Review Article - Interventional Cardiology (2011) Volume 3, Issue 5
Controversies surrounding renal artery intervention: making sense of the confusion
- Corresponding Author:
- Michael R Jaff
Massachusetts General Hospital 55 Fruit St, Boson, MA 02114, USA
Tel: +1 617 726 3784
Fax: +1 617 724 0371
E-mail: mjaff@partners.org
Abstract
Keywords
atherosclerosis,renal artery stenosis,renal artery stent
Atherosclerotic renal artery stenosis (ARAS) is common, found to affect 6.8% of elderly (>65 years of age) participants in the Cardiovascular Health Study [1]. A retrospective analysis of 395 arteriograms noted a high prevalence of renal artery stenosis (RAS) associated with atherosclerotic disease in other vascular beds ranging from 33 to 70% [2]. Prevalence in patients with peripheral artery disease was reported to be as high as 59% [3,4]. An analysis of 434 patients with hypertension (HTN) who underwent serial renal artery duplex ultrasonography over 3 years noted a prevalence of 20.6% [5]. In another retrospective study of 127 patients undergoing lower extremity arterial revascularization, 57 patients (44.9%) had RAS [6].
Current guidelines suggest that treatment of ARAS should be offered to symptomatic patients only. Patients with hemodynamically significant ARAS and otherwise unexplained cardiopulmonary disturbance syndromes, such as unstable angina, recurrent unexplained congestive heart failure or sudden unexplained pulmonary edema should be treated [3,4]. A class IIb recommendation was assigned to treating patients with hemodynamically significant RAS and accelerated, resistant or malignant HTN, HTN with an unexplained unilateral atrophic kidney, and HTN with intolerance to medication. Other indications included progressive chronic kidney disease with bilateral RAS or RAS to a solitary functioning kidney [3,4]. Despite these recommendations, there is controversy regarding which patients benefit from intervention, as not all patients respond favorably to such treatment. In addition, there is no accepted tool that is uniformly used in everyday practice to predict which patients will respond with improved blood pressure control and prevention of deterioration in renal function.
ARAS correlates with clinically significant end points in some, but not all, patients
RAS has been implicated as a cause for renovascular HTN, deteriorating renal function and cardiac disturbance syndromes (recurrent unexplained congestive heart failure, refractory angina and sudden ‘flash’ pulmonary edema).
The basic pathophysiologic mechanism for HTN associated with unilateral renovascular HTN is well delineated. The kidney with RAS responds by secreting renin, which ultimately stimulates production of angiotensin II. Angiotensin II promotes sodium and water retention and HTN via intense vasoconstriction via the renin–angiotensin–aldosterone pathway. The contralateral kidney reacts by secreting sodium and contracting the extracellular fluid volume. This in turn causes the diseased kidney to secrete more renin as its perfusion pressure is further reduced. Eventually the nonstenotic kidney cannot further compensate via pressure natriuresis and HTN ensues. This mechanism is referred to as the Goldblatt kidney [7].
That having been said, it is difficult in clinical practice to determine the contribution of ARAS to HTN or to renal function deterioration. Unfortunately, there is no linear relationship between the severity of ARAS and HTN or renal dysfunction [8]. Certainly the majority of patients with ARAS and HTN do not have classic ‘renovascular HTN’. Certainly, the majority of patients with ARAS do not progress to endstage renal disease (ESRD) [9]. Confounding factors that may also lead to renal failure include longstanding systemic HTN, renal ischemia, recurrent atheromatous embolization from an atherosclerotic aorta and contrast nephropathy from recurrent contrast exposure [10].
From a clinical perspective, ARAS is found more often in patients with ESRD than in the general population. In a prospective study of patients with RAS, 2 year dialysis-free survival was 97.3% for patients with unilateral RAS, 82.4% for patients with bilateral RAS and only 44.7% in patients with renovascular disease and a solitary functioning kidney. Renal atrophy was also noted as a consequence of ARAS [11]. In a Medicare population ARAS was found in 9.2% of patients with ESRD. This was higher than in the elderly population, where it was reported in 6.8% and in the general Medicare population, in which the incidence was only 3.7 per 1000 patient years [9,12].
ARAS is also progressive, leading to more severe stenosis and occlusion of the renal artery with decrease in ipsilateral renal size. In a study of 204 kidneys in 122 subjects with varying degrees of RAS (ranging from normal to >60%) patients were followed for an average of 33 months. In 49% the renal arteries had baseline stenosis of >60% and 21% were normal. Ipsilateral renal atrophy was detected more often in kidneys with >60% RAS at baseline when compared with patients with normal renal arteries (20.8 vs 5.5%; p = 0.009). In addition, 44% of patients progressed from stenosis <60% to >60% during the observation period [13]. A retrospective analysis reported the outcome of renal angiography in 85 subjects (126 arteries) with ARAS. After a mean follow-up of 52 months, 44% demonstrated progression of stenosis severity and 16% progressed to renal artery occlusion. Compared with normal arteries or arteries with mild stenosis, both progression of disease and progression to occlusion were more prevalent and more rapid when baseline stenosis >75% was present [14]. Progression has also been observed despite optimal medical therapy. In the aforementioned cohort of hypertensive patients, a progression of stenosis to occlusion was described in 2.3% and new-onset RAS in 9.1% over 3 years despite medical treatment for HTN [5]. In another retrospective review, 104 patients with RAS were examined to assess the effect of statins. A total of 68 patients received statins while the remaining 36 did not. Mean time to doubling of serum creatinine or deteriorating to ESRD was 27 months in patients without statins and 122 months in patients who received them at all levels of glomerular filtration rates (GFR) [15].
The majority of patients with ARAS have HTN. Renovascular HTN, should be suspected in individuals who are either young (suggesting a nonatherosclerotic etiology, such as fibromuscular dysplasia) or older than 55 years at the time of onset of HTN [3,4]. A second presentation is the patient with primary, resistant HTN, defined as the inability to achieve goal blood pressure lower than 140/90 mmHg, despite the use of three antihypertensive medications at maximum tolerable doses used in appropriate combinations [16]. Systolic blood pressure elevation is typical [8].
Endovascular interventions to treat ARAS
Currently, treatment for ARAS incorporates comprehensive medical interventions including antihypertensive, lipid lowering and antidiabetes medications, tobacco cessation, and antiplatelet medications or this regimen combined with intervention in selected patients. An early report of percutaneous transluminal renal angioplasty (PTRA) for ARAS described longterm (42 months) improvement in blood pressure control and stabilization or improvement in renal function in ten patients with HTN and azotemia following surgery or PTRA [17].
Endovascular renal artery stent revascularization (ERASR) offers an acute procedural success rate of up to 98% [18]. A report of a pooled cohort from two centers in two countries in Europe compared medical therapy to medical therapy and ERASR. At 1 year of follow-up, blood pressure and renal function were lower in the intervention group. The benefits of intervention were most pronounced in patients with more advanced chronic kidney disease [19].
ERASR success rate is higher than that for PTRA [20]. A prospective, nonrandomized trial, AutoPulse Assisted Prehospital International Resuscitation (ASPIRE)-2, reported the 2 year effects of the Palmaz® balloon-expandable stent (Cordis, a Johnson & Johnson Company, NJ, USA) in 208 patients with ARAS and uncontrolled HTN in whom PTRA failed (either dissection or residual stenosis ≥50%). Compared with baseline, systolic and diastolic blood pressure decreased after 2 years of follow-up [21]. A meta-analysis compared studies of ERASR and PTRA between the years 1995 and 1998 [22]. Cured HTN was higher in the stent group (20 vs 10%; p < 0.001), but the percentage of patients with improved blood pressure was similar between groups (49 vs 53%). The rate of improved renal function was lower in the stent group (30 vs 38%; p < 0.001). The restenosis rate was lower after stent placement than after PTA (17 vs 26%; p < 0.001) after a mean follow-up of 17 and 19 months, respectively. In the more contemporary Stenting in Renal dysfunction caused by Atherosclerotic Renal Artery Stenosis (STAR) trial only 2 out of 62 (3.2%) patients experienced restenosis by the end of follow-up [23], while in the Angioplasty and Stent for Renal Artery Lesions (ASTRAL) trial, restenosis rates were not reported [24]. The most recent report of ERASR for RAS causing uncontrolled HTN came from the HERCULES trial. This is a prospective, multicenter trial evaluating the safety and effectiveness of the RX Herculink Elite® Renal Stent System (Abbott Vascular, Santa Clara, California). Of 241 lesions, re-stenosis was noted in 10.5% of patients at 9 months. Clinical success rate was 98%, correlating with a reduction in systolic BP (162 ± 18 vs 145 ± 21; p < 0.0001) [25].
Comparison of endovascular interventions & medical treatment for RAS
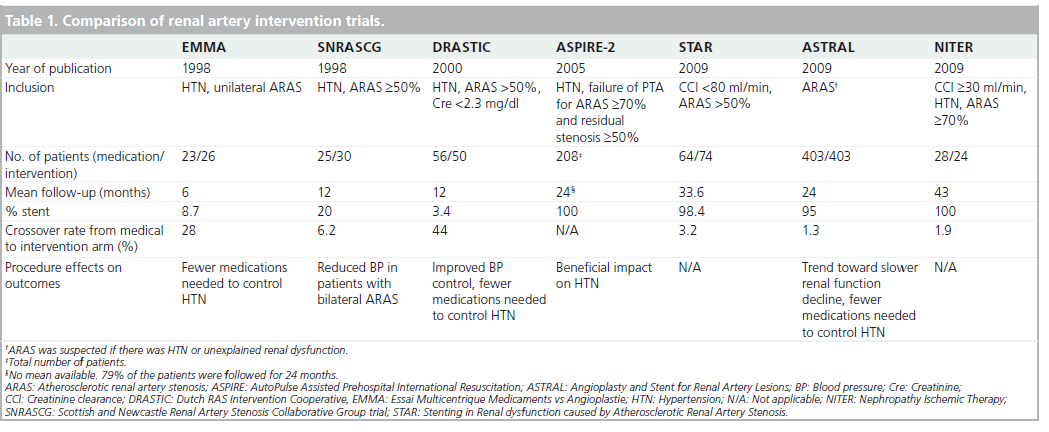
Several prospective trials have examined the efficacy of renal artery angioplasty with and without stenting as compared with medical treatment (Table 1).
