Research Article - International Journal of Clinical Rheumatology (2022) Volume 17, Issue 6
Choroidal Vascularity Index Changes in Children with FMF
Sezer Taha*, Teberik Kuddusi
Department of Ophthalmology, Duzce University School of Medicine, Duzce, Turkey
- *Corresponding Author:
- Sezer Taha
Department of Ophthalmology, Duzce University School of Medicine, Duzce, Turkey
E-mail: drtahasezer@gmail.com
Abstract
Purpose: To evaluate choroidal thickness (CT) and choroidal vascularity index (CVI) changes in children with familial Mediterranean fever (FMF).
Methods: This retrospective study evaluated the CT and CVI values of 46 children with FMF patients according to their clinical and genetic data and in comparison with 46 controls. All eye examinations and EDI-OCT imaging were included in the analysis. The genetic subtypes of the FMF patients were determined. The acute phase reactants (fibrinogen, ESR, CRP) were examined.
Results: The mean CVI was 73.5% ± 4.8% in the FMF group and 74.7% ± 5.2% in the control group (p>0.05). CT and CVI were not significantly correlated with the genetic subtype in the FMF group (p>0.05).
Conclusion: There was no statistically significant difference in CVI and CT between pediatric FMF patients in remission and the control group. CVI and CT showed no correlation with FMF genetic subtype or acute phase reactants in FMF patients.
Keywords
familial mediterranean fever ● choroidal vascularity index ● EDI-OCT ● acute phase reactants
Introduction
Familial Mediterranean fever (FMF) is an autosomal recessive inflammatory disease characterized by recurrent episodes of fever, peritonitis, and polyserositis [1]. It is called Mediterranean fever due to its prevalence among Turks, Armenians, Arabs, and Jews living in the Mediterranean basin. Most cases are diagnosed in childhood and adolescence, although some patients are diagnosed in adulthood [2].
The gene responsible for the disease is MEFV (Mediterranean FeVer), a 10-exon gene located on the short arm of chromosome 16. The MEFV gene encodes a 781-amino acid protein called pyrin, which has antiinflammatory action. It is believed that defective pyrin due to an MEFV mutation is unable to suppress inflammation, resulting in the clinical presentation of an inflammatory disease with fever, serositis, and elevated acute phase reactants (APRs) [3-5].
The choroid is the vascular layer of the eye that is situated between the retina and sclera to form the posterior uveal tract. It consists of networks of various sized vessels and connective tissue containing melanin, fibroblasts, and mast cells. The choroidal vessels narrow in diameter from the sclera to the retina, with the thinnest and innermost layer, the choriocapillaris, adjacent to the retinal pigment epithelium and therefore to the retina. The choriocapillaris has a key role in the metabolic support of the outer third of the retina, including the retinal pigment epithelium [6].
Ocular involvement is rarely seen due to FMF. It can cause inflammatory diseases such as anterior or posterior uveitis, especially involving the uvea. FMF can also lead to diseases such as episcleritis, retinal vasculitis [7]. It is especially important to examine the pathophysiological effects of a disease that progresses with recurrent attacks and chronic inflammation on a tissue rich in vascular and stromal structures such as the choroid.
Choroidal imaging can now be performed non-invasively with enhanced depth imaging optical coherence tomography (EDI-OCT) [8]. Imaging with this technique had revealed changes in choroidal thickness (CT) with many drugs and diseases. CT is especially affected by factors such as smoking, the use of sildenafil, decongestants, and antihistamine drugs, the person’s age and axial length,inflammatory diseases such as Vogt-Koyanagi-Harada, and central serous chorioretinopathy [9-12]. Previous studies have examined CT in both pediatric and adult FMF patients [13-15]. However, recent studies have more frequently evaluated choroidal vascularity index (CVI), which is less affected by such factors [16,17]. This new parameter is determined from EDI-OCT images through automatic detection of the luminal and stromal areas of the choroid [18,19].
