of symptom during the COVID-19 pandemic.
Keywords
COVID-19 • fibromyalgia syndrome • trigger • SARS-CoV-2 • ACE
Introduction
The novel coronavirus disease 2019 (COVID-19) has swept the world and caused a global pandemic [1] coupled with challenges that should be prepared for [2]. Streamlining of workflows for rapid diagnosis, isolation, clinical management, and infection prevention will matter not only to patients with COVID-19, but also to patients who at risk and health-care workers [2]. In the highly interconnected world, the vastly transmissible COVID-19 causes relatively high mortality, particularly in aging populations [3]. The diagnosis for Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection is largely based on Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) with high rate of false-negative results and has been found largely inferior to that of a chest computed tomography [4]. To date, much public health messaging regarding COVID-19 has focused on social distancing, hand hygiene, Personal Protective Equipments (PPE) for health care workers, and the need for increased testing [5].
In COVID-19, the innate immune system is activated producing and releasing proinflammatory cytokines such as interleukins (IL-6, IL-1β and IL-8) and tumor necrosis factor-α and other chemokines. The excessive inflammatory response may result in Cytokine Storm syndrome that causes coagulation abnormalities, excessive oxidation and mitochondrial permeability, vital organ damage, immune system failure and eventually disseminated intravascular coagulation [1]. The clinical manifestations of COVID-19 include fever, cough, fatigue, muscle pain, diarrhea, and pneumonia, which can develop to acute respiratory distress syndrome, metabolic acidosis, septic shock, coagulation dysfunction, and organ failure [6]. However, most patients recover, some experience disease progression. Clinical outcomes of COVID-19 are considerably worse in persons with medical comorbidities and immunosuppression [7]. The long term effects of the pandemic in patients with rheumatic diseases will not be known until much later and will likely include stressors flaring disease, flaring due to stopping medications and less physician visits with subsequent under-treatment [8]. A previous study conducted by the Egyptian College of Rheumatology (ECR) study group found that patients with rheumatoid arthritis faced remarkable difficulty to obtain their medications with subsequent change in their disease status during COVID-19 pandemic [9]. Most commonly reported neuronal presentations associated with SARS-CoV-2 infection involved headache, nausea, vomiting, anosmia and ageusia, impaired visual acuity, pain and muscular symptoms like fibromyalgia. Anxiety, anger, confusion, post-traumatic stress symptoms, and cognitive impairment were observed [10]. Acute or chronic stress may trigger or aggravate symptoms of Fibromyalgia Syndrome(FMS) [11].
Fibromyalgia syndrome is fairly common reaching a prevalence of 2% to 3% worldwide. It comprises not only chronic widespread pain, fatigue and sleep alterations but also autonomic disturbances, cognitive dysfunction, hypersensitivity to external stimuli, somatic symptoms and psychiatric disorders [8]. FMS and chronic fatigue syndrome are distinct conditions that share a common pathophysiological etiology involving central sensitization [7] and many other factors such as genetic, immunological, and hormonal [12]. Interestingly, inhibition of ACE 1 can enhance pain by blocking the degradation of substance P and bradykinin, besides enhancing kinin receptors signaling leading to fibromyalgia-like symptoms [13]. Furthermore, a remarkable association of Angiotensin- Converting Enzyme 1 (ACE-1) gene polymorphism with the development of FMS has been addressed [14]. ACE/ACE2 balance is critical in the regulation of electrolyte homeostasis, vascular and cardiac remodeling and inflammation [15].
Attention for the possible development of FMS during post COVID-19 syndrome has risen. Few studies were conducted during the COVID-19 pandemic had a variable impact on the well-being of patients with FMS. While all FMS symptoms were more severe in patients with FMS and COVID-19 infections [16], for others, this period brought the opportunity to introduce changes in daily lifestyle, which resulted in improvement in well-being. Accordingly, this complicated relation has induced attention for the possible relationship of FMS and COVID-19 syndrome and the way we deliver health care. In this article we report out of the ordinary scenarios and outcomes for three cases who developed primary fibromyalgia syndrome following the recovery of COVID-19 infection (post-COVID-19).
Case reports
Three female patients with no previous history of FMS or any other rheumatic disease, were referred for rheumatology consultation regarding the persistence of symptoms after recovery from COVID-19 infection.