▪ Percutaneous renal artery angioplasty
The Essai Multicentrique Medicaments vs Angioplastie (EMMA) trial compared medical treatment to PTRA in 49 patients with HTN and unilateral ARAS [26]. Patients were enrolled if they were hypertensive and had normal renal function. Most patients had RAS of 60–75% and a positive lateralization test (lateralized intravenous pyelography, renal scintigraphy or renal vein renin determination). A large proportion of patients in each group received fewer than two antihypertensive medications (46 and 65% in the control and treatment groups, respectively). While blood pressure was not reduced when measured by a blinded method, patients treated with PTRA needed fewer medications to achieve goal blood pressure (p = 0.009). Crossover rate was high and by the end of follow-up, 7 out of 25 (28%) patients in the control group underwent PTRA due to refractory HTN. A central limitation of this study was a low randomization rate (one out of three patients declined randomization, as they preferred PTRA).
The Scottish and Newcastle Renal Artery Stenosis Collaborative Group trial (SNRASCG) randomized 55 patients to medical therapy alone or to medical therapy and renal artery intervention (either PTRA, nephrectomy or venous bypass) for treatment of HTN [27]. Inclusion criteria included HTN despite at least two antihypertensive medications and angiographically proven ARAS ≥50%. Patients had either bilateral (n = 28) or unilateral (n = 27) ARAS. No reduction in the number of antihypertensive medications used or in serum creatinine was noted following renal artery intervention. Of note, nephrectomy was performed in one patient in the bilaterally treated group and two in the unilateral RAS group. Venous bypass was performed in one patient in each group.
The Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) trial examined the efficacy of PTRA in 106 patients with ARAS for treatment of HTN [28]. Inclusion criteria included HTN and ARAS (defined as >50% stenosis via angiography) and serum creatinine concentration of <2.3 mg/dl (200 μmol/l). Patients were randomized to undergo PTRA or medical treatment only. Patency and blood pressure measurements were assessed after 12 months. By the end of the trial 22 patients had crossed over from the medication to the intervention group because of failure to adequately control the blood pressure with medication alone. Only two patients in DRASTIC received a stent. The remainder assigned to the intervention group received PTRA. In an intention-to-treat analysis, there was no blood pressure difference between the two groups. Nevertheless, fewer medications were required in the PTRA group despite restenosis of ≥50% in 72% of patients. In addition, blood pressure improved in more subjects in the intervention group, deteriorated in fewer (p = 0.002), and was cured more often. Furthermore, in a retrospective analysis, ten patients were found to have RAS of less than 50% at entry and no pressure measurements were available to suggest a more significant degree of stenosis. These patients were unlikely to benefit from a revascularization procedure as they did not have true renovascular HTN.Furthermore, in those patients who crossed over to the PTRA cohort due to uncontrollable HTN or deteriorating renal function, there was a benefit of intervention.
▪ Percutaneous renal artery angioplasty with stent
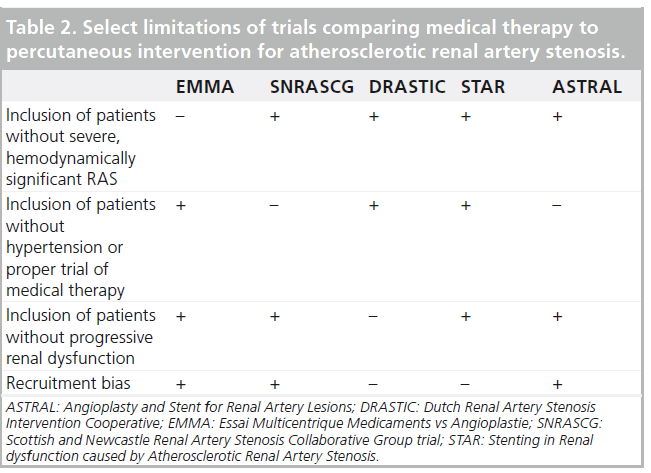
The STAR trial was a prospective, randomized trial comparing medical treatment to medical treatment plus stenting in patients with ARAS and chronic kidney disease for at least 2 years [23]. The primary end point was a decrease of more than 20% in creatinine clearance. Of 140 patients, 76 were assigned to medical therapy (antihypertensive agents, a statin and aspirin) and the remainder were randomized to ERASR. Results revealed a nonstatistically significant excess of events in the medication-only group (22 vs 16%). Despite the importance of the STAR trial, it was not without multiple methodologic flaws that hinder the widespread adoption of its results (Table 2). As RAS was defined by noninvasive methods, without core laboratory verification, many patients had nonhemodynamically significant lesions that were ultimately not stented [29]. Only 46 of the 64 patients in the intervention group actually received a stent; 12 of those who did not were found to have ARAS <50% during the procedure. Coupled with a low event rate, which can also be attributed to the relatively mild RAS and the lenient definition of renal impairment (80 ml/min instead of the customary 60 ml/min) without including the rate of deterioration in renal function, these limitations may have resulted in an underpowered trial [30].
The largest published trial evaluating the management of ARAS to date was the Angioplasty and Stent for Renal Artery Lesions (ASTRAL) [24]. ASTRAL was a prospective, randomized comparison of optimal medical therapy alone or with renal artery stenting in 806 patients with ARAS. Patients were followed for a median of 34 months. The primary outcome was the change in renal function as defined by the reciprocal of serum creatinine. During the follow-up period, there was a trend toward a lower rate of progression of renal impairment in the revascularization group (−0.07 × 10-3 l/μmol per year in the revascularization group, as compared with −0.13 × 10-3 l/μmol in the medical-therapy group; p = 0.06). Neither intention-to-treat nor per-protocol analyses showed a difference in blood pressure control, which decreased in both treatment groups. A post hoc analysis revealed that patients in the revascularization cohort required fewer antihypertensive medications than the medication only group (average of 2.97 vs 2.77; p = 0.03). Complications were unexpectedly high in the intervention group, including two deaths and three lower extremity amputations. As a result, the authors concluded that the risks of ERASR outweighed the benefits. The ASTRAL trial also had several flaws (Table 2). First, patients were recruited only if their treating physicians were unsure about their potential to benefit from the procedure. It is likely that many patients who may have benefited from randomization were not recruited. In addition, many patients (41%) did not have ARAS greater than 70% and ‘borderline lesions’ were not tested for hemodynamic significance. Only 83% of the patients randomized to intervention underwent the procedure. Furthermore, 25% of patients in both groups had a creatinine clearance rate of over 50 ml/min and do not fit the profile of patients with ischemic nephropathy. These patients should not have been included in the trial. Finally, as for the procedures, on average there were very few patients enrolled per center (two/year), which may result from either inexperience of centers/operators or selection bias. The notion of operator inexperience is supported by low procedural technical success rates (79%) and unusually high complication rates (8%).
A recently published meta-analysis compared clinical outcomes, blood pressure and renal function between medical therapy and renal artery intervention in an analysis of six trials [31]. The authors included randomized trials that assigned 1208 patients with ARAS ≥50% to medical therapy with or without PTRA or stent placement. Mean follow-up was 29 months. While blood pressure (both systolic and diastolic) and clinical outcomes were not affected by the procedure, fewer antihypertensive medications were required in the intervention group (p < 0.001). There was a trend toward improved renal function in the intervention group (p = 0.06). Study limitations are shown in Table 2.
In addition to the obvious promise of renal artery intervention and the aforementioned limitations of currently available data, there are also other theoretical reasons that may explain the disappointing results of renal artery stent placement. First, there may be an overlap between conditions causing atherosclerotic RAS and renal parenchymal disease. Thus, while treating ARAS, the renal parenchyma may be beyond salvage. Selecting those patients who still have preserved renal function should therefore lead to better results [31]. Furthermore,as medical therapy improves, especially with the use of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers [32] and statins [15], demonstrating a benefit for percutaneous intervention is more difficult.
Safety of endovascular renal artery procedures
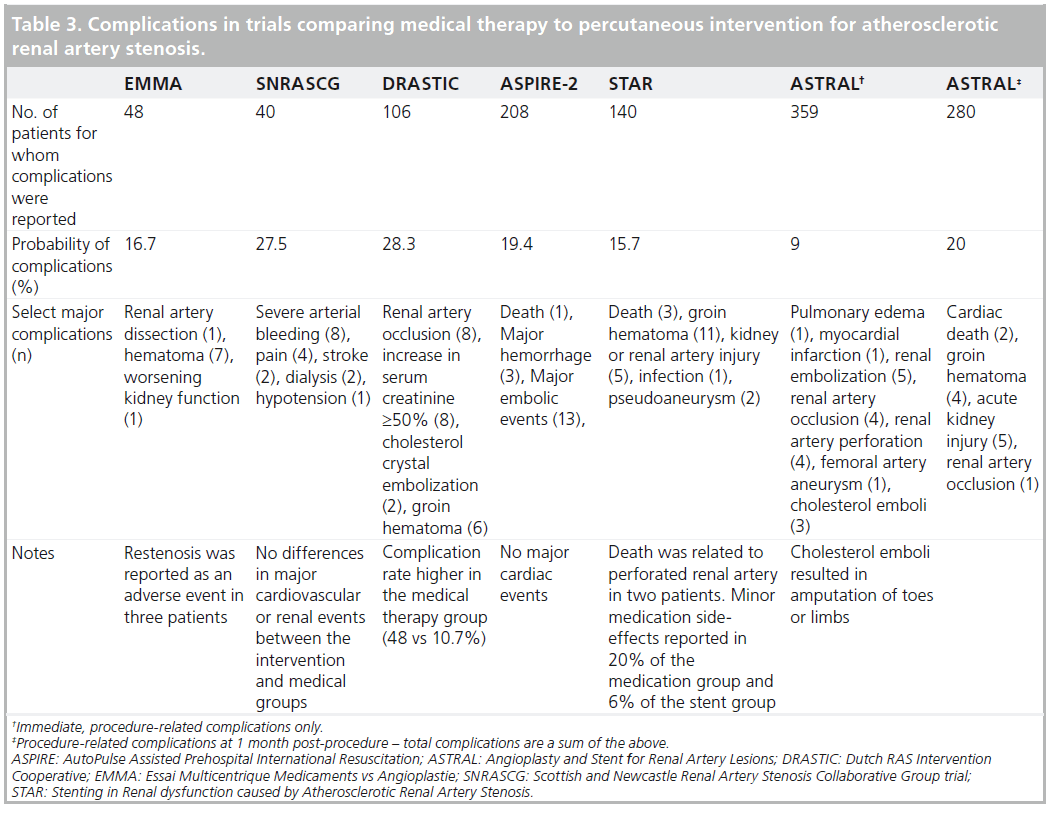
Renal artery interventions are not without risk. This needs to be considered when considering these procedures (Table 3).
Other than risk, these procedures have inherent limitations. The incidence of contrast nephropathy may be as high as 50% in high-risk individuals (e.g., baseline chronic kidney disease, diabetes mellitus and dehydration). These comorbidities are common among patients being considered for ERASR [10]. Atheromatous embolization may also play a role in progressive deterioration in renal function following ERASR. The RESIST trial was a 2 × 2 factorial design prospective trial comparing emboli protection device (EPD) to no EPD and abciximab to placebo in 100 total patients. While EPD alone did not show benefit, the combination of an EPD with abciximab demonstrated stabilization of renal function [33].
Selecting patients who will benefit from intervention
Identifying patients in whom ARAS is actually causing HTN and/or chronic kidney disease remains very challenging. Incidentally discovered RAS is never reason enough for interventional treatment. Intervention should only be considered when there is a correlation between an imaging finding and clinical manifestations,including difficult-to-treat HTN, renal dysfunction that is otherwise unexplained and cardiac disturbance syndromes, as mentioned above [3,4,29].
Various invasive and noninvasive factors have been proposed to correlate between RAS, difficult to control HTN and impaired renal function. Utilizing these measures diligently may result in more accurate patient selection and more predictable results. A summary of potential predictors of success of renal artery stenting is presented in Figure 1.
Figure 1: Choice of treatment for patients with suspected atherosclerotic renal artery stenosis. †CT and MRA are valid choices depending on local expertise and patient characteristics. Captopril renal scintigraphy has an acceptable sensitivity but a low specificity for the diagnosis of ARAS. ‡Validated for patients with HTN. §Validated for a general group of patients with ARAS. ACE-I: Angiotensin-converting enzyme inhibitor; ARAS: Atherosclerotic renal artery stenosis; ARB: Angiotensin receptor blocker; BNP: Brain natriuretic peptide; CHF: Congestive heart failure; CT: Computed tomography; DUS: Duplex ultrasonography; ERASR: Endovascular renal artery stent revascularization; FFR: Fractional flow reserve; HTN: Hypertension; MRA: Magnetic resonance angiography; RAS: Renal artery stenosis; RFC: Renal frame counts; RI: Resistive index.
First, there must be significant RAS, typically >70% by noninvasive imaging studies, even to consider revascularization. Hemodynamic significance may be inferred from a translesional pressure gradient that may offer greater correlation with clinical success following ERASR than angiographic assessment of stenosis or intravascular ultrasound measures. This was shown in a study of 62 individuals with RAS undergoing stent placement, where a hyperemic systolic gradient across the stenotic lesion of ≥21 mmHg was the single best predictor of sustained blood pressure response after 12 months (sensitivity 82%, specificity 84% and accuracy 84%). These patients also required fewer antihypertensive medications [34]. In another study of 53 individuals with moderate–severe ARAS undergoing ERASR, baseline stenosis severity was not predictive of clinical outcomes, while responders were predicted by measuring a dopamine enhanced pressure gradient across the stenosis [29].
It is critical to determine with certainty that the etiology of chronic kidney disease is ischemic and potentially reversible in nature. It is generally believed that patients who demonstrate gradual deterioration in renal function over years do not respond as well to ERASR as those who have rapidly deteriorating renal function over the previous 8–12 weeks [30]. This was reinforced in a retrospective analysis of prospectively collected data regarding 129 patients followed for an average of 544 days after renal artery stenting. Chronic kidney disease (estimated glomerular filtration rate [eGFR] <40 ml/min) was associated with poor blood pressure response to ERASR [35]. Reversibility may be deduced from renal mass. Consequently, patients with atrophic kidneys (conventionally, less than 7 cm in poleto- pole length) are not considered candidates for ERASR, while patients with global renal ischemia (bilateral severe RAS or stenosis to a solitary functioning kidney) with renal length >8 cm, may experience clinical benefit. Current data is of poor quality in this regard [36,37]. ERASR for unilateral RAS may be particularly successful if the nonstenotic kidney has intrinsic renal disease [29,38]. Captopril renal scintigraphy, while having an acceptable sensitivity for the screening of RAS, has not been consistently shown to predict outcome, largely due to difficulties in patients with bilateral disease or those with chronic kidney disease [37,39]. It is not recommended by current guidelines [3,4].
The renal resistive index is another duplex ultrasonography measure used to evaluate the reversibility of renal injury. The resistive index is calculated by Equation 1.
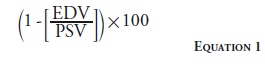
A baseline renal resistive index <0.8 may suggest better clinical outcomes after ERASR [40], although this has been challenged by more recent publications [41].
Elevated serum brain natriuretic peptide (BNP) has been shown to correspond to favorable blood pressure response following renal artery stenting in a small series of patients [42]. In another prospective trial, BNP was evaluated as a predictor of response to endovascular treatment in 127 patients. Inclusion criteria were lenient; patients were included if they had 50% RAS and there was no cut-off for the number of antihypertensive medications. Blood pressure improvement at 6 months correlated with pre-procedure BNP and BNP at 1 day post-procedure [43]. However, the aforementioned HERCULES trial, representing the largest cohort of patients in whom BNP levels were correlated to blood pressure response, enrolled 202 patients with uncontrolled HTN and failed to show a predictive value for pretreatment BNP [25].
