Perspective - Interventional Cardiology (2015) Volume 7, Issue 3
Bioresorbable stents for pediatric practice: where are we now?
- Corresponding Author:
- Damien Kenny
Rush Center for Congenital & Structural Heart Disease; Rush University Medical Center, 1653 West Congress Parkway,Chicago, IL 60612, USA
Tel: +1 312 942 6800
Fax: +1 312 942 6801
E-mail: damien_kenny@rush.edu
Abstract
Children born with congenital heart disease may outgrow materials used to palliate their heart defects. Application of evolving bioresorbable technologies to these children may assist in providing minimally invasive options that lead to restoration of vascular function, arterial remodeling and vessel growth. The availability of this technology for children may be even more vital than its use in adults as early reversal of vessel stenosis in children may have a profound impact on lifetime vessel development, and the disappearance of the scaffold over time ensures that somatic development should not lead to stent outgrowth. This review evaluates the progress of bioresorbable scaffold development as applied to children with congenital heart disease.
Keywords
bioresorbable,congenital heart disease,pediatric,stenting,surgery
Nonsurgical therapeutic strategies for patients with congenital heart disease have evolved greatly over the past 20 years. Endovascular stenting is now the treatment of choice for coarctation of the aorta [1] and pulmonary artery stenosis [2] in older children and adolescents where balloon expandable stents dilatable up to adult sizes can be delivered safely and provide long-term luminal patency. Stent endurance is also excellent with low reintervention rates for stent fractures with contemporary stent designs [3,4]. Application of this approach to smaller infants has been more challenging due to the inability to deliver larger profile metal stents required for serial dilation to ensure adequate vessel ‘growth’ occurs throughout childhood. Indeed the requirements for a suitable stent have evolved as increasingly complex congenital surgical repairs and palliations undertaken in neonatal life have led to a greater need for smaller vessel rehabilitation, particularly in the pulmonary arteries. Varying endovascular strategies have been attempted including highpressure balloon fracture of smaller lower profile stents [5] and novel stent designs [6] but to date, these approaches have not led to widespread acceptance due to concerns regarding vessel growth or damage. Bioresorbable coronary scaffolds have been tested preclinically with early outcome clinical trial data published demonstrating excellent immediate results with no early in stent restenosis seen at 6-month follow-up [7,8]. Longerterm data are also available evaluating comparable ischemia driven major cardiac event rates at 3 years follow-up showing favorable results compared with metal stents. Data from these trials have demonstrated return of physiological responses to vasoactive stimuli once the scaffold has resorbed [9]. This potential for re-establishment of functional capabilities of a vessel is very attractive. However the available scaffold diameters and lengths for coronary stenting may not be suitable for application in the aorta or pulmonary arteries of pediatric patients. A number of scaffold designs and materials have been evaluated. These include biocorrodible metals such as magnesium and polymers of polycarbonate or polylactic acid. The majority of contemporary clinical trials are evaluating scaffolds made with polylactic acid, as results with corrodible metals have been disappointing [10]. Indeed a resorbable magnesium scaffold has been evaluated in a pediatric patient with a stenotic aorto-pulmonary collateral [11], and although initial increase in vessel diameter was demonstrated, significant restenosis was seen at 4 months mirroring adult coronary studies. Therefore the thrust of contemporary efforts is focused on a polymer-based scaffold providing adequate radial support up to 6 months postimplantation with an absorption time of up to 2 years. This review will focus on how progress with coronary stent technology has percolated through to the pediatric world and what modifications are necessary to ensure the unique challenges seen in congenital heart disease patients with vessel stenosis are addressed.
The evolution of bioresorbable scaffolds
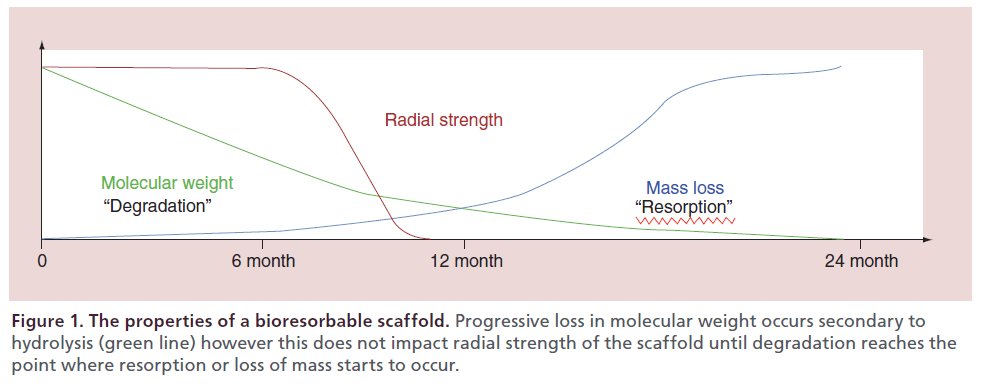
Although the revolution of coronary artery stenting was still on an upward slope in the early 1990s, there was already an understanding of the limitations of placing a bare metal stent to overcome vessel stenoses. Albeit the challenges of acute occlusion and recoil seen with balloon angioplasty were overcome, subacute thrombosis, vessel remodeling and neointimal hyperplasia became relevant. Hence although the stent achieved its goal, there became a point at which it started to generate its own chronic and detrimental influences on the vessel. Therefore the search for a stent that could mitigate against the acute and subacute challenges of vessel stenosis, yet dissolve over time, began. The first polymeric (nonbioresorbable polyethylene terephthalate) braided mesh stent was designed in 1992. Preclinical evaluation in porcine models demonstrated that the developed polymeric stent had comparable safety and efficacy to the cotemporary metallic stent [12]. Since this time, extensive development and testing of polymers and bioresorbable metals has taken place. These polymers can be manipulated through chemical processes to produce the polymer that has the optimal properties for the intended purpose of the device (Table 1). For a coronary stent or indeed a stent used for any vessel stenosis, radial strength must be maintained for an adequate period of time despite ongoing degradation of the stent, which will ultimately lead to stent resorption and loss of mass (Figure 1). Of the agents evaluated the aliphatic polymers such as poly-l-lactic acid (PLLA) provide the most favorable degradation/resorption profile in the polymer group while magnesium has evolved as the most promising biocorrodible metal (Table 2).implantation (Figure 2). The key element is ensuring that radial strength is maintained despite progressive loss in molecular weight until a point at when progressive loss of mass takes place and the stent begins to fragment into segments of lower molecular weight. Once the polymer chains have been hydrolyzed into shorter segments, they become increasingly hydrophilic accelerating resorption with the soluble monomer (l-Lactate) converted into pyruvate, which is further converted to carbon dioxide and water through the Krebs cycle.