In this study, it was evaluated whether there was a difference in CVI in the remission periods of FMF patients in parallel with previous studies in CT. Because CVI provides more detailed and reliable information about the choroid than CT, in this study we evaluated differences in CVI in pediatric patients with different genetic subtypes of FMF compared to a control group.
Materials and Methods
The study included 46 FMF patients and 46 healthy controls with no systemic or ocular disease. Patients with any systemic disease other than FMF and those who used drugs that may affect CT were not included in the study.
The diagnosis of FMF was confirmed by genetic testing. MEFV gene profiles were determined with pyrosequencing and direct Sanger sequencing [20].
The FMF patients’ erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level, and fibrinogen level were assessed to evaluate the relationship between choroidal parameters and APRs.
The study was approved by the Institutional Review Board and Ethics Committee of Düzce University and adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent for the use of their clinical data.
Ophthalmologic examination
All patients and control subjects underwent a detailed ophthalmological examination performed by the same ophthalmologists (S.T.). Refraction, best corrected visual acuity (BCVA), intraocular pressure measured by non-contact tonometry, and fundus examinations were performed.
Exclusion criteria were history of ocular trauma or surgery, congenital eye malformation, amblyopia, and refractive defects of ± 1 diopter (D) or greater. Data from the right eyes of all patients and controls were used for analysis. EDI-OCT images were obtained at the same time of the day (9:00-10:00 am) to minimize the effect of diurnal variations in the choroid [21].
Choroidal thickness measurements
CT measurements were performed by the same experienced ophthalmologists (S.T. and T.K.) using the Spectralis spectral domain OCT instrument (Heidelberg Engineering, Heidelberg, Germany). Images were acquired after pupil dilation, using a standardized imaging protocol with a 9-mm fovea-centered horizontal image. Image quality was enhanced by activating eye tracking mode and obtaining an average of 100 B-scans for all sections to improve the signal-to-noise ratio.
CT was measured from the outer edge of the hyperreflective line corresponding to the retinal pigment epithelium (RPE) to the hyporeflective line corresponding to the choroidal-scleral interface. CT was measured subfoveally and at 500, 1000, and 1500 μm nasal and temporal of the fovea.
Choroid Vascularity Index
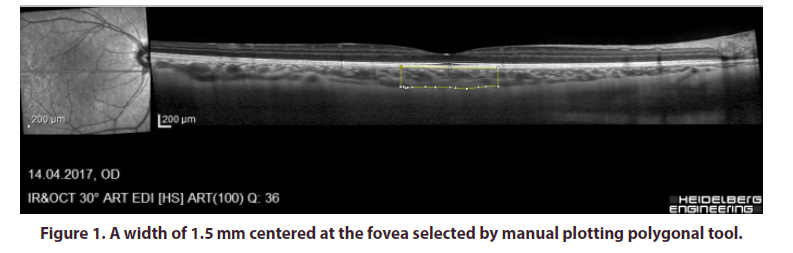
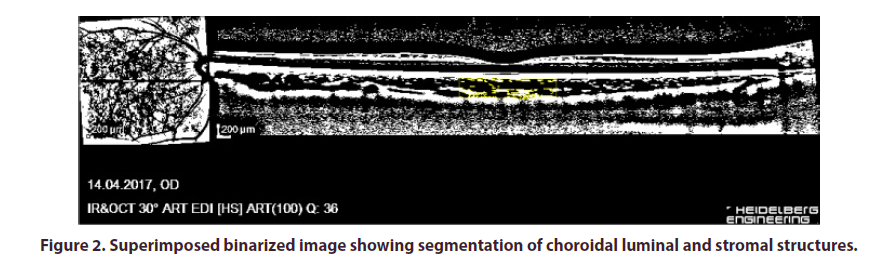
CVI was calculated using image binarization technique described by Sonoda et al. as modified by Agrawal et al [18,22]. Image processing was done using the opensource ImageJ software (version 1.47; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/ij/). In brief, 1 pixel EDI-OCT images were opened in ImageJ and the scale was set at 200 μm. A total choroidal area (TCA) 1.5 mm in diameter and centered on the fovea was selected using the manual plotting polygonal tool (Figure 1). The upper and lower borders of the choroid were marked at the RPE and the choroid-sclera junction, respectively. The entire length of the OCT B-scan was used for analysis. The EDI-OCT B-scan was then converted to 8-bit images using the default setting and Niblack's automated local threshold tool was applied to determine the boundaries of the luminal area (LA) and stromal area (SA). The image was then converted back to RGB (red, green, blue) for computation of the LA using the color threshold tool (Figure 2). CVI was calculated as the proportion of LA to TCA. All CVI measurements were performed by the same physician (S.T and T.K.).