Proteinuria may indicate low likelihood for success of renal artery intervention. A single-center, retrospective analysis reviewed the outcomes of 83 patients undergoing revascularization for ARAS [44]. Stenting was performed in 88% of patients and PTA in the remaining patients. The indication for the procedure was both HTN and renal dysfunction. Preprocedural proteinuria predicted lack of response to the procedure.
Aside from a translesional gradient, other measurements performed during angiography may predict which patients respond to ERASR. The fractional flow reserve represents the fraction of the normal maximal tissue flow that can be achieved despite arterial stenosis. It is derived from the ratio of pressures after and before a lesion [45]. It has been shown to add predictive value in ERASR patients in an elegant trial performed in 17 individuals, all with severe RAS. At a follow-up of 10 ± 2 months, those with abnormal fractional flow reserve required fewer antihypertensive medications, and more of these patients noted sustained46 blood pressure responses (71 vs 10%) [46].
A third set of angiographic measures is a combination of renal frame count and renal blush grades, which are measures of renal perfusion and are reduced in most patients with HTN. An elevated renal frame count predicted response to ERASR in a retrospective analysis of hypertensive patients undergoing ERASR [47].
It is noteworthy that many of the aforementioned predictors of outcome have not been tested in a comprehensive model in which they were validated in conjunction with other measures. Therefore, it remains unknown how these predictors should be used together, especially when contradictory indices are present in a particular patient.
Catheter-based renal artery sympathetic denervation
Catheter-based renal artery sympathetic denervation (RSD) is a novel intervention poised to become a central treatment option for patients with medication-resistant HTN. Currently, denervation can be achieved with the radiofrequency Symplicity® catheter (Medtronic, MN, USA). The Symplicity HTN-2 trial compared blood pressure response for hypertensive patients undergoing RSD to controls [48]. Of note, patients with significant RAS were excluded from this trial. At 6 months, both systolic and diastolic blood pressures were lower in the treatment group (32/12 vs 1/0 mmHg; p < 0.0001) and a larger proportion of treated patients experienced a reduction in the amount of anti-HTN medication they needed (20 vs 8%; p = 0.04). There were no serious complications recorded with the procedure. Blood pressure control using the postsympathetic denervation technique has been shown to be durable for 2 years in the long-term follow-up of the Symplicity HTN-1 trial [49]. Concern has been raised regarding the potential for RSD to cause local tissue damage and resultant RAS. This has not been reported. Increased sympathetic tone has actually been shown to have a trophic effect on blood vessels in rats. Denervation may thus have a beneficial effect on renal artery luminal diameter [50]. In addition to the obvious benefit of blood pressure reduction, RSD may offer other systemic benefits, including improved glycemic control [51].
Conclusion
The prerequisite for treatment of ARAS is when there is a correlation between an imaging finding and clinical manifestations. This includes patients with difficult-to-treat HTN, renal dysfunction that is otherwise unexplained or cardiac disturbance syndromes unexplained by any other etiology. Recent trials comparing medical management to ERASR have suggested that there is little, if any benefit of ERASR. This is thought to be largely due to poor patient selection and other serious methodological flaws. Various technical reasons may have also contributed to these poor results, including imperfections of the procedure, procedural complications, atheromatous embolization and renal injury from contrast. Other factors that may contribute to procedural failure relate to improper patient surveillance such as delayed response to artery restenosis or inadequate post-procedural blood pressure management. In addition, identifying patients in whom irreversible renal damage exists and subsequently avoiding unneeded procedures in them is still a challenge. As more knowledge accumulates regarding patient selection, and technical as well as clinical aspects of ERASR are perfected, we expect this procedure to remain an important component of the armamentarium for treatment of ARAS.
Future perspective
Despite two decades of development and research, the field of percutaneous renal artery revascularization is still evolving. As patient selection improves and catheters and stents are being specifically designed for renal intervention, more predictable patient outcomes can be expected. The Cardiovascular Outcomes in Renovascular Atherosclerotic Lesions (CORAL) trial is a large, multicenter, prospective, randomized study and aims to compare the effects of angioplasty with stenting and optimal medical therapy to medical therapy alone on a composite of cardiovascular and renal events. In the trial, design emphasis has been given to optimal medical therapy. Primary end point results are expected in early 2012. Renal artery denervation, while taking a different approach to catheter-based renal artery intervention, shows great promise in treating some of the consequences of clinically significant ARAS.
Financial & competing interests disclosure
MR Jaff is a non-compensated consultant for Abbott Vascular, Cordis Corporation, Covidien and Medtronic Vascular, and a board member at VIVA Physicians Inc, a 501 (c) 3 education and research organization. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪▪ Atherosclerotic renal artery stenosis is progressive and often linked to renovascular hypertension (HTN), progressive renal dysfunction and cardiac disturbance syndromes.
▪▪ Percutaneous renal artery intervention is indicated in the presence of atherosclerotic renal artery stenosis (ARAS) for recurrent, unexplained cardiac disturbance syndromes.