| Polymer | Melting Pt (°C) | Tg (°C) | Modulus (Gpa) | Elongation (%) | Degradation time (months) |
|---|---|---|---|---|---|
| Polyglycolide: PGA | 225–230 | 35–40 | >7.0 | 15–20 | 6–12 |
| Poly(ɛ-Caprolactone): PCL | 55–65 | -65 to -60 | 0.2–0.34 | 300–500 | >24 |
| Poly(l-Lactide): PLL | 170–180 | 60–65 | 2.8–4.0 | 5–10 | >24 |
| Poly(d,l-lactide): PDLL | Amphorous | 45–55 | 1.4–2.7 | 3–10 | 12–16 |
| 85:15 DLPLG | Amphorous | 45–52 | 1.4–2.8 | 3–10 | 5–6 |
| 75:25 DLPLG | Amphorous | 45–52 | 1.4–2.8 | 3–10 | 4–5 |
| 65:35 DLPLG | Amphorous | 45–50 | 1.4–2.8 | 3–10 | 3–4 |
| 50:50 DLPLG | Amphorous | 30–45 | 1.4–2.8 | 3–10 | 1–2 |
Varying the polymer properties will affect the elasticity or stiffness of the material ([Young’s] Modulus) and also the degradation times. It is measured here in Gpa or gigapascals.
Table 1. Properties of polymers with potential application to a bioserbable scaffold.
| Scaffold | Company | Status | Strut material | Coating material | Drug elution | Design | Resorption time (months) |
|---|---|---|---|---|---|---|---|
| Igaki–Tamai | Kyoto | CE mark | PLLA | Nil | Nil | Helical zigzag | 24 |
| Medical | coils with | ||||||
| straight bridges | |||||||
| Absorb BVS | Abbott | CE mark | PLLA | PDLLA | Everolimus | In-phase hoops | 24–36 |
| with straight | |||||||
| links | |||||||
| DREAMS 1.0 | Biotronik | Clinical studies | Magnesium | PLGA | Paclitaxel | 6-crown | 12 |
| DREAMS 2.0 | – | Preclinical | Magnesium | PLLA | Sirolimus | 6-crown | – |
| ReZolve™ | REVA | Clinical studies | Poly-tyrosine | Nil | Sirolimus | Spiral slide-and- | 24 |
| Medical | derived | lock | |||||
| polycarbonate | |||||||
| DeSolve™ | Elixir | CE mark | PLLA | – | Novolimus | In-phase hoops | 24–36 |
| myolimus | with straight | ||||||
| links | |||||||
| BTI Ideal™ | Xenogenics | Clinical studies | Polymer salicylate | Salicylate + | Nil | Tube with laser- | – |
| +linker | different linker | sirolimus | cut voids | ||||
| ART18Z | ART | Clinical studies | PLLA | Nil | Nil | Creep resistant | 18–24 |
| hinge | |||||||
| Amaranth | Amaranth | Clinical studies | PLLA | Nil | Nil | In-phase hoops | 12–24 |
| with straight | |||||||
| links | |||||||
| Xinsorb | Huaan | Preclinical | PLA, PCL, PGA | – | Sirolimus | – | – |
| Biotech | |||||||
| Acute | OrbusNeich | Preclinical | PLCL, PDLD, PLLA | – | Sirolimus | Single ringlet | – |
| +CD4 Ab | Helical linked | ||||||
| double ringlet | |||||||
| MeRes™ | Meril | Preclinical | PLA | – | Merilimus | Hybrid scaffold | – |
| sirolimus | geometry | ||||||
| FADES | Zorion | Preclinical | PLGAMagnesium | Nil | Nil | – | 6 |
| Medical |
BVS: Bioresorbable vascular scaffold; DREAMS: Drug-eluting absorbable metallic stents; PDLLA: Poly-d,l-lactide acid; PLCL: Poly-l-lactide-co-ɛ-caprolactone; PLGA: Poly-lactide-co-glycolide; PLLA: Poly-l-lactic acid.
Table 2. Major bioresorbable scaffolds designed for coronary application.
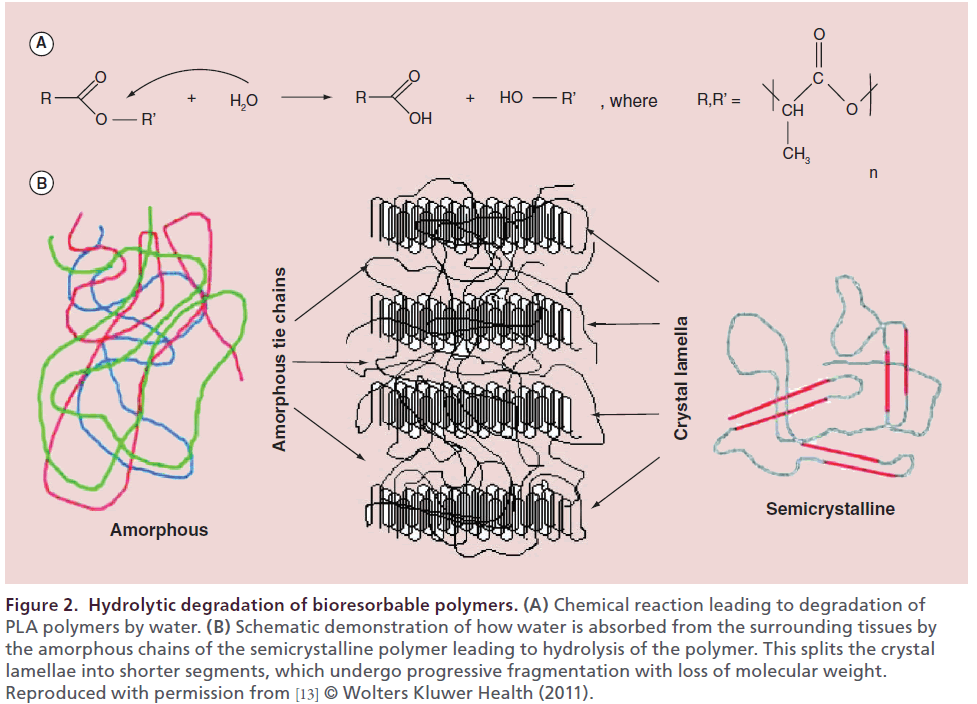
Figure 2: Hydrolytic degradation of bioresorbable polymers. (A) Chemical reaction leading to degradation of PLA polymers by water. (B) Schematic demonstration of how water is absorbed from the surrounding tissues by the amorphous chains of the semicrystalline polymer leading to hydrolysis of the polymer. This splits the crystal lamellae into shorter segments, which undergo progressive fragmentation with loss of molecular weight. Reproduced with permission from [13] © Wolters Kluwer Health (2011).
Magnesium alloys
.Magnesium ions are essential to all living cells, where they play a major role in manipulating important biological polyphosphate compounds like ATP, DNA and RNA. The absorbable magnesium stent (AMS) (Biotronik, Berlin Germany) was the first biocorrodible metal stent to be implanted in humans and has evolved through a number of iterations from the initial AMS-1 stent which was plagued by vessel recoil and restenosis thought to be due to early loss of radial force secondary to rapid stent degradation [14]. Design modifications have been made with development of two newer stents – AMS-2 and AMS-3. The AMS-2 is made with a different magnesium alloy that has a higher collapse pressure and a slower degradation time with the AMS-3 incorporating a bioresorbable matrix for controlled release of an antiproliferative drug onto the AMS-2 stent with the anticipation this may mitigate against neointimal proliferation. Other investigators are also evaluating alternative magnesium alloys to improve radial force and degradation times. Zorion (IN, USA) have developed a small diameter (100–125 μm) magnesium alloy wire with a thin walled l-PLA absorbable polymer sleeve laser-cut to provide stability and flexibility to the stent. Preclinical results evaluating stent performance are awaited.
Iron & zinc
Both iron and zinc have been evaluated as biocorrodible alternatives to magnesium stents. Iron has a slow degradation time and leads through oxidation to ‘rust’ within the vessel wall that may reduce the cross sectional area of the vessel lumen. Consequently iron has not been studied extensively in the coronary arteries. However preclinical assessment of its performance in the descending aorta for a potential pediatric application demonstrated comparable follow-up rates of vessel patency and neointimal proliferation to a stainless steel control [15]. Nevertheless as corrosion rates are difficult to regulate it may be that modification through alloys or alternative coatings may be necessary to ensure more predictable degradation rates [16] before application to clinical practice.
Zinc is an essential element for basic biological function, and participates in nucleic acid metabolism, signal transduction, apoptosis regulation and gene expression. Six-month follow-up assessment of a zinc stent in the abdominal aorta of a rat demonstrated desirable aspects of both iron and its alloys, namely in vivo longevity, with the harmless degradation of magnesium and its alloys [17]. However, due to the low tensile strength of pure zinc, alloying with another agent may be necessary to promote strength.
Other stent materials
Tyrosine polycarbonate polymers have been used in the REVA stent with reported resorption times of between 12 and 18 months depending on the molecular weight of the polymer. The polymer degrades into carbon dioxide, water and ethanol. Further stent modifications have taken place to counter the focal mechanical failures that were seen in early clinical trials resulting in the second-generation ReZolve scaffold, which has a Sirolimus coating and a more robust polymer.
Salicylate-based polymer stents have also been developed on the expectation that the anti-inflammatory properties of salicylic acid may prevent neointimal proliferation. Iterations of the IDEAL stent are undergoing clinical trials but it is unclear if these will ever have any application in the pediatric market.
The unique properties and preclinical and early clinical outcomes of each of the numerous coronary scaffolds in development are beyond the remit of this review. To date only three of these stents have achieved CE marking and none of these has received US FDA approval in the USA. Relevant clinical data will be discussed in relation to those stents currently with a potential pediatric application.
Bioresorbable scaffolds for pediatric practice
Bioresorbable scaffold implantation in both the airway and the esophagus of children has been reported however experience and follow-up has been limited to small case series [18–20]. These systems have generally been self-expanding stents. Vondrys et al. [20] reported on the use of a custom-made polydioxanone stent in the airways of four children. Stent migration and premature degradation requiring repeat intervention was necessary in all patients and underlies some of the challenges involved with adequate radial force and rates of degradation. Similar issues exist with developing an appropriate stent for children with congenital heart disease. In particular the issues in developing a larger diameter scaffold for pediatric practice relate to:
• Lower radial strength of larger diameter bioresorbable scaffolds;
• Need for thicker stent struts to counter lower radial strength leading to larger stent profile;
• Brittle nature of semi-crystalline polymers particularly when crimping thicker stents for lower profile delivery;
• Potential need for drug elution with uncertain systemic impact of antiproliferative drug in smaller infants;
• Uncertainty regarding potential for stent fragment embolization with nonendothelialization of nonopposed areas of the stent in irregular vessel stenoses;
• Lack of a naturally occurring animal model (such as coarctation of the aorta) to mimic vessel stenoses seen in infants;
• Limited likelihood of return on investment for industry due to limited clinical application.
To date, the majority of the limited clinical experience in infants with congenital heart disease has been with magnesium coronary scaffolds in either the branch pulmonary arteries or aorta of small infants [11,21–22]. The durability of these early iterations of magnesium stents in the reported cases has been called into question with the appearance of early restenosis, however selective use of these stents to act as a bridge to surgery in critically ill infants may be an acceptable approach [22]. Pathological assessment of a magnesium scaffold 5 months following placement to treat severe left pulmonary artery stenosis in a preterm infant did not demonstrate detrimental arterial remodeling following stent implantation [23]. Macroscopic assessment demonstrated degradation of the magnesium scaffold and disintegration of the metal struts leaving small bulks of calcium-phosphate crystals. The scaffold implantation diameter was 3 mm however at explantation, the diameter of the stented vessel segment was 3.7 mm demonstrating some vessel growth after degradation of the resorbable scaffold. However the unaffected right pulmonary artery was 7 mm suggesting that a more durable polymer that may be redilatable to keep up with the rapid growth phase in the first year of life, may be preferable for a pediatric application. Limited intimal proliferation with no stent-related inflammatory reaction was noted, an important finding particularly considering the lack of drug-eluting properties of this stent. Indeed the importance of a drug-eluting design in mitigating against neointimal proliferation in a stent for pediatric practice has sparked debate, as there may be added complexities in evaluating drug levels and proving safety in smaller infants in a clinical trial setting. A recent case report describing serum drug levels after use of an everolimus drug-eluting stent in an infant with recurrent pulmonary vein stenosis did not demonstrate toxic drug levels [24] and this has spurned some further clinical reports of these scaffolds in congenital heart disease infants with vessel stenoses [25]; however, the risk benefit of drug eluting properties in larger diameter scaffolds vessels with growth potential remains unclear. This highlights one of the many differences in the requirements of a bioresorbable scaffold for an adult coronary application versus those properties required for implantation in major arteries in small infants, outside of the very obvious differences in vessel size. This is why a dedicated pediatric design is required to ensure these requirements are appropriately addressed. At present here are a number of prototypes with preclinical data being assessed (Table 3).
| Company/center | Status | Strut material | Drug elution | Size, design and profile | Resorption time (months) |
|---|---|---|---|---|---|
| Akesys Medical | Preclinical trial | PLLA | No | 6 × 20 mm | 24 |
| underway | Balloon expandable | ||||
| 6 Fr delivery | |||||
| University of Texas | Preclinical data | PLLA | No | 3–6 mm diameter | NA |
| SMC | published | Balloon expandable | |||
| 5 and 6 Fr delivery | |||||
| 480 Biomedical | Preclinical data | PLGA coated | No | 6–10 mm diameter | NA |
| presented | with cross- | 10–25 mm length | |||
| linked polyester/ | Self-expanding | ||||
| polyurethane | 6 Fr delivery | ||||
| elastomer | |||||
| UCLA | Ongoing design | PLLA | No | 6 Fr delivery | NA |
| modification |
NA: Not available; PLGA: Poly-lactide-co-glycolide; PLLA: Poly-l-lactic acid.
Table 3. Current bioresorbable scaffolds for pediatric application.
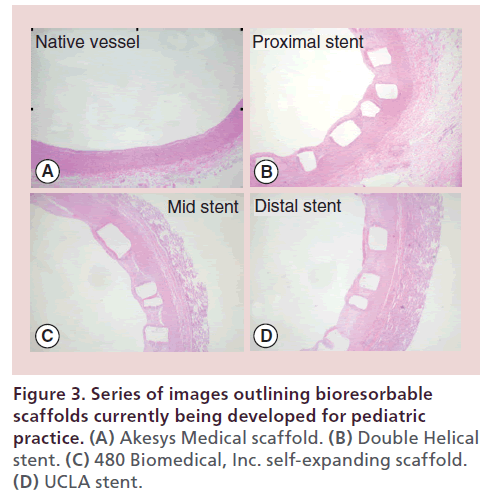
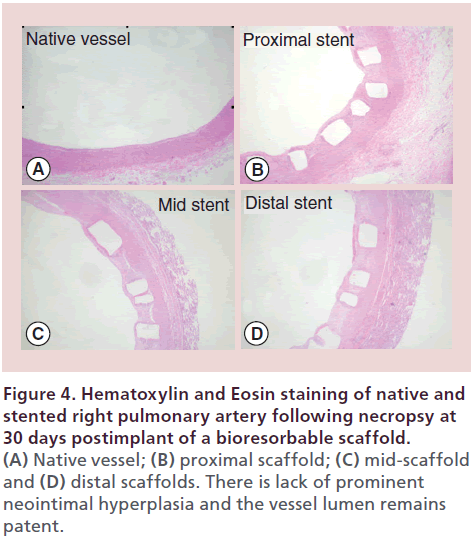
Akesys medical pediatric bioresorbable scaffold The DESolve stent (Elixir Medical, CA, USA) is a Novolimus-eluting bioresorbable coronary scaffold fabricated from a PLLA-based polymer and is one of only two bioresorbable PLLA scaffolds with CE marking. Impressive clinical results in 126 adults with coronary artery disease have been demonstrated with late luminal loss measurements at 6 months comparable to drug-eluting stents. Indeed both the mean scaffold area and mean luminal area of the stented vessel were shown to have increased over 6 months, a finding that suggests early vessel remodeling. The stent degrades over one year with full resorption by 2 years. A larger iteration of the scaffold without drug, is being designed by Akesys Medical (CA, USA) for application to pediatric practice (Figure 3A). The stent is premounted and deliverable through a 6-Fr sheath although there are ongoing efforts to reduce the profile to 5 Fr without impacting on the radial strength of the stent. Thirty-day pathology assessment of this 6 × 20 mm scaffold in a piglet model following implantation into the branch pulmonary arteries and the descending abdominal aorta have demonstrated no vessel inflammation with minimal vessel injury based on standard Schwartz scores [26] (Figure 4). A larger three-month survival study is underway to further assess the properties of this larger scaffold prior to considering a clinical trial.
Figure 4: Hematoxylin and Eosin staining of native and stented right pulmonary artery following necropsy at 30 days postimplant of a bioresorbable scaffold.(A) Native vessel; (B) proximal scaffold; (C) mid-scaffold and (D) distal scaffolds. There is lack of prominent neointimal hyperplasia and the vessel lumen remains patent.
Double helical (DH) coil stent
The DH stent (Figure 3B) has been designed and tested in a preclinical model by a group at the University of Texas Southwestern Medical Center. PLLA 32 pellets were melted to extrude a 0.25 mm diameter fiber that was further reduced to 100 μm diameter. Four double opposed helical stent geometries with varying expandable diameters (3–6 mm) were fashioned and crimped onto appropriate sized balloons
The stent design consists of three internally coiled loops that unwind with expansion adding to the overall final diameter of the stent. Platinum markers are used at either end of the stent as the polymer is not radio-opaque. The 3–5 mm stents are deliverable through a 5-Fr system with the 6 mm scaffold requiring a 6-Fr delivery sheath. Initial bench testing evaluated collapse pressures and elastic recoil and demonstrated collapse pressures inversely related to the stent diameter with elastic recoil lower for smaller diameter stents.
Initial preclinical testing evaluated the DH stent in the external iliac arteries of six adult female rabbits weighing 3.4–3.9 kg [27]. Stent deployment was successful in all cases with 1-month follow-up data available on two animals. Follow-up angiography and intravascular ultrasound demonstrated mild neointimal restenosis with mild mid-stent luminal loss of 28 and 19%. There was minimal neointimal proliferation noted with IVUS.
A further follow-up assessment of larger diameter (6 mm) DH stents in the descending aorta of seven New Zealand white rabbits out to nine months has been published [28]. Delivery was via a 6-Fr sheath and the follow-up angiography, intravascular ultrasound and histopathology demonstrated good stent apposition to the vessel wall, minimal luminal loss and minimal neointimal hyperplasia. The lack of significant neointimal proliferation without the need for drug elution is a positive finding and it may be that these larger diameter, growing vessels react differently to adult coronaries in this regard. Two of the rabbits had intentional distal embolization of PLLA stent fragments, which lodged in the right common iliac and the left internal iliac arteries, respectively. This goal was to mimic potential for fragment embolization, which may occur secondary to poor endothelialization with incomplete scaffold apposition in patients with poststenotic vessel dilation. Similar concerns have been expressed in a clinical report demonstrating early fracture of a bioresorbable scaffold with enhanced mobility of the proximal end of the scaffold following placement in an infant with severe right pulmonary artery stenosis [25]. Mimicking the impact of variable stent apposition in an animal model may prove challenging, however clinical coronary trials have not indicated this to be an issue although scaffold apposition is more likely to be complete along the length of the scaffold in this setting. Further experimental design testing with this stent has been reported evaluating the impact of anti-inflammatory coating with dexamethasone comparing a porous versus a nonporous PLGA coating. Cumulative drug release was significantly higher with the porous coating design without any impact on mechanical properties of the PLLA fiber [29], and it remains to be seen whether this design will add weight to inclusion of a drug-eluting agent to scaffolds designed for infants with congenital heart disease.
480 Biomedical self-expanding stent
480 Biomedical, Inc. (MA, USA) have tested a selfexpanding scaffold (Figure 3C) in a swine model for potential clinical application to small children with aortic coarctation and pulmonary artery stenosis. The self-expanding scaffold is a composite of braided, bioresorbable poly(l-lactide-co-glycolide) monofilaments coated with a cross-linked polyester/polyurethane elastomer. Radiopaque edge markers are incorporated into the device for fluoroscopic visualization. Pediatricspecific requirements were achieved by iterating base fiber (number, diameter, geometry, pattern and angle) and elastomer (cross-link density, coating mass and morphology, as well as hydrophobicity). The stent is deliverable via a 6-Fr delivery system, with expanded diameter of 6–10 mm and length 10–25 mm.
Bench testing has demonstrated the scaffold provides acute radial strength comparable to balloonexpandable stents commonly used in larger children (Palmaz Genesis, Cordis), sustained radial strength for 6 months, and completion of active resorption by 12 months. Radial resistive force was shown to be greater than a Genesis stent comparator (984 ± 39 mmHg vs 830 mmHg; data presented at PICS-AICS, Chicago, IL, USA 2014). The chronic outward force was 293 ± 13 mmHg, whereas conventional balloon-expandable stents have no outward force and therefore minimal ability to self-expand as the vessel grows. In simulated 6 month in vitro testing, radial resistive and chronic outward forces declined 10–20% per month.
Stents have also been tested in vivo up to 2 weeks in a 21 kg swine. There was no acute or follow-up thrombosis, constrictive remodeling or branch-loss. Endothelialization was 95–100% complete in all histologic sections. In-stent stenosis or inflammation was mild. Further more extensive preclinical data are expected.
UCLA stent
The group at University of California, Los Angeles have developed a PLLA scaffold designed specifically for children with congenital heart disease. The stents are fabricated from PLLA tubes generated using a robotic sprayer and rotating mandrel which allows for layering of multiple polymers. The tubes are then laser cut into the desired stent geometry (Figure 3D) and crimped down on a balloon catheter for stent delivery. Due to initial stents being prone to fracture during crimping, stent geometry has been analyzed using FEM stresstesting (Autodesk Inventor 2014). These tests indicated the greatest concentrated stresses were present in the original curved design and these data have led to design modifications with optimal stress distribution and crimping ability. Studies are underway to optimize heat-setting and crimping protocol for stent deliverability.
Applications to pediatric practice
Multiple studies have demonstrated that pulmonary artery stenting in children is safe, effective in decreasing right ventricular afterload and improving the size of the branch pulmonary arteries [2,30–31]. However limitations on stent size exist within infants and smaller children. Multiple conditions requiring surgery in infancy including truncus arteriosus, tetralogy of Fallot and transposition of the great arteries are associated with significant rates of postoperative pulmonary artery distortion leading to stenosis [30,32–33]. Balloon angioplasty has been demonstrated to be less effective than stenting in providing long-term relief of these stenosis, and therefore although technically less challenging than stenting in small infants, the results are often suboptimal. Smaller sized low profile stents may be used and have been effective in providing shorter-term relief of stenoses, however these stents are often limited by maximum inflation diameters due to their low profile and hence the stent is soon outgrown providing a therapeutic conundrum for treating physicians and surgeons. Conventional treatment has involved surgical removal of the stent when somatic growth has led to restriction in further pulmonary artery growth however longitudinal transection of the stent with patching of the artery has been associated with high requirements for further reintervention [34]. More recently endovascular options have been described with use of ultra-high pressure balloons to disrupt smaller diameter stents with some degree of success however the response is somewhat unpredictable and the potential for progressive longer-term vessel damage uncertain [5].
Hence, there is significant need for a therapeutic alternative to low-profile small diameter bare metal stents for pulmonary artery stenoses in infants and smaller children and this is one of the main clinical conditions in which a suitable bioresorbable scaffold could treat stenosis without committing the infant to further intervention or surgery when somatic growth has outstripped maximum stent diameter.
The greatest benefit of a bioresorbable scaffold in the setting of small infants is the potential for reestablishment of normal vasomotor function and vessel growth over the lifetime of the vessel if early remodeling occurs. Neointimal ingrowth is less likely to lead to vessel restenosis and the impact of covering of sidebranches is clinically less relevant. Artifact caused by permanent metallic materials which may impact upon follow-up noninvasive imaging such as cardiac MRI is also less of an issue with a bioresorbable scaffold [35].
The other major congenital heart defect likely to benefit from an appropriate sized bioresorbable scaffold is coarctation of the aorta (CoA), accounting for 6–8% of live births with congenital heart disease, and represented by discrete constriction of the aortic isthmus. Untreated CoA has a significant early mortality with one report identifying CoA in 17% of neonates dying from congenital heart disease [36]. Therapeutic options in infancy include balloon angioplasty, stent implantation or surgical repair, with surgery having evolved into the treatment of choice as balloon angioplasty has demonstrated and aneurysm formation [37]. Stent implantation has been performed and reported as a rescue therapy in those too unwell for surgery [13], however similar longer-term limitations exist in relation to somatic growth of the patient exceeding the dilatable diameter of the stent. A bioresorbable scaffold could be used to treat neonatal coarctation avoiding the need for a thoracotomy which has been associated with damage to the thoracic duct, laryngeal nerve damage and longer term risk of scoliosis, and potentially restoring native aortic growth potential and physiologic properties with scaffold resorption. Other potential pediatric applications include arterial duct stenting in duct dependent lesions and right ventricular outflow tract stenting in infants with tetralogy of Fallot.