Statistical Analysis
Statistical analysis was performed using SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables are presented as median (min– max) and categorical variables as number (n) and percentage (%). Normality of all data distributions was assessed with Shapiro–Wilk test. For the comparison of the means of two groups, Student’s t-test was used when normally distributed and the Mann–Whitney U test was used when the samples did not show normal distribution. The correlation between subfoveal CT and FMF variables was evaluated by Pearson correlation or Spearman’s rho test, depending on the data distribution. Statistical significance was defined as p < 0.05.
Results
The mean age of the 46 FMF patients (28 girls, 18 boys) was 12 ± 3.3 (range 6-18) years and that of the 46 controls (28 girls, 18 boys) was 11.7 ± 3.3 (range 6-17). There was no significant difference in age between the groups (p=0.655). BCVA was 0.00 logMAR (20/20 Snellen equivalent) in all eyes. The mean axial length was 23.3 ± 0.8 mm (range 21.4–25.5) in the FMF group and 22.9 ± 0.9 mm (range 21.1–25) in the control group (Table 1).
| Characteristics | FMF group | Control group | p value |
|---|---|---|---|
| Age, years | 12 ± 3.3 | 11.7 ± 3.3 | 0.655 ** |
| Gender (male/female) | 19/27 | 19/27 | |
| BCVA | 20/20 | 20/20 | |
| AL (mm) | 23.3 ± 0.8 | 22.9 ±0.9 | 0.117* |
| Fibrinogen, mg/dl | 286.28 ± 51.12 | ||
| ESR, mm/h | 18.6 ± 10.56 | ||
| CRP, mg/dl | 2.5 ± 3.9 | ||
| Proteinüri, n (%) | 42 (91.3%) | ||
| Amiloidozis, n (%) | 39 (84.8%) | ||
| * Student t-test | |||
| ** Mann Whitney U test | |||
Table 1. Demographic data of patients and controls.
Subfoveal CT was 333.87 ± 81.83 μm in the FMF group and 348.22 ± 82.96 μm in the control group (p>0.05). CT measured at 500, 1000, and 1500 μm nasal and temporal of the fovea also showed no significant differences between the groups (p>0.05). The mean CVI was 73.5% ± 4.8% in the FMF group and 74.7% ± 5.2% in the control group. There was no significant difference in mean CVI between the FMF and control groups (p>0.05). Only LA differed significantly in the FMF group compared to the control group (p=0.026) (Table 2).