▪▪ Treatment of other adverse effects of ARAS (e.g., HTN and chronic kidney disease) has shown promise in early trials, however, while offering supportive results, recent trials including DRASTIC, STAR and ASTRAL have suggested that there is no advantage of renal artery intervention for ARAS.
▪▪ Though no unified model exists, various noninvasive and interventional measures have independently been shown to predict endovascular renal artery stent revascularization outcomes. Utilizing these measures diligently may result in more accurate patient selection and more predictable results.
▪▪ Renal artery sympathetic denervation with the Symplicity® catheter is a promising emerging treatment for patients with resistant HTN unrelated to ARAS.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Hansen KJ, Edwards MS, Craven TE et al. Prevalence of renovascular disease in the elderly: a population-based study. J. Vasc. Surg. 36(3), 443–451 (2002).
- Olin JW, Melia M, Young JR et al. Prevalence of atherosclerotic renal artery stenosis in patients with atherosclerosis elsewhere. Am. J. Med. 88(1N), 46N–51N (1990).
- Hirsch AT, Haskal ZJ, Hertzer NR et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/ AHA task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease). Circulation 113(11), e463–e654 (2006).
- Rooke TW, Hirsch AT, Misra S et al. 2011 ACCF/AHA focused update of the guidelines for the management of patients with peripheral artery disease (updating the 2005 guidelines): a report of the American College of Cardiology Foundation/ American Heart Association task force on practice guidelines. Circulation DOI:10.1016/ j.jacc.2011.08.023 (2011) (Epub ahead of print).
- Davis RP, Pearce JD, Craven TE et al. Atherosclerotic renovascular disease among hypertensive adults. J. Vasc. Surg. 50(3), 564–571, e1–e3; discussion 571 (2009).
- Missouris CG, Buckenham T, Cappuccio FP et al. Renal artery stenosis: a common and important problem in patients with peripheral vascular disease. Am. J. Med. 96(1), 10–14 (1994).
- Garovic VD, Textor SC. Renovascular hypertension and ischemic nephropathy. Circulation 112(9), 1362–1374 (2005).
- Chrysochou C, Kalra PA. Epidemiology and natural history of atherosclerotic renovascular disease. Prog. Cardiovasc. Dis. 52(3), 184–195 (2009).
- Guo H, Kalra PA, Gilbertson DT et al. Atherosclerotic renovascular disease in older US patients starting dialysis, 1996 to 2001. Circulation 115(1), 50–58 (2007).
- Manske CL, Sprafka JM, Strony JT et al. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am. J. Med. 89(5), 615–620 (1990).
- Guzman RP, Zierler RE, Isaacson JA et al. Renal atrophy and arterial stenosis. prospective study with duplex ultrasound. Hypertension 23(3), 346–350 (1994).
- Kalra PA, Guo H, Kausz AT et al. Atherosclerotic renovascular disease in united states patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int. 68(1), 293–301 (2005).
- Caps MT, Zierler RE, Polissar NL et al. Risk of atrophy in kidneys with atherosclerotic renal artery stenosis. Kidney Int. 53(3), 735–742 (1998).
- Schreiber MJ, Pohl MA, Novick AC. The natural history of atherosclerotic and fibrous renal artery disease. Urol. Clin. N. Am. 11(3), 383–392 (1984).
- Silva VS, Martin LC, Franco RJ et al. Pleiotropic effects of statins may improve outcomes in atherosclerotic renovascular disease. Am. J. Hypertens. 21(10), 1163–1168 (2008).
- Textor SC. Current approaches to renovascular hypertension. Med. Clin.Am. 93(3), 717–732, (2009).
- Ying CY, Tifft CP, Gavras H et al. Renal revascularization in the azotemic hypertensive patient resistant to therapy. Engl. J. Med. 311(17), 1070–1075(1984).
- Simon JF. Stenting atherosclerotic renal arteries: time to be less aggressive. Cleve. Clin. J. Med. 77(3), 178–189 (2010).
- Kalra PA, Chrysochou C, Green D et al. The benefit of renal artery stenting in patients with atheromatous renovascular disease and advanced chronic kidney disease. Catheter Cardiovasc. Interv. 75(1), 1–10 (2010).
- van de Ven PJ, Kaatee R, Beutler JJ et al. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomised trial. Lancet 353(9149), 282–286 (1999).
- Rocha-Singh K, Jaff MR, Rosenfield K. ASPIRE-2 trial investigators. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. Am. Coll. Cardiol. 46(5), 776–783 (2005).
- Leertouwer TC, Gussenhoven EJ, Bosch JL et al. Stent placement for renal arterialstenosis: where do we stand? A meta-analysis. Radiology 216(1), 78–85 (2000).
- Bax L, Woittiez AJ, Kouwenberg HJ et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann. Intern. Med. 150(12), 840–848,W150–W151 (2009).
- Wheatley K, Ives N, Gray R et al. Revascularization versus medical therapy for renal-artery stenosis. ASTRAL investigators. Engl. J. Med. 361(20), 1953–1962 (2009).
- Jaff MR, Textor SC, HERCULES Executive Committee and Investigators. Does elevated brain natriuretic peptide (BNP) predict outcomes for patients with uncontrolled hypertension in renal artery stenting? results from the HERCULES trial. Presented at: The Society for Cardiovascular Angiography and Intervention. Baltimore, MD, USA, 4–7May 2011.
- Plouin PF, Chatellier G, Darne B et al. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai multicentrique medicaments vs angioplastie (EMMA) study group. Hypertension 31(3), 823–829 (1998).
- Webster J, Marshall F, Abdalla M et al. Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. Hum. Hypertens. 12(5), 329–335 (1998).
- van Jaarsveld BC, Krijnen P, Pieterman H et al. The effect of balloon angioplasty onhypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group Engl. J. Med. 342(14), 1007–1014(2000).
- White CJ. Optimizing outcomes for renal artery intervention. Circ. Cardiovasc. Interv. 3(2), 184–192 (2010).
- Muray S, Martin M, Amoedo ML et al. Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis.Am. J. Kidney Dis. 39(1), 60–66 (2002).
- Kumbhani DJ, Bavry AA, Harvey JE et al. Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: a meta-analysis of randomized controlled trials. Am. Heart J. 161(3), 622–630.e1 (2011).
- Losito A, Errico R, Santirosi P et al. Long-term follow-up of atherosclerotic renovascular disease beneficial effect of ACE inhibition. Nephrol. Dial. Transplant. 20(8), 1604–1609 (2005).
- Cooper CJ, Haller ST, Colyer W et al. Embolic protection and platelet inhibition during renal artery stenting. Circulation 117(21), 2752–2760 (2008).
- Leesar MA, Varma J, Shapira A et al. Prediction of hypertension improvement after stenting of renal artery stenosis: comparative accuracy of translesional pressure gradients, intravascular ultrasound, and angiography. J. Am. Coll. Cardiol.53(25), 2363–2371 (2009).
- Beck AW, Nolan BW, De Martino R et al. Predicting blood pressure response after renal artery stenting. J. Vasc. Surg. 51(2), 380–385 discussion 385 (2010).
- Bommart S, Cliche A, Therasse E et al. Renal artery revascularization: predictive value of kidney length and volume weighted by resistive index. Am. J. Roentgenol. 194(5), 1365–1372 (2010).
- Soulez G, Therasse E, Qanadli SD et al. Prediction of clinical response after renal angioplasty: respective value of renal doppler sonography and scintigraphy. Am. J. Roentgenol. 181(4), 1029–1035 (2003).
- White CJ. Catheter-based therapy for atherosclerotic renal artery stenosis. Circulation 113(11), 1464–1473 (2006).
- Vasbinder GB, Nelemans PJ, Kessels AG et al. Diagnostic tests for renal arterystenosis in patients suspected of having renovascular hypertension: a meta-analysis. Ann. Intern. Med. 135(6), 401–411 (2001).
- Radermacher J, Chavan A, Bleck J et al. Use of doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N. Engl. J. Med. 344(6), 410–417 (2001).
- Zeller T, Frank U, Muller C et al. Predictors of improved renal function after percutaneous stent-supported angioplasty of severe atherosclerotic ostial renal artery stenosis. Circulation 108(18), 2244–2249 (2003).
- Silva JA, Chan AW, White CJ et al. Elevated brain natriuretic peptide predicts blood pressure response after stent revascularization in patients with renal artery stenosis. Circulation 111(3), 328–333 (2005).
- Staub D, Zeller T, Trenk D et al. Use of B-type natriuretic peptide to predict blood pressure improvement after percutaneous revascularisation for renal artery stenosis.Eur. J. Vasc. Endovasc. Surg. 40(5), 599–607(2010).
- Chrysochou C, Cheung CM, Durow M et al. Proteinuria as a predictor of renalfunctional outcome after revascularization in atherosclerotic renovascular disease (ARVD). Q JM 102(4), 283–288 (2009).
- Pijls NH, De Bruyne B, Peels K et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N. Engl. J. Med. 334(26), 1703–1708 (1996).
- Mitchell JA, Subramanian R, White CJ et al. Predicting blood pressure improvement in hypertensive patients after renal artery stent placement: renal fractional flow reserve. Catheter. Cardiovasc. Interv. 69(5), 685–689(2007).
- Mahmud E, Smith TW, Palakodeti V et al. Renal frame count and renal blush grade: quantitative measures that predict the success of renal stenting in hypertensive patients with renal artery stenosis. JACC Cardiovasc. Interv.1(3), 286–292 (2008).
- Esler MD, Krum H, Sobotka PA et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity HTN-2 trial): a randomised controlled trial. Symplicity HTN-2 Investigators. Lancet 376(9756), 1903–1909 (2010).
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 57(5), 911–917 (2011).
- Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation and systemic hypertension. Am. J. Cardiol. 105(4), 570–576 (2010).
- Mahfoud F, Schlaich M, Kindermann I et al. Effect of renal sympatheticdenervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 123(18), 1940–1946 (2011).
▪ Comprehensive guidelines outlining the treatment of patients with peripheral artery disease, including atherosclerotic renal artery stenosis (RAS).
▪ ASTRAL is, to date, the largest comparative trial of treatment of RAS with medical treatment or medical treatment with renal artery stent placement.
▪ Up-to-date summary and meta-analysis of most trials pertaining to renal artery intervention for the treatment of RAS.
▪▪ The Symplicity® catheter and renal artery sympathetic denervation are promising emerging techniques for the treatment of resistant hypertension.