Conclusion & future perspective
As the complexity of surgical interventions for congenital heart disease increases along with the demand for less invasive solutions to both primary and recurrent vessel stenoses, the need for a robust, appropriate diameter low-profile bioresorbable scaffold to provide a long-term solution to vessel rehabilitation in children is vital. There are numerous stents undergoing preclinical assessment but it is unclear if these scaffolds will perform adequately in the setting of congenital or acquired large artery stenosis. Questions remain regarding the ideal resorption properties, the need for and potential deleterious effects of drug-coating and whether these scaffolds will perform adequately if balloon re-expansion during phases of rapid infantile growth is required. However there is almost universal agreement on the potential benefits of a bioresorbable scaffold applicable to pediatric practice. Although industry support may be limited due to likely poor financial return on any capital investment, investigators must continue to find collaborative approaches to ensure that this need in children with CHD is met. Extension of this technology to stent valves and mergence of stem cell technologies to valve tissue mounted a bioresorbable scaffold could herald the way for a fully integrated valve system with growth potential, implanted without the need for surgery. Either way, continued commitment and focus from clinicians, scientists and industry are essential so that advances in this technology are filtered through to the population likely to benefit most.
Financial & competing interests disclosure
Hijazi is an investor with Elixir Medical. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Limitations of current interventions in congenital heart disease
• Congenital heart disease is the most common congenital defect affecting just under 1 in 100 children.
• A significant proportion of these infants will require surgery to correct or palliate their defects with implications of these surgeries leading to future scar and vessel stenoses.
• Current options for vessel stenosis in smaller infants include further surgery, or stenting, which may limit future vessel growth and commit the patient to recurrent interventions and further vessel scarring.
Application of bioresorbable technology to children with congenital heart disease
• Use of bioresorbable scaffolds for coronary artery disease is becoming more commonplace due to a number of attractive features leading ultimately to the potential for restoration of normal vasomotor function.
• Application of this technology to children with congenital heart disease may reverse the implications of vessel stenosis on longer-term vessel growth and restore normal physiology at an early stage.
• A number of scaffolds are in development for application to children with congenital heart disease. A number of questions remain unanswered however continued commitment to development of these scaffolds for children is likely to provide alternatives that may limit the need for surgery and longer-term morbidity.
References
- Forbes TJ, Kim DW, Du W et al. CCISC Investigators. Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: an observational study by the CCISC (Congenital Cardiovascular Interventional Study Consortium). J. Am. Coll. Cardiol. 58(25), 2664–2674 (2011).
- Law MA, Shamszad P, Nugent AW et al. Pulmonary artery stents: long-term follow-up. Catheter Cardiovasc. Interv. 75(5), 757–764 (2010).
- Chakrabarti S, Kenny D, Morgan G et al. Balloon expandable stent implantation for native and recurrent coarctation of the aorta‐‐prospective computed tomography assessment of stent integrity, aneurysm formation and stenosis relief. Heart 96(15), 1212–1216 (2010).
- Peters B, Ewert P, Berger F. The role of stents in the treatment of congenital heart disease: current status and future perspectives. Ann. Pediatr. Cardiol. 2(1), 3–23 (2009).
- Excellent review of the currently benefits and limitations of current stent practice in children with congenital heart disease.
- Maglione J, Bergersen L, Lock JE, McElhinney DB. Ultra-high-pressure balloon angioplasty for treatment of resistant stenoses within or adjacent to previously implanted pulmonary arterial stents. Circ. Cardiovasc. Interv. 2(1), 52–58 (2009).
- Ewert P, Peters B, Nagdyman N, Miera O, Kühne T, Berger F. Early and mid-term results with the Growth Stent‐‐a possible concept for transcatheter treatment of aortic coarctation from infancy to adulthood by stent implantation? Catheter Cardiovasc. Interv. 71(1), 120–6 (2008).
- Diletti R, Farooq V, Girasis C et al. Clinical and intravascular imaging outcomes at 1 and 2 years after implantation of absorb everolimus eluting bioresorbable vascular scaffolds in small vessels. Late lumen enlargement: does bioresorption matter with small vessel size? Insight from the ABSORB cohort B trial. Heart 99(2), 98–105 (2103).
- Ghimire G, Spiro J, Kharbanda R et al. Initial evidence for the return of coronary vasoreactivity following the absorption of bioabsorbable magnesium alloy coronary stents. EuroIntervention 4(4), 481–484 (2009).
- Waksman R, Erbel R, Di Mario C et al. PROGRESS-AMS (Clinical Performance Angiographic Results of Coronary Stenting with Absorbable Metal Stents) Investigators. Early-and long-term intravascular ultrasound and angiographic findings after bioabsorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc. Interv. 2(4), 312–320 (2009).
- Van der Giessen WJ, Slager CJ, van Beusekom HM et al. Development of a polymer endovascular prosthesis and its implantation in porcine arteries. J. Interv. Cardiol. 5(3), 175–185 (1992)
- McMahon CJ, Oslizlok P, Walsh KP. Early restenosis following biodegradable stent implantation in an aortopulmonary collateral of a patient with pulmonary atresia and hypoplastic pulmonary arteries. Catheter Cardiovasc. Interv. 69(5), 735–738 (2007).