| Parameter | FMF group Mean± SD Median (min-max) |
Control group Mean± SD Median (min-max) |
p value |
|---|---|---|---|
| Subfoveal CT (µm) | 333.87 ±81.83 328 (204-537) |
348.22 ± 82.96 340 (155-599) |
0.333** |
| N500 CT (µm) | 305.07 ± 75.4 301(186-523) |
324.98 ± 76.90 314 (152-564) |
0.207** |
| N1000 CT (µm) | 282 ± 71.39 286 (171-497) |
293.96 ±76.63 283 (101-559) |
0.380** |
| N1500 CT (µm) | 252.22 ± 70.50 246 (145-472) |
259.02 ± 74.71 247 (75-527) |
0.714** |
| T500 CT (µm) | 335.37 ± 77.93 334 (194-534) |
347.78 ± 84.49 341 (148-547) |
0.466* |
| T1000 CT (µm) | 336.39 ± 77.63 338 (212-511) |
336.11 ± 78.44 325 (173-519 |
0.986* |
| T1500 CT (µm) | 326.93±76.20 325 (216-492) |
331.67±78.67 331 (140-503) |
0.696** |
| Total Choroidal Area (mm2) | 0.552 ±0.137 0.544 (0.364-0.938) |
0.597 ±0.145 0.583 (0.255-0.958) |
0.069** |
| Luminal Area (mm2) | 0.404 ±0.100 0.399 (0.249-0.713) |
0.444±0.104 0.450 (0.188-0.713) |
0.026** |
| Stromal Area (mm2) | 0.147 + 0.04 0.139 (0.06-0.253) |
0.152 + 0.05 0.139 (0.06-0.308) |
0.821** |
| CVI (%) | %73.5 ± 4.8 %73.1 (64.7-84.7) |
%74.7 ± 5.2 %74.5 (64.6- 83.5) |
0.254* |
| * Student t-test | |||
| ** Mann Whitney U test |
Table 2. The mean choroidal thickness measurements and TCA, LA, SA, CVI values of FMF and control groups.
In the correlation analysis of CT and CVI with APRs, fibrogen level was found to be negatively correlated with CT at nasal 1500 μm (r=-0.326, p=0.043) and total choroidal area (r=-0.344, p=0.032) (Table 3).
| Parameter | FMF group Mean± SD Median (min-max) |
Control group Mean± SD Median (min-max) |
p value |
|---|---|---|---|
| Subfoveal CT (µm) | 333.87 ±81.83 328 (204-537) |
348.22 ± 82.96 340 (155-599) |
0.333** |
| N500 CT (µm) | 305.07 ± 75.4 301(186-523) |
324.98 ± 76.90 314 (152-564) |
0.207** |
| N1000 CT (µm) | 282 ± 71.39 286 (171-497) |
293.96 ±76.63 283 (101-559) |
0.380** |
| N1500 CT (µm) | 252.22 ± 70.50 246 (145-472) |
259.02 ± 74.71 247 (75-527) |
0.714** |
| T500 CT (µm) | 335.37 ± 77.93 334 (194-534) |
347.78 ± 84.49 341 (148-547) |
0.466* |
| T1000 CT (µm) | 336.39 ± 77.63 338 (212-511) |
336.11 ± 78.44 325 (173-519 |
0.986* |
| T1500 CT (µm) | 326.93±76.20 325 (216-492) |
331.67±78.67 331 (140-503) |
0.696** |
| Total Choroidal Area (mm2) | 0.552 ±0.137 0.544 (0.364-0.938) |
0.597 ±0.145 0.583 (0.255-0.958) |
0.069** |
| Luminal Area (mm2) | 0.404 ±0.100 0.399 (0.249-0.713) |
0.444±0.104 0.450 (0.188-0.713) |
0.026** |
| Stromal Area (mm2) | 0.147 + 0.04 0.139 (0.06-0.253) |
0.152 + 0.05 0.139 (0.06-0.308) |
0.821** |
| CVI (%) | %73.5 ± 4.8 %73.1 (64.7-84.7) |
%74.7 ± 5.2 %74.5 (64.6- 83.5) |
0.254* |
| * Student t-test | |||
| ** Mann Whitney U test |
Table 3. Correlation analysis between the choroidal thickness at the measurement points, TCA, LA, SA, CVI and fibrinogen, CRP, ESR.
Genetic analysis of the FMF patient group revealed 17 combined heterozygous, 21 heterozygous, and 8 homozygous mutations. The R202Q mutation was most common, followed by the M694V mutation (Table 4).
| Parameter | Homozigot (n= 8) |
Heterozigot (n= 21) |
Com. Heter (n= 17) |
p value |
|---|---|---|---|---|
| Subfoveal CT (µm) | 349.67 ± 61.61 | 323.90 ± 85.92 | 337.24 ± 88.84 | 0.727* |
| N500 CT (µm) | 313 ± 45.32 | 300.65 ± 75.93 | 306.06 ± 89.74 | 0.757** |
| N1000 CT (µm) | 297.56 ± 40.30 | 271.7 ± 68.22 | 285.88 ± 87.80 | 0.258** |
| N1500 CT (µm) | 264.78 ± 35.69 | 247.7 ± 68.80 | 259 ± 87.07 | 0.521** |
| T500 CT (µm) | 341.78 ± 40.11 | 327.15 ± 83.54 | 341.65 ± 88.52 | 0.828* |
| T1000 CT (µm) | 337.67 ± 59.42 | 331.15 ± 84.24 | 341.88 ± 81.78 | 0.918* |
| T1500 CT (µm) | 323.89 ± 47.07 | 328.95 ± 85.68 | 326.18 ± 80.59 | 0.986* |
| Total Choroidal Area(mm2) | 0.572 ± 0.074 | 0.564 ± 0.157 | 0.527 ± 0.138 | 0.442** |
| Luminal Area (mm2) | 0.429 ±0.055 | 0.410 ± 0.118 | 0.386 ± 0.094 | 0.282** |
| Stromal Area (mm2) | 0.142 ± 0.033 | 0.153 ± 0.048 | 0.141 ± 0.054 | 0.776* |
| CVI (%) | %73.6 ± 5.3 | %72.8 ± 4.7 | %75.1 ± 4.2 | 0.613* |
| * One way ANOVA | ||||
| ** Kruskal Wallis |
Table 4. The mean choroidal thickness measurements and TCA, LA, SA, CVI values of homozigot, heterozigot, compound teterozigot FMF groups.
CT and CVI were not significantly correlated with genetic subtype in the FMF group (p>0.05).
Discussion
In this study, we observed no significant difference between pediatric FMF patients and the control group in terms of CT and CVI. In vivo choroidal imaging by EDI-OCT, CVI has been increasingly used to evaluate the choroidal structure in recent years and is believed to provide more detailed information than CT [16,17]. This is because CVI involves a detailed assessment of the choroidal LA and SA and more accurately reveals which structures are responsible for changes in the choroidal structure. For example, CVI shows whether an increase or decrease in CT is caused by changes in LA or SA.
FMF is an autoinflammatory disease, and although eye involvement is uncommon, it can cause ocular diseases such as episcleritis, posterior uveitis, retinal vasculitis, and optic nerve edema [23]. Its autoinflammatory nature and ability to cause ocular inflammation suggest that it may affect the choroidal structure by causing luminal or stromal changes. In addition, because FMF causes vasculopathy, vascular changes such as increased atherosclerosis, intima media thickness, and endothelial dysfunction may occur [24,25]. Especially considering the inflammatory process, Agrawal et al. evaluated the CVI data of 19 patients with panuveitis and a control group over 3 months and reported no significant differences in TCA and SA but statistically significant decreases in LA and especially in CVI [26]. However, in order to make a comparison about choroidal structures in this regard, it is necessary to examine FMF patients, especially in the attack period, not the remission period.
In a study of children with FMF conducted by Erdurmuş et al., no statistically significant difference in CT was found compared to the control group [13]. Yener et al. compared randomized selected eyes of 20 pediatric FMF patients with those of a control group and detected no statistically significant difference in CT between the two groups [27]. Considering the age group in these studies is similar to the age group in our study, our findings are consistent with the CT data in previous studies. In children, CT increases with age and is inversely proportional to axial length. No sex-based difference in CT has been observed [28]. However, we could find no study regarding changes in CVI in the pediatric age group.
Optical coherence tomography angiography (OCTA) is increasingly used in clinical practice and is a superior diagnostic method to OCT in regards to retinal and choroidal vascular flow [29]. In a study conducted with adult FMF patients, Karaca et al. found no statistical difference in choriocapillary layer blood flow [30]. Çavdarli et al. compared 10 adult FMF patients with homozygous and 28 heterozygous M694V gene mutations with 40 healthy controls in their OCTA study and reported no significant difference in choriocapillary density [31]. However, in a study by Bicer et al. including 50 adult FMF patients, central and nasal CT values were found to be thinner compared to the control group. The authors stated that chronic inflammation may cause choroidal thinning in adults as a result of atrophy of the choroidal structures due to reduced vascular function. Vasculitis, atherosclerosis, or amyloidosis were also listed as possible causes [15]. Gundogan et al. evaluated 50 adult FMF patients during an attack and observed significant choroidal thickening in all areas measured. They stated that this may be a result of increased vascular permeability and exudation during the FMF attack [14].
Leukocytosis, ESR, CRP, and fibrinogen levels increase during FMF attacks and are important in diagnosis [32]. However, the course of APRs during attacks and non-attack periods varies considerably. For example, CRP may be high in 30% to 90% of patients, even in patients under colchicine treatment in the attack-free period. Many studies have demonstrated that subclinical inflammation persists in FMF patients, including high ESR and fibrinogen levels [33-35]. Although we examined FMF patients in attack-free periods in this study, we observed no significant difference in inflammatory markers or CT and CVI. Only a negative correlation was detected between fibrinogen and TCA, and even that cannot be considered clinically significant. Our review of the literature yielded no studies examining the relationship between APR and CT and CVI in pediatric FMF patients. Gundogan et al. found a significant positive correlation between CT and CRP in 50 adult FMF patients evaluated during an acute attack [14]. Similarly, CT was also significantly positively correlated with ESR and fibrinogen levels. Together with the significant correlations between CT and APR observed in this study, these findings suggest that CT and perhaps CVI are primarily affected by acute disease exacerbations rather than a chronic disease process in attack-free periods. This should be more accurately assessed with studies of CT and CVI in pediatric patients during acute attacks.
Another issue we evaluated in this study was whether genetic mutation subtypes were associated with differences in CT and CVI in FMF patients. Numerous genetic mutations are seen in FMF, with mutations in the M694V allele being especially common [36,37]. Similarly, R202Q allele mutations were most frequent in our study, followed by M694V allele mutation. Previous studies evaluating whether there is a clinical correlation with genetic mutations in FMF indicated that patients with a M694V mutation are more likely to have amyloidosis and show a more severe clinical course [38,39]. In our study, no statistically significant difference in CVI was found between the combined heterozygous, heterozygous, and homozygous subtypes.
This study has certain limitations. One of these is that our analysis included a small number of patients for a disease with high genetic diversity like FMF. The data may not represent all children with MEFV gene mutations. Multicenter studies with large patient groups should be conducted to evaluate whether genetic subtypes are associated with differences in CT and CVI. In addition, the evaluation of patients especially in the remission period rather than in the attack period is another limitation. Moreover, limitation is that APRs were evaluated during periods of disease remission. Studies of APRs during acute attacks may provide more detailed information in terms of their relationship with CT and CVI. However, we believe the fact that CT and CVI did not change in an FMF patient group that had a relatively shorter duration of chronic inflammation compared to the adult group and was in remission, and that these results are consistent with previous CT studies, makes a contribution to the literature.
In conclusion, the results of our study revealed no statistically significant difference in CVI and CT between pediatric FMF patients in remission and the control group. In addition, CVI and CT showed no correlation with FMF genetic subtype or AFR in FMF patients.
Acknowledgement
The authors would like to thank all the patients and their families.
Conflict of interest
Author Taha Sezer declares that he has no conflict of interest. Author Kuddusi Teberik declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by Duzce University Institutional Review Board.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- Ben Chetrit E, Levy M. Familial mediterranean fever. The Lancet. 351,659-664 (1998).
- Gershoni Baruch R, Shinawi M, Leah K et al. Familial Mediterranean fever: prevalence, penetrance and genetic drift. Eur J Hum Genet. 9(8),634-637 (2001).
- Cazeneuve C, Ajrapetyan H, Papin S et al. Identification of MEFV-Independent Modifying Genetic Factors for Familial Mediterranean Fever. Am J Hum Genet. 67(5),1136-1143 (2000).
- The French FMFC, Bernot A, Clepet C et al. A candidate gene for familial Mediterranean fever. Nat Genet. 17(1),25-31 (1997).
- Centola M, Wood G, Frucht DM et al. The gene for familial Mediterranean fever, MEFV, is expressed in early leukocyte development and is regulated in response to inflammatory mediators. Blood. 95(10),3223-3231 (2000).
- Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 29(2),144-168 (2010).
- Petrushkin H, Stanford M, Fortune F et al. Clinical Review: Familial Mediterranean Fever-An Overview of Pathogenesis, Symptoms, Ocular Manifestations, and Treatment. Ocul Immuno Inflamm. 24(4),422-430 (2016).
- Sezer T, Altınışık M, Koytak İA et al. The Choroid and Optical Coherence Tomography. Turk J Ophthalmol. 46(1),30-37 (2016).
- Vance SK, Imamura Y, Freund KB. The effects of sildenafil citrate on choroidal thickness as determined by enhanced depth imaging optical coherence tomography. Retina. 31(2),332-335 (2011).
- Sizmaz S, Küçükerdönmez C, Pinarci EY et al. The effect of smoking on choroidal thickness measured by optical coherence tomography. Br J Ophthalmol. 97(5),601-604 (2013).
- Chung SE, Kang SW, Lee JH et al. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 118(5),840-845 (2011).
- Maruko I, Iida T, Sugano Y et al. Subfoveal choroidal thickness after treatment of Vogt-Koyanagi-Harada disease. Retina. 31(3),510-517 (2011).
- Erdurmuş M, Bekdaş M, Demircioğlu F et al. Retinal and Choroidal Thickness in Children with Familial Mediterranean Fever. Ocul Immunol Inflamm. 22(6),444-448 (2014).
- Gundogan FC, Akay F, Uzun S et al. Choroidal Thickness Changes in the Acute Attack Period in Patients with Familial Mediterranean Fever. Ophthalmologica. 235(2),72-77 (2016).
- Bicer T, Celikay O, Kosker M et al. Retinal and Choroidal Thickness in Adult Patients with Familial Mediterranean Fever. Ophthalmic Epidemiol. 24(5),346-351 (2017).
- Agrawal R, Chhablani J, Tan KA et al. Choroidal vascularity index in central serous chorioretinopathy. Retina. 36(9),1646-1651 (2016).
- Agrawal R, Ding J, Sen P et al. Exploring choroidal angioarchitecture in health and disease using choroidal vascularity index. Prog Retin Eye Res. 77,100829 (2020).
- Agrawal R, Gupta P, Tan KA et al. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci Rep. 6,21090 (2016).
- Breher K, Terry L, Bower T et al. Choroidal Biomarkers: A Repeatability and Topographical Comparison of Choroidal Thickness and Choroidal Vascularity Index in Healthy Eyes. Transl Vis Sci Technol. 9(11),8 (2020).
- Moorthie S, Mattocks CJ, Wright CF. Review of massively parallel DNA sequencing technologies. Hugo J. 5(1),1-12 (2011).
- Tan CS, Ouyang Y, Ruiz H et al. Diurnal Variation of Choroidal Thickness in Normal, Healthy Subjects Measured by Spectral Domain Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 53(1),261-266 (2012).
- Sonoda S, Sakamoto T, Yamashita T et al. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am J Ophthalmol. 159(6),1123-1131.e1 (2015).
- Maccora I, Marrani E, Mastrolia MV et al. Ocular involvement in monogenic autoinflammatory disease. Autoimmun Rev. 20(11),102944 (2021).
- Akdogan A, Calguneri M, Yavuz B et al. Are familial Mediterranean fever (FMF) patients at increased risk for atherosclerosis? Impaired endothelial function and increased intima media thickness are found in FMF. J Am Coll Cardiol. 48(11),2351-2353 (2006).
- Bilginer Y, Ozaltin F, Basaran C et al. Evaluation of intima media thickness of the common and internal carotid arteries with inflammatory markers in familial Mediterranean fever as possible predictors for atherosclerosis. Rheumatol Int. 28(12),1211-1216 (2008).
- Agrawal R, Salman M, Tan KA et al. Choroidal Vascularity Index (CVI)--A Novel Optical Coherence Tomography Parameter for Monitoring Patients with Panuveitis? PLoS One. 11(1),e0146344 (2016).
- Yener A, Tayfur A Posterior Segment Ocular Parameters in Children with Familial Mediterranean Fever. Ocul Immunol Inflamm. 29(3),615-620 (2021).
- Bidaut Garnier M, Schwartz C, Puyraveau M et al. Choroidal thickness measurement in children using optical coherence tomography. Acta Ophthalmologica. 91(2013).
- Spaide RF, Fujimoto JG, Waheed NK et al. Optical coherence tomography angiography. Prog Retin Eye Res. 64,1-55 (2018).
- Karaca EE, Ozek D, Omma A et al. Comparison of optical coherence tomography angiography results of adult patients with Familial Mediterranean fever and healthy individuals. Ther Adv Ophthalmol. 11 (2019).
- Çavdarli C, Çavdarli B, Topcu-Yilmaz P et al. Optical coherence tomography-angiographic vascular densities in Familial Mediterranean Fever (FMF) Patients with M694V Mutations. Ophthalmic Genet. 41(3),257-262 (2020).
- Korkmaz C, Ozdogan H, Kasapçopur O et al. Acute phase response in familial Mediterranean fever. Ann Rheum Dis. 61(1),79-81 (2002).
- Özcakar ZB, Yalçinkaya F, Yüksel S et al. Possible effect of subclinical inflammation on daily life in familial Mediterranean fever. Clin Rheumatol. 25(2),149-152 (2006).
- Ben-Zvi I, Livneh A. Chronic inflammation in FMF: markers, risk factors, outcomes and therapy. Nat Rev Rheumatol. 7(2),105-112 (2011).
- Lachmann HJ, Şengül B, Yavuzşen TU et al. Clinical and subclinical inflammation in patients with familial Mediterranean fever and in heterozygous carriers of MEFV mutations. Rheumatology. 45(6),746-750 (2006).
- Yilmaz E, Ozen S, Balcı B et al. Mutation frequency of familial Mediterranean fever and evidence for a high carrier rate in the Turkish population. Eur J Hum Genet. 9(7),553-555 (2001).
- Mansour I, Delague V, Cazeneuve C et al. Familial Mediterranean fever in Lebanon: mutation spectrum, evidence for cases in Maronites, Greek orthodoxes, Greek catholics, Syriacs and Chiites and for an association between amyloidosis and M694V and M694I mutations. Euro J Hum Genet. 9(1),51-55 (2001).
- Touitou I, Sarkisian T, Medlej‐Hashim M et al. Country as the primary risk factor for renal amyloidosis in familial Mediterranean fever. Arthritis Rheum. 56(5),1706-1712 (2007).
- Livneh A, Langevitz P, Shinar Y et al. MEFV mutation analysis in patients suffering from amyloidosis of familial Mediterranean fever. Amyloid. 6(1),1-6 (1999).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref