Mini Review - Journal of Experimental Stroke & Translational Medicine (2022) Volume 14, Issue 2
Clinically Actionable Strategies for Studying Neural Influences in Cancer
Sara Lucia*
University of Manitoba, Winnipeg, Manitoba, Canada
University of Manitoba, Winnipeg, Manitoba, Canada
E-mail: saraluciaedu@org
Received: 04-March-2022, Manuscript No. JESTM-22-80773; Editor assigned: 07-March-2022, PreQC No. JESTM-22-80773 (PQ); Reviewed: 21-March-2022, QC No. JESTM-22-80773; Revised: 25-March-2022, Manuscript No. JESTM-22-80773 (R); Published: 31-March-2022, DOI: 10.37532/ jestm.2022.14(2).13-19
Abstract
Neuroglial activation is a recently identified hallmark of growing cancers. Targeting tumor hyper innervation in preclinical and small clinical trials has yielded promising antitumor effects, highlighting the need of systematic analysis of neural influences in cancer (NIC). Here, we outline the strategies translating these findings from bench to the clinic.
Introduction
Cancer cells induce sprouting of peripheral nerve fibers termed axonogenesis its underlying mechanism is thought to be similar to cancer’s ability to initiate angiogenesis. Increased and functionally altered innervation has been shown to be associated with worse prognosis in several cancers, such as pancreatic, prostate gastric, colorectal, head and neck, and hematological cancers. Exploitation of neuronal activity by cancer cells has recently been viewed as a central and common patho-mechanism for progression in both solid and hematological cancers and has emerged as a dynamic research field at the intersection of oncology and neuroscience [1].
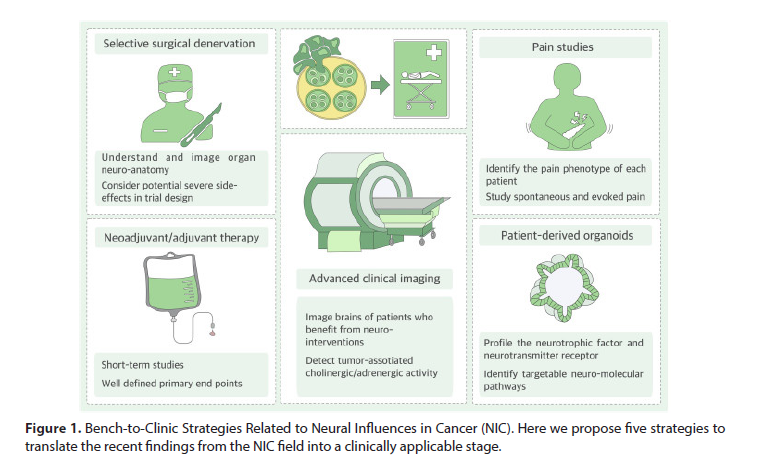
Given the recent advances made in the study of neural influences in cancer (NIC), it is now the time to define concrete, clinically actionable strategies for targeting NIC and for turning axonogenesis into an oncologically relevant target, similar to angiogenesis (Figure 1).
Figure 1: Bench-to-Clinic Strategies Related to Neural Influences in Cancer (NIC). Here we propose five strategies to translate the recent findings from the NIC field into a clinically applicable stage.
Targeting NIC: What Is the Therapeutic Potential?
Denervation as a Therapeutic Approach in Cancer
There is accumulating evidence for the dependence of growing cancers on innervation.
Numerous animal studies have convincingly shown that complete or selective surgical, pharmacological, or genetic denervation of solid cancers leads to deceleration of tumor growth.
Denervation approaches have taught us the pathophysiology of NIC, yet translating denervation approaches into the human setting might be technically difficult. Th ere are examples of successful attenuation of cancer progression following chemical denervation during surgery but also failed trials without effect on cancer growth despite promising preclinical findings. Denervation may be effective over a limited time period in tumor pathogenesis, given the complex, multifactorial nature of the tumor milieu. Furthermore, there are key anatomic considerations for each organ. For organs like the colon or rectum, which receive a complex innervation from multiple extrinsic and intrinsic sources, it may be difficult to identify the primary driver of nerve-cancer interactions in an organ-specific manner. The side effects of denervation, as known from radical resection approaches for cancers directly infiltrating neural plexus, can be associated with severe postoperative complaints such as intractable diarrhea, sexual dysfunction, or incontinence [2]. Similarly, denervation in tumors of the head and neck region can be associated with severe organ dysfunction. Therefore, future clinical studies that plan to perform such selective denervation approaches in cancer patients should consider the potential ethical and clinical consequences of these innovative techniques on patient quality of life.
Targeting Autonomic Nervous Activity
An alternative approach for modulating neural activity in cancers may be to repurpose medications known to regulate sympathetic or parasympathetic signaling, such as selective or non-selective beta blockers, or Para sympathomimetic agents as adjuvant therapy for cancer patients. Here, it is important to consider the differences in the cholinergic response among cancers, such as gastric versus pancreatic cancer. Indeed, a number of retrospective studies have shown improved outcomes for patients taking beta blockers in a number of cancer types [3]. New clinical trials are emerging targeting the autonomic nervous system with muscarinic agonists and beta blockers in both nonmetastatic patients and metastatic patients. Despite these advances, large randomized controlled trials are currently lacking and will be needed to demonstrate a benefit from these agents.