- Gorenflo M, Boshoff DE, Heying R et al. Bailout stenting for critical coarctation in premature/critical/complex/early recoarcted neonates. Catheter Cardiovasc. Interv. 75(4), 553–561 (2010).
- Van der Giessen WJ, Slager CJ, van Beusekom HM et al. Development of a polymer endovascular prosthesis and its implantation in porcine arteries. J. Interv. Cardiol. 5(3), 175–185 (1992)
- Peuster M, Hesse C, Schloo T, Fink C, Beerbaum P, von Schnakenburg C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 27(28), 4955–4962 (2006).
- Huang T, Cheng J, Zheng YF. In vitro degradation and biocompatibility of FE-Pd and Fe-Pt composites fabricated by spark plasma sintering. Mater. Sci. Eng. C. Mater. Bio. Appl. 35, 43–53 (2014).
- Bowen PK, Drelich J, Goldman J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 25(18), 2577–2582 (2013).
- Vandenplas Y, Hauser B, Devreker T, Urbain D, Reynaert H. A biodegradable esophageal stent in the treatment of a corrosive esophageal stenosis in a child. J. Pediatr. Gastroenterol. Nutr. 49(2), 254–257 (2009).
- 19 Martín Cano F, Rodríguez Vargas J, Velasco Sánchez B, Herrera Montes I. Use of self-expandable prosthesis in esophageal stenosis in children. Cir. Pediatr. 25(4), 207–210 (2012).
- Vondrys D, Elliott MJ, McLaren CA, Noctor C, Roebuck DJ. First experience with biodegradable airway stents in children. Ann. Thorac. Surg. 92(5), 1870–1874 (2011).
- Zartner P, Cesnjevar R, Singer H, Weyand M. First successful implantation of a biodegradable metal stent into the left pulmonary artery of a preterm baby. Catheter Cardiovasc. Interv. 66(4), 590–594 (2005).
- Schranz D, Zartner P, Michel-Behnke I, Akintürk H. Bioabsorbable metal stents for percutaneous treatment of critical recoarctation of the aorta in a newborn. Catheter Cardiovasc. Interv. 67(5), 671–673 (2006).
- Zartner P, Buettner M, Singer H, Sigler M. First biodegradable metal stent in a child with congenital heart disease: evaluation of macro and histopathology. Catheter Cardiovasc. Interv. 69(3), 443–446 (2007).
- Muller MJ, Krause U, Paul T, Schneider HE. Serum levels after everolimus-stent implantation and paclitaxel-balloon angioplasty in an infant with recurrent pulmonary vein obstruction after repaired total anomalous pulmonary vein connection. Pediatr. Cardiol. 32(7), 1036–1039 (2011).
- McCrossan BA, McMahon CJ, Walsh KP. First reported use of drug-eluting bioabsorbable vascular scaffold in congenital heart disease. Catheter Cardiovasc. Interv. doi:10.1002/ccd.25768 (2014) (Epub ahead of print).
- Schwartz RS, Koval TM, Edwards WD et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J. Am. Coll. Cardiol. 19(2), 267–274 (1992).
- Veeram Reddy SR, Welch TR, Wang J et al. A novel biodegradable stent applicable for use in congenital heart disease: bench testing and feasibility results in a rabbit model. Catheter Cardiovasc. Interv. 83(3), 448–456 (2014).
- Veeram Reddy SR, Welch TR, Wang J et al. A novel design biodegradable stent for use in congenital heart disease: mid-term results in rabbit descending aorta. Catheter Cardiovasc. Interv. 85(4), 629–639 (2014)
- Goodfriend AC, Welch TR, Barker G et al. Novel bioresorbable stent coating for drug release in congenital heart disease applications. J. Biomed. Mater. Res. A 103(5), 1761–1770 (2014).
- McMahon CJ, El Said HG, Vincent JA et al. Refinements in the implantation of pulmonary arterial stents: impact on morbidity and mortality of the procedure over the last two decades. Cardiol. Young 12(5), 445–452 (2002).
- Gonzalez I, Kenny D, Slyder S, Hijazi ZM. Medium and long-term outcomes after bilateral pulmonary artery stenting in children and adults with congenital heart disease. Pediatr. Cardiol. 34(1), 179–184 (2013).
- McMahon CJ, El Said HG, Vincent JA et al. Refinements in the implantation of pulmonary arterial stents: impact on morbidity and mortality of the procedure over the last two decades. Cardiol. Young 12(5), 445–452 (2002).
- Formigari R, Santoro G, Guccione P et al. Treatment of pulmonary artery stenosis after arterial switch operation: stent implantation vs. balloon angioplasty. Catheter Cardiovasc. Interv. 50(2), 207–211 (2000).
- Law MA, Breinholt JP 3rd, Shamszad P et al. The outcome of pulmonary artery stents following surgical manipulation. Catheter Cardiovasc. Interv. 77(3), 390–394
(2011). - Serruys PW, Garcia-Garcia HM, Onuma Y. From metallic cages to transient bioresorbable scaffolds: change in paradigm of coronary revascularization in the upcoming decade? Eur. Heart J. 33(1), b16–b25 (2012).
- Izukawa T, Mulholland HC, Rowe RD et al. Structural heart disease in the newborn. Changing profile: comparison of 1975 with 1965. Arch. Dis Child. 54(4), 281–285 (1979).
- comparison of 1975 with 1965. Arch. Dis Child. 54(4), 281–285 (1979).angioplasty and surgery for native coarctation of the aorta in childhood. Circulation 111(25), 3453–3456 (2005).