Case 1
A 39-year-old housewife presented in June 2020 with moderate grade fever, watery diarrhea, nausea, severe abdominal pains, sudden loss to the sense of taste and smell, anorexia, fatigue, and generalized muscle pains. Two days later she developed dry cough and mild dyspnea. There was no history of hypertension or diabetes. Her investigations revealed: Hemoglobin (Hb) 11.9 g/dl, mild microcytic hypochromic anemia, Total Leucocytic Count (TLC) of 2.4 x103/mm3 with relative monocytosis (18%), absolute neutropenia (29%), platelet count of 203 x103/mm3. The Erythrocyte Sedimentation Rate (ESR) was 54mmHg/1st hour, C-reactive protein (CRP) was positive (7.9 mg/L), serum ferritin: 361 ng/mL (normal 13-150), serum lactate dehydrogenase (LDH): 169 U/L and D-dimer 0.48 mg/L (normal <0.5 mg/L). A nasopharyngeal swab was analyzed by RT-PCR and confirmed a SARS-CoV- 2 infection. Computerized Tomography (CT) chest and echocardiography were normal. She was given azithromycin 500mg/day orally for 5 d, followed by ceftriaxone 1 gm/day IM for a week, prednisolone 20 mg/day with gradual weaning off over one month, rivaroxaban 15 mg/day for 27 d, low dose aspirin 81 mg/day for 3 m, proton pump inhibitor, L-carnitine, iron supplement and Co-enzyme Q (10 mg/day). There was improvement in her gastrointestinal symptoms, body temperature normalized with partial regain in the sense of smell and taste. There was persistence of fatigue even on mild exertion, with generalized muscle pains.
In September 2020, she reported new symptoms as anxiety, migraine headache, generalized paresthesia especially in the upper limbs, allodynia, non- restorative sleep and lack of concentration. On examination: The Body Mass Index (BMI) was 25.4 and there was no joint tenderness or swelling. 11/18 tender points for fibromyalgia were positive. Other systems were normal. Patient global assessment of pain on a 100 mm Visual Analogue Scale (VAS) was 100. Her laboratory tests then showed Hb (13.6 g/dl), TLC 8.5 x103/mm3, platelets 319 x103/mm3, ESR 18 mmHg/1st hour, CRP 5.4 mg/L. Hepatitis C-virus antibodies were negative, Antinuclear Antibodies (ANA) negative,Rheumatoid Factor (RF) Negative, Thyroid Stimulating Hormone (TSH) 0.7 mlU/L, 25(OH) vitamin-D 18.5 ng/ ml, Alanine Transaminase (ALT) 25U/L, Aspartate Transaminase (AST) 24U/L, creatinine 0.9 mg/dl, HbA1c:5.5%, serum ferritin 35 ng/ml and D-dimer: 0.3 mg/L. A diagnosis of primary fibromyalgia was held according to the 2010 ACR preliminary diagnostic criteria [17]. The patient was given duloxetine 30 mg/ day however, she developed gastritis with marked GIT upset and dizziness after few days so it was discontinued and replaced by gabapentin 300 mg twice daily, vitamin D3 injection 200,000 I.U./month for 3 consecutive months, vitamin C 1 g/day, omega 3 capsule/day, vitamin B injection/10 days for two months and paracetamol tablets when required in case of pain and headache. On follow up four months later, January 2021, the patient’s general wellbeing improved with less paresthesia and only mild sleep disturbances. The VAS became 60. On clinical examination, there were fewer tender points 7/18. All routine investigations were within normal range and 25(OH) vitamin D became 35.9 ng/ml. General instructions were provided to the patient for proper exposure to sunlight 10 minutes/ day over her limbs, proper hydration with at least 10 cups of water/day, follow healthy food habits and walk regularly. She continued on gabapentin capsule 300 mg/d, paracetamol tablet when required and would also regularly consider meditation.
Case 2
A 44-year-old housewife presented on March 2020 with severe chest pain, dyspnea, fever and dry cough. She was a chronic heavy cigarette smoker 2 packs/week and was not known to be diabetic or hypertensive. She was accustomed to travel abroad and since the COVID-19 pandemic she was completely locally locked down. Her investigations revealed normocytic normochromic anemia, leukocytosis 12.3 x103/mm3 with a shift to left and CRP 12.8 mg/L. Chest x-ray revealed exaggerated bronchovascular markings. High resolution CT chest showed pneumonic patches for which she received multiple antibiotics but with mild improvement. Two nasopharyngeal swabs were analyzed by RT-PCR, the first was negative but the second confirmed a SARSCoV- 2 infection. Her D-dimer was high normal 0.48 mg/L. She received azithromycin 500 mg/day orally for 5 d, hydroxychloroquine 400 mg/day, lactoferrin, zinc, vitamin C and vitamin D supplements, mast cell stabilizer and nasal steroid drops with moderate improvement. As chest tightness persisted, she quit smoking. Five months later, August 2020, she developed insomnia, restless leg syndrome, severe generalized muscle pains, allodynia and hyperacusis. The VAS was 100 mm.A bone scan and tumor markers were all negative. The condition progressed with panic attacks and depression, so she consulted a psychiatrist and was prescribed Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs), venlafaxine 75mg/day for three weeks with mild improvement. After a rheumatological consultation, she was diagnosed to have primary FMS according to the 2010 ACR criteria [17] with 18/18 tender points. Duloxetine 60 mg/day at night was added to her current intake of venlafaxine 75 mg/day. The patient was advised to lead a stress-free lifestyle and zinc supplements were provided. On follow up three months later, November 2020, the general wellbeing of the patient improved with almost normal sleep rhythm and restless leg syndrome recovered. On examination there were fewer tender points 12/18 and her VAS became 60. The patient continued on the same medications and started to receive cognitive therapy.
Case 3
A 41-years-old health worker, on June 2020, started to develop fever, severe chest pain, fatigue, generalized muscle and bone pain and dry cough. She was known to have controlled type 2 diabetes and hypertension. Her investigations revealed: Hb 12.5 g/dl, TLC of 9.0 x103/ mm3 with relative lymphopenia, platelets of 226 x103/ mm3. The ESR was 44 mm/1st h, the CRP was positive (24.3 mg/L), serum ferritin: 184 ng/mL, D-dimer 0.5 mg/L, ALT 18 U/l, AST 21 U/l, and HbA1c: 9.7%. Two PCRs for COVID-19, 21 days apart, were positive. CT chest was normal. She was diagnosed as a case of COVID-19 infection and was given azithromycin 500 mg/day, low dose aspirin 81 mg/d for 3 months, iron, Zinc, vitamin C and vitamin D supplement. There was improvement in her chest symptoms, normalized body temperature. However, there was persistence of fatigue, with generalized muscle and bone pain especially on the morning. Two months later, August 2020, she developed progressive insomnia, mood disturbance with depressive symptoms and panic attacks. The patient was advised to receive pain relives and to have a stress-free lifestyle but with no improvement. February 2021, investigations revealed Hb 13.7 g/dl, TLC 13.5 x103/mm3, platelets of 292 x103/mm3. CRP: 23.7 mg/L, serum ferritin: 128.1 ng/mL, D-dimer 0.5 mg/L, ANA and RF were negative. After a rheumatologic consultation, the diagnosis of primary FMS was considered [17] with 15/18 tender points. Gabapentin capsule 400 mg/day was started, and a month later there was mild improvement in her well-being and mood status.
Discussion
Fibromyalgia syndrome is a stress–related disorder; psychological and other types of stressors are frequent fibromyalgia drivers [18]. The global FMS symptoms were significantly more severe, and impairment was higher in patients with COVID-19 infection [16]. Meanwhile, the COVID-19 pandemic has generated long-lasting widespread assorted stressful events with direct influence of the imposed lifestyle changes on FMS symptoms [18]The spike protein on this virus binds the ACE2 receptor, mediating entry into human cells and provoking antagonistic pathways of the renin-angiotensin system and imbalance of the ACE/ Angiotensin II and its receptor as well as the ACE2/ angiotensin (1–7)/Mas receptor pathways. By inducing a decrease of membrane ACE2 receptors on host cells, an imbalance of ACE/ACE2 occurs that contributes critically to the pathogenesis of SARS-CoV-2 infection and triggers severe lung injury [19]. Nevertheless, the ACE/ACE2 imbalance could be the common pathway (theory) to explain the underestimated and overlooked link between the development of FMS and COVID-19. In this work three cases are presented with a peculiar onset of FMS post-COVID-19 and the diagnostic dilemma due to the overlap of symptoms before consulting a rheumatologist are presented.
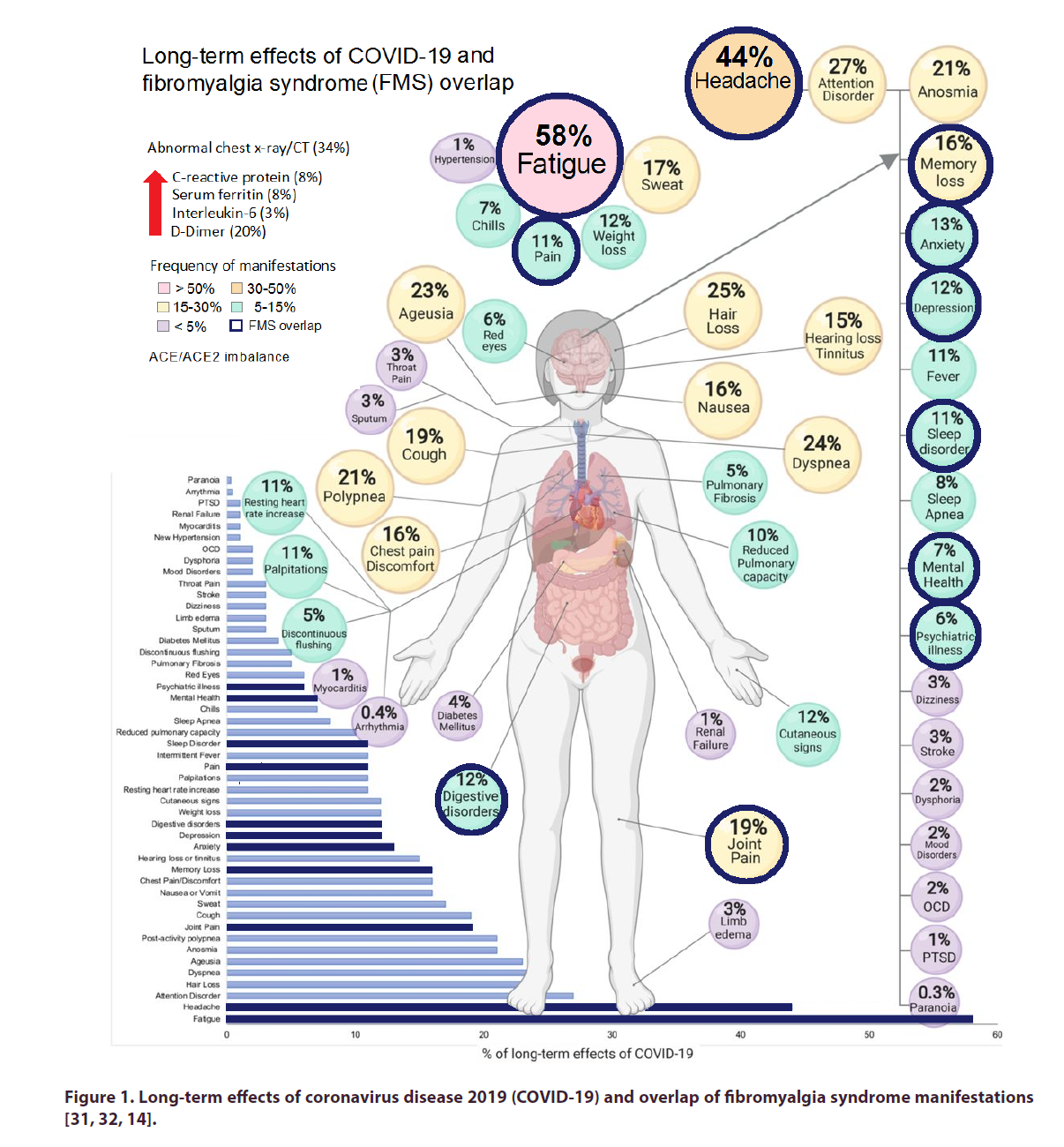
Patients with FMS often experience clinical impairment with stress [20] and adverse mental and physical outcomes during the COVID-19 outbreak have been reported. Factors such as stopping current treatments may play a central role and social support was found to be protective [11]. However, others reported no clinical impairment due to COVID-19 confinement. The perceived worsening among FMS patients relied primarily on how patients cope with their disease, without a real impact on clinical manifestations [20]. It has been suggested that the post-COVID syndrome with persisting fibromyalgia-like symptoms will potentially join the spectrum of rheumatic disorders [21]. Interestingly, during lock-down due to COVID-19 pandemic, 64.5% of patients presenting with increased musculo-skeletal pain had a low vitamin D3 levels and 45% developed symptoms of FMS [22].Fibromyalgia was reported as an independent factor associated with greater pain severity as a result of social isolation during the COVID-19 pandemic [23] Long term effects of COVID-19 have been reported and are presented in Figure 1 with adaptation to refer to overlapping manifestations of FMS [12, 19, 24]. Non-hospitalized ‘long hauler’ patients with COVID-19 experience “prominent and persistent” neurological symptoms, including brain fog and fatigue and is considered an important emerging entity that requires a multidisciplinary approach to care [25]. The COVID-19 pandemic has a substantial impact on rheumatology and is back to the lime-light [21]. With predicted worsening of symptoms, we anticipate direct medical expenses, and further loss of productivity. Further, given the highly comorbid nature of mood disorders in patients with FMS, we fear that this pandemic will add detrimental impact on mood and could lead to increased suicidal ideations, a trend that is already being seen during the COVID-19 pandemic in the general population. This potential impact should be further investigated in patients with FMS.
Figure 1. Long-term effects of coronavirus disease 2019 (COVID-19) and overlap of fibromyalgia syndrome manifestations [31, 32, 14].
The restraints imposed by the lockdown during the COVID-19 pandemic had a variable impact on the well-being of patients with FMS. Physicians should be mindful of these individual differences and discuss with FMS patients how beneficial changes might be maintained or even implemented in the aftermath of the pandemic [24]. Patients with chronic medical conditions need routine assessment; patients with FM should be treated no differently. Thus, we recommend routine follow-up visits, whether done virtually (telehealth) or in person. These visits will allow for assessing current symptoms, adherence to medications, and the presence of any red-flag concerns. Furthermore, these encounters will help to promote a supportive environment, and take the opportunity to further reinforce the utility of effective nonpharmacologic treatment options. Nonpharmacologic treatment should include healthy lifestyle, meditation, cognitive behavioral therapy, biofeedback therapy, and ongoing patient education. Patients should be made aware of these convenient options and encouraged to participate. Interestingly, in Spain, a telerehabilitation program based on aerobic exercise in women with FMS during the lockdown declared due to the COVID-19 pandemic lead to remarkable improvements in pain intensity, mechanical pain sensitivity, and psychological distress [19]. In the treatment for FMS, some analgesics, particularly pregabalin and most antidepressants were associated with COVID-19 incidence, whereas duloxetine presented a negative relation [25]. It may be also considered that since hypertension is a frequent late effect of COVID-19 [24] and is a comorbidity affecting FMS patients, in such cases hypertension treatment with ACE inhibitors should be avoided and other classes of antihypertensive drugs should be preferable [18]. Although there are reports that COVID-19 infection may result in a cytokine storm with a massive release of various cytokines particularly interleukins, biologics may be potentially effective in treating severe cases [26]. As a consequent lesson from the COVID-19 pandemic, a shift from investing primarily in disease-specific resource targets is emerging toward decoding the human immune system [3].
In conclusion, as we are in the midst of an unprecedented pandemic, we can only speculate the long-term effects and implications of COVID-19. Among persons with COVID-19, the development of FMS is anticipated as a consequence to pandemic-associated stressors, isolation and uncertainties that further dysregulate the underlying ACE. Consequently, the health of these people could be profoundly and negatively affected. Hopefully the detailed stories of the three cases end up throwing light on the newly emerging trigger for FMS and may thus help the world in its fight against this fierce enemy. Such association should be considered in the planning, development, and prioritization of interventions to improve pain care and to prevent worsening of symptoms during the continuing COVID-19 pandemic. In the middle of this pandemic, this vulnerable group should not be overlooked.
Conflicts of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Phua J, Weng L, Ling L et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet. Respir. Med. 8, 506–517(2020).
- Carbone M, Green JB, Bucci EM et al. Coronaviruses: Facts, Myths, and Hypotheses. J. Thorac. Oncol. 15, 675–678(2020).
- Yokota S, Miyamae T, Kuroiwa Y et al. Novel Coronavirus Disease 2019 (COVID-19) and Cytokine Storms for More Effective Treatments from an Inflammatory Pathophysiology. J. Clin. Med. 10, (2021).
- Zhang W, Du R-H, Li B et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microbes. Infect. 9, 386–389(2020).
- Koff WC, Williams MA. Covid-19 and Immunity in Aging Populations - A New Research Agenda. N. Engl. J. Med. 383, 804–805(2020).
- Wang D, Hu B, Hu C et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 323, 1061–1069(2020).
- Mohabbat AB, Mohabbat NML, Wight EC. Fibromyalgia and Chronic Fatigue Syndrome in the Age of COVID-19. Mayo. Clin. Proc. Innov. Qual. Outcomes. 4, 764–766(2020).
- Pope JE. What Does the COVID-19 Pandemic Mean for Rheumatology Patients?. Curr. Treat. Options. Rheumatol. 1–4(2020).
- Abualfadl E, Ismail F, Shereef RRE et al. Impact of COVID-19 pandemic on rheumatoid arthritis from a Multi-Centre patient-reported questionnaire survey: influence of gender, rural-urban gap and north-south gradient. Rheumatol. Int. 41, 345–353(2021).
- Javed A. Neurological associations of SARS-CoV-2 infection: A Systematic Review. CNS. Neurol. Disord. Drug. Targets.(2021).
- Aloush V, Gurfinkel A, Shachar N et al. Physical and mental impact of COVID-19 outbreak on fibromyalgia patients. Clin. Exp. Rheumatol.(2021).
- Bellato E, Marini E, Castoldi Fet al. Fibromyalgia syndrome: etiology, pathogenesis, diagnosis, and treatment. Pain. Res. Treat. 2012, 426130(2012).
- Brusco I, Justino AB, Silva CR et al. Inhibitors of angiotensin I converting enzyme potentiate fibromyalgia-like pain symptoms via kinin receptors in mice. Eur. J. Pharmacol. 895, 173870(2021).
- Inanir A, Yigit S, Tekcan A et al. Angiotensin converting enzyme and methylenetetrahydrofolate reductase gene variations in fibromyalgia syndrome. Gene. 564, 188–192(2015).
- Guney C, Akar F. Epithelial and Endothelial Expressions of ACE2: SARS-CoV-2 Entry Routes. J. Pharm. Pharm. Sci. 24, 84–93(2021).
- Chaudhary M. COVID-19 susceptibility: potential of ACE2 polymorphisms. Egypt. J. Med. Hum. Genet. 21, 1–8(2020).
- Wolfe F, Clauw DJ, Fitzcharles M-A et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis. Care. Res. 62, 600–610(2010).
- Hügle T. [Rheumatology update 2020: the focus was on Covid-19]. Rev. Med. Suisse. 17, 214–218(2021).
- Hernando-Garijo I, Ceballos-Laita L, Mingo-Gómez MT et al. Immediate Effects of a Telerehabilitation Program Based on Aerobic Exercise in Women with Fibromyalgia. Int. J. Environ. Res. Public. Health. 18, (2021).
- Dhatt SS, Kumar V, Neradi D et al. Need for Testing and Supplementation of Vitamin D3 After Release of COVID-19 Lockdown in Patients with Increased Musculoskeletal Pain. Indian. J. Orthop. 1–4(2021).
- Hruschak V, Flowers KM, Azizoddin DR et al. Cross-sectional study of psychosocial and pain-related variables among patients with chronic pain during a time of social distancing imposed by the coronavirus disease 2019 pandemic. Pain. 162, 619–629(2021).
- Lopez-Leon S, Wegman-Ostrosky T, Perelman C et al. More Than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis. Res. Sq.(2021).
- Su S, Cui H, Wang T et al. Pain: A potential new label of COVID-19. Brain. Behav. Immun. 87, 159–160(2020).
- Cavalli G, Cariddi A, Ferrari Jet al. Living with fibromyalgia during the COVID-19 pandemic: mixed effects of prolonged lockdown on the well-being of patients. Rheumatol. Oxf. Engl. 60, 465–467(2021).
- Blanch-Rubió J, Soldevila-Domenech N, Tío L et al. Influence of anti-osteoporosis treatments on the incidence of COVID-19 in patients with non-inflammatory rheumatic conditions. Aging. 12, 19923–19937(2020).
- Gheita TA, Kenawy S. Egypt’s groundwork blessing during the COVID-19 pandemic curse: Rheumatologic experience. Eur. J. Rheumatol. 7, S134–S136(2020).