Molecular Targets Pertinent to NIC
It is imperative to identify the specific target molecules relevant for the impact of the nervous system in different cancers. In addition to generating more specific drug inhibitors or activators, it seems highly relevant to generate and apply specific molecular inhibitors of the protumorigenic neural pathways. Examples for such neuronor glia-specific classes of molecules are neurotrophic factors and neurotransmitters with their corresponding receptors. For example, targeting the nerve growth factor (NGF) signaling via tanezumab is an effective method for analgesia and bears the potential of treating NGF-overexpressing cancer [4]. Tyrosine kinase receptors expressed by cancer cells are also potential targets to prevent neural dissemination by cancer. There is an urgent need to identify specific neuronor glia-derived mediators of nerve-cancer interactions across cancer types. In-depth profiling of the transcriptomic, proteomic, and epigenetic signature of tumors at the single-cell level will help achieve this goal.
Strategies for Future Bench-to- Clinic Studies on NIC
Integrate Oncologists
Although NIC is a relative young field of research, the available findings already allow the performance of clinical trials. Indeed, such trials that make use of beta-blockers, botulinum toxin, or betanechol, for example, are already running, and the first results are expected within the next three years. We recommend the consideration of a simple, rather than complex, clinical study design. To see the effectiveness of co-administration of neuro-modulatory drugs with other established anti-cancer agents, clear-cut primary endpoints are necessary [5]. For solid tumors, one can consider the administration of the agents in neoadjuvant (preoperative) setting, and the primary endpoint can be the margin-free (R0) resection rate, the extent of perineural invasion (Pn), or lymph node metastasis (N status). Such short-term study designs can not only have major impact on the prognosis of the patients but also lay the foundation for subsequent large-scale, longterm oncological studies.
Integrate Surgeons
For solid tumors, surgeons have the unique opportunity to directly expose the tumor during surgical exploration. This approach enables several maneuvers such as selective surgical denervation, chemical denervation, or the local direct injection of neuromodulatory agents or placement of neurostimulatory devices around or into the tumor. For solid tumors, such surgical maneuvers should be considered for downsizing the tumors that are initially found to be unresectable.
Profile Nervous-System-Related Targets on Cancer Cells
Organoids grown in a 3D culture environment can better mimic the natural activation status and molecular signature of cancer cells. Molecular profiling of patientderived organoids (PDOs) is currently being tested within clinical trials for identifying patient-specific molecular vulnerabilities of cancer cells [6]. For some cancers, genetic alterations in nervous-system-related pathways are among the most prominently activated molecular signatures. Identifying the individual “neuro-molecular profile” of patients may yield valuable clues for individualized, neuro-targeting therapies for specific patient groups.
Advance Clinical Imaging Technologies
Advanced imaging technologies such as positron-emission tomography (PET) using specific neuro-molecular targets can be very useful to identify patients who exhibit, for example, cholinergic activity in the tumor or at distant sites. The uptake of PET tracers binding to cholinergic nerves is considerably higher in prostate cancer tissues with a high severity. Development of novel neuromolecular tracers and performance of such specific neuro-tracer-PET imaging within clinical studies may help identify patients who may specifically benefit from neuromodulatory therapies [7].
Study Cancer-Associated Pain
Studying pain in the context of human cancer requires detailed prospective analyses comprising not only questionnaires but also quantitative sensory testing as well as diligent attention to the choice of the correct preclinical models for reverse translation. While pain studies in human patients reveal pain phenotypes and their temporal relation to dynamic tumor progression, animal models allow the identification of underlying mechanisms. With the large number of available transgenic mouse lines, it is possible to study individual genes in cancerassociated pain behavior [8]. However, behavioral analyses in animal models require significant experience to ensure data reproducibility. Both evoked hypersensitivity and spontaneous pain behaviors should be assessed, as the potential pharmacology to treat evoked versus breakthrough pain can differ. The contribution of sensory neuron subpopulations and corresponding sensory neurotransmission in animal models may differ in patients; therefore, target proteins and neuron subpopulations should be verified in human specimens [9]. In addition, in certain cancers, there is a strong variation of pain-associated behavior depending on the cancer stage. The pain signaling in the early stages may be masked by the opioid signaling mediated by the immune system and the endogenous antinociceptive pathways. These pathways may be overwhelmed later in the disease, leading to aggressive, difficult-to-treat breakthrough pain that may be driven by a combination of inflammatory and neuropathic pain features. Assessing differences in painful versus painless cancers may reveal important clues to pain control in cancer [10].
Conclusions
Dependence of cancer cells on nerves for their evolution and spread and the promising anticancer effects of surgical or pharmacological denervation or modulation approaches have provided a solid basis for future translational investigations in the NIC field. Here, we outline key clinically actionable strategies for advancing the NIC field into a “clinically applicable” stage. Upon successful implementation of these strategies, axonogenesis research may contribute to strong clinical utility similar to angiogenesis research. The NIC group is actively calling for integration of talented scientists and clinicians worldwide into this collaborative effort.
References
- Moller T, Boddeke HB Glial cells as drug targets: what does it take? Glia.64, 1742-1754 (2016).
- Danbolt NC Glutamate uptake. Prog Neurobiol. 65, 101-105 (2001).
- Anderson CM, Swanson RA Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 32, 1-14 (2000).
- Clausen T Potassium and sodium transport and pH regulation. Can J Physiol Pharmacol. 70, 219-222 (1992).
- Simard M, Nedergaard M The neurobiology of glia in the context of water and ion homeostasis. Neurosci. 129, 877-896 (2004).
- Mantovani A, Sica A, Sozzani S et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677-686 (2004).
- Nash B, Thomson CE, Linington C et al. Functional duality of astrocytes in myelination. J Neurosci. 31, 13028-13038 (2011).
- McKimmie CS, Graham GJ Astrocytes modulate the chemokine network in a pathogen-specific manner. Biochem Biophys Res Commun. 394, 1006-1011 (2010).
- Allaman I, Belanger M, Magistretti PJ et al. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 34, 76-87 (2011).
- Lan X, Han X, Li Q, Yang QW et al. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol. 13, 420-433 (2017).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref