Research Article - Interventional Cardiology (2025) Volume 17, Issue 4
Atrial fibrillation and COVID-19 vaccination: Current understanding and insights from the literature
- Corresponding Author:
- Rosanna Ruggiero Campania Regional Centre for Pharmacovigilance and Pharmacoepidemiology, Napoli, Italy
E-mail: federica.fraenza@unicampania.it
Received: 05-Jul-2024, Manuscript No. FMIC-24-140810; Editor assigned: 09-Jul-2024, PreQC No. FMIC-24-140810 (PQ); Reviewed: 23-Jul-2024, QC No. FMIC-24-140810; Revised: 05-Aug-2025, Manuscript No. FMIC-24-140810 (R); Published: 12-Aug-2025, DOI: 10.37532/1755- 5310.2025.17(4).1016
Abstract
In the battle against the COVID-19 pandemic, vaccines and mass vaccination programs played a fundamental role in reducing the total number of COVID-19 related severe cases, hospitalizations, and deaths. During the pre-authorization studies, it was observed that anti-COVID-19 vaccine side effects were generally mild and resolved within a few days after vaccination. However, their wide administration revealed some possible, albeit rare, adverse events related to COVID-19 vaccines. Among the rare adverse events the cardiac ones arose. Beyond carditis, other cardiac adverse events were described in the literature as suspected adverse events to COVID-19 vaccines. These include cardiac arrhythmias. Some articles published in the literature described Atrial Fibrillation (AF) episodes that occurred in vaccinated subjects, suggesting that COVID-19 vaccination may lead to this arrhythmia. For this reason, we review the data published in the literature describing AF as a suspected adverse event that occurred following the administration of a COVID-19 vaccine. Data regarding AF occurrence following COVID-19 vaccination mainly come from case reports and pharmacovigilance database analyses. The possible underlying mechanisms remain unclear. Aberrant inflammatory responses and activation or dysregulation of the immune system could be involved in the biological plausibility of AF induced by COVID-19 immunization. However, it is essential to balance these risks against the substantial benefits of vaccination, particularly as COVID-19 infection itself is associated with significant cardiovascular risks, including new-onset AF. Ongoing pharmacovigilance and further research are necessary to understand this hypothetical association better. Despite some reported cases, current regulatory assessments do not indicate a clear causal relationship.
Keywords
Atrial fibrillation • Suspected adverse event following immunization • COVID-19 vaccines • COVID-19 vaccination • Cardiac arrhythmia
Introduction
COVID-19 and vaccines
From December 2020 to May 2, 2024, a total of 5.63 billion people were vaccinated against CoronaVirus Disease 19 (COVID-19) worldwide [1]. The global impact of the COVID-19 pandemic, caused by the coronavirus SARS-CoV-2, has reshaped the world’s landscape. From its emergence, in late 2019, to its global spread, the virus has posed immense challenges to public health, economies, and societies worldwide [2].
In the battle against the COVID-19 pandemic, vaccines and mass vaccination programs played a fundamental role in reducing the total number of COVID-19-related severe cases, hospitalizations, and deaths [3]. Harnessing innovative technologies and scientific advancements, all the authorized vaccines represented a unique approach to controlling the spread of the virus by stimulating immune responses against the new SARS-CoV-2 virus and protecting public health. Against COVID-19, different vaccines have been produced, based on various platforms and technologies. Some of them have been subsequently withdrawn at the request of the marketing authorization holder for commercial reasons (e.g. the inactivated, adjuvanted COVID-19 Vaccine Valneva, the recombinant Vaxzevria, and the recombinant, adjuvanted VidPrevtyn Beta).
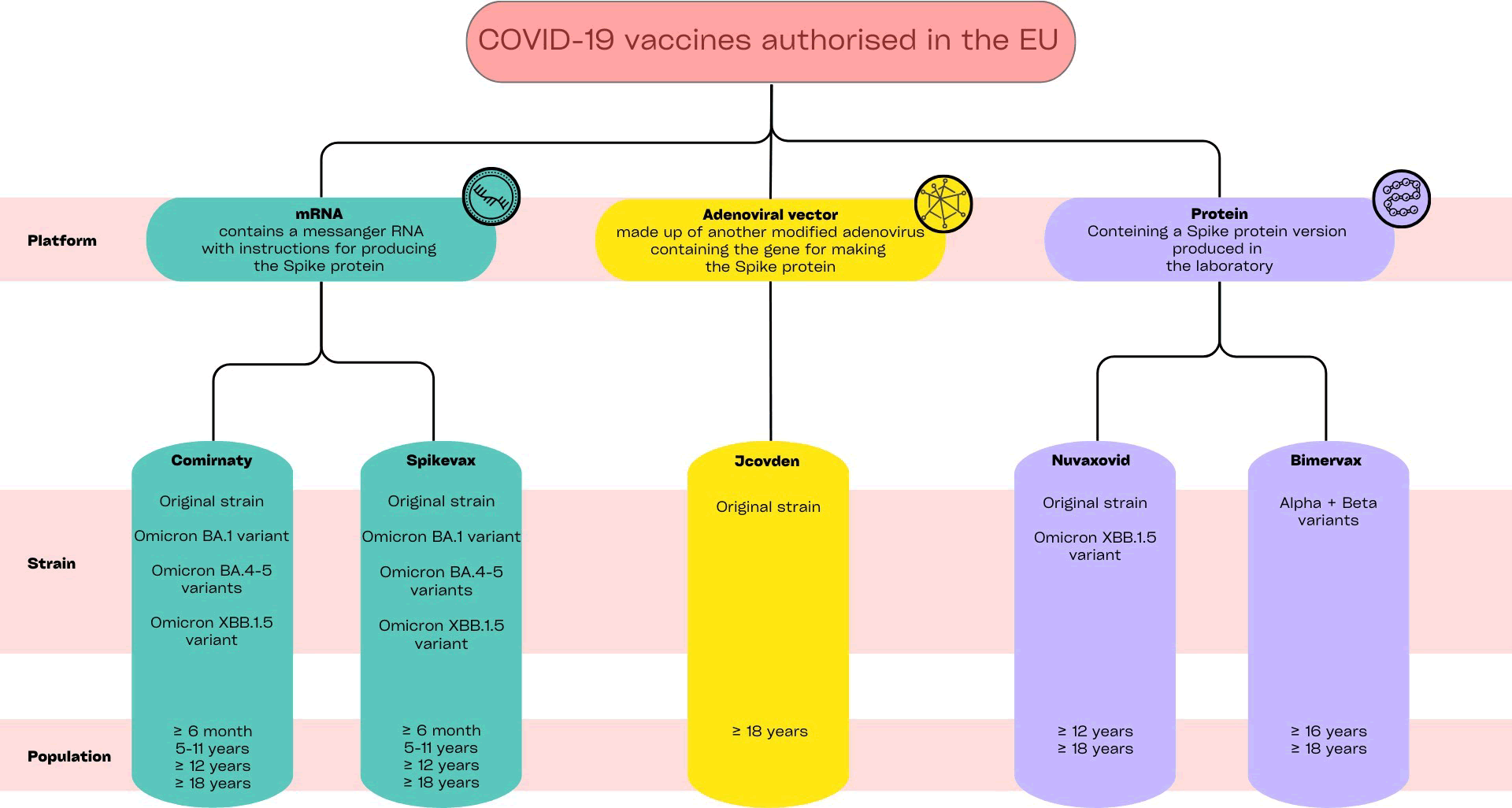
To date, five COVID-19 vaccines are authorized for use in the European Union (Figure 1). Comirnaty, produced by Pfizer-BioNTech, and Spikevax, by Moderna, are two vaccines consisting of messenger RNA (mRNA) included in lipid particles, that leverage the genetic information to produce viral spike protein and, consequently, trigger the host’s immune defenses [4,5]. Additionally, the European Medicine Agency (EMA) approved Jcovden, by Johnson & Johnson, an adenoviral vector vaccine, delivering genetic instructions for spike protein production [6], and two adjuvanted vaccines, named Nuvaxovid and Bimervax, containing recombinant forms of the SARS-CoV-2 spike protein produced in the laboratory [7,8]. During the pandemic, vaccines have been updated and adapted considering the new circulating variants of SARS-CoV2, such as Omicron BA.1, and Omicron BA.4-5, providing broader protection against COVID-19. Post-marketing safety monitoring and cardiac safety aspect of COVID-19 vaccines.
Figure 1: Available COVID-19 vaccines in Europe, authorized by the European Medicines Agency. Data updated to June 2024.
Post-marketing safety monitoring and cardiac safety aspect of COVID-19 vaccines
In this context, pharmacovigilance, consisting of the activities of monitoring, assessing, understanding, and preventing adverse effects of pharmaceutical products, emerged as an essential cornerstone for guaranteeing public health. With the rapid development and deployment of COVID-19 vaccines and treatments, pharmacovigilance activities were amplified to ensure the safety and efficacy of these critical interventions [9]. Firstly, post-marketing surveillance of COVID-19 vaccines and treatment safety has been of paramount importance to continuously monitor their safety in real-world settings. Secondly, robust reporting and analysis mechanisms were in place to facilitate the timely collection and evaluation of safety data. Moreover, sophisticated signal detection techniques were used to identify potential safety signals amidst a large dataset, and communication and transparency were key to maintaining public trust and confidence [10]. As for all medicines (drugs or vaccines), post-marketing monitoring of COVID-19 vaccines’ safety brought to light the rarest adverse reactions that did not emerge in clinical studies. During the preauthorization studies, it was observed that anti-COVID-19 vaccine side effects were generally mild and resolved within a few days after vaccination. The most common were pain at the injection site, tiredness, headache, and nausea or vomiting. However, their wide administration revealed some possible, albeit rare, adverse events related to COVID-19 vaccines. Among the rare adverse events the cardiovascular ones arose. Some cardiac safety aspects emerged during the vaccinal campaign, especially associated with the mRNA ones. Myocarditis and pericarditis were the main cardiac events following COVID-19 immunization, on which the scientific and general community mainly focused. Even if these two cardiac inflammatory events did not emerge during the clinical trials, a causal relationship with vaccines was confirmed thanks to the pharmacovigilance activities, including the post-marketing safety signals analysis. Molecular mimicry against the S protein and hypersensitivity reactions were some of the proposed mechanisms involved in the occurrence of these adverse events related to the mRNA vaccines. On the other hand, induced platelet aggregation and thrombus formation in cardiac blood vessels were proposed as possible mechanisms for the cardiovascular adverse events induced by the adenoviral COVID-19 vaccines. In light of the biological and temporal plausibility, the EMA’s safety committee (PRAC) concluded that myocarditis and pericarditis can occur following COVID-19 vaccination as very rare events, primarily occurring within 14 days after vaccination, more often in younger adult men and after the second dose. Recently, increased carditis risks were also detected within 28 days after all three doses of mRNA vaccine (Comirnaty). In light of these, the product information was updated. Even if some cases required intensive care or with fatal outcomes have been observed, available data indicate that the majority of these cardiac adverse events were resolved.
Moreover, attention has also shifted to other rare cardiac adverse events. Beyond carditis, other cardiac adverse events were described in the literature as suspected adverse events to COVID-19 vaccines.These include ischemic and non-ischemic heart disease, Takotsubo cardiomyopathy, Brugada syndrome, and cardiac arrhythmias. In particular, these latter included Atrioventricular (AV) block, ventricular tachycardia or fibrillation as well as supraventricular tachycardia. According to the results of a study including 38,615,491 vaccinated individuals in England, the authors found an increased risk of cardiac arrhythmia following a second dose of Spikevax. At the same time, it was decreased after the first and second doses of Vaxzevria and Comirnaty. Among cardiac arrhythmias described following COVID-19 vaccination, Atrial Fibrillation (AF) emerged as the most frequently reported one. Some articles published in the literature described AF episodes that occurred in vaccinated subjects, suggesting that COVID-19 vaccination may lead to this arrhythmia. For this reason, we focused our attention on this arrhythmia, reviewing the data published in the literature describing AF as a suspected adverse event that occurred following the administration of a COVID-19 vaccine.
Materials and Methods
Published data on AF following COVID-19 vaccination were identified through searches in Pubmed using the search term “atrial fibrillation” in association with the search terms “COVID-19 vaccination” or “COVID-19 vaccine*” or “Spikevax” or “Comirnaty” or “vaccine*” or “mRNA vaccine*” or “ChAdOx1-S” or “tozinameran” or “elasomeran” or “NVX- CoV2373” or “NVX- CoV2373” or “AD26.COV2.S” or “viral vector vaccines”. Only articles published in the English language were considered. All articles deemed eligible for inclusion based on titles and abstracts were read in full.
Focus on AF and COVID-19 vaccination
Data regarding AF occurrence following COVID-19 vaccination mainly come from case reports and pharmacovigilance database analyses. Based on our literature review, we identified twelve articles describing AF that occurred following COVID-19 vaccination. These are summarized in Table 1.
| S. no. | Authors | Publication year | Type vaccine | COVID-19 vaccine | Article type |
| 1 | Choi JY et al. | 2024 | mRNA vaccines | BNT162b2, mRNA-1273 | VAERS database analysis |
| 2 | Marques MC et al. | 2023 | viral vector vaccine | ChAdOx1-S NCoV-19 | Online survey |
| 3 | Ruggiero R et al. | 2023 | viral vector-based, recombinant subunit protein, mRNA-based vaccines | Ad26.Cov2.S, ChAdOx1-S NCoV-19 NVX-CoV2373, mRNA-1273, BNT162b2 | EV database analysis |
| 4 | Takedani K et al. | 2023 | mRNA vaccine | BNT162b2 | Case report |
| 5 | Zhou M et al. | 2023 | mRNA vaccine | BNT162b2 | Case report |
| 6 | Chen CY et al | 2023 | mRNA vaccine | mRNA-1273 | Case report |
| 7 | Kumar A et al. | 2022 | viral vector-based, mRNA-based vaccines | Ad26.COV2.S mRNA-1273, BNT162b2 | VAERS database analysis |
| 8 | Theodorou A et al. | 2022 | N/A | N/A | Case report |
| 9 | Scheuermeyer FX et al. | 2022 | mRNA vaccine | mRNA-1273 | Case report |
| 10 | Choi YK et al. | 2022 | viral vector vaccine | ChAdOx1-S NCoV-19 | Case report |
| 11 | Yamamoto K et al. | 2021 | mRNA vaccine | BNT162b2 | Case report |
| 12 | Li K et al. | 2021 | inactivated vaccine | VeroCell | Case report |
Table 1: Published work reporting atrial fibrillation occurred following COVID-19 vaccination.
The majority of the identified articles were case reports relating to mRNA vaccines and AF that occurred following their administration. One of these was a case report of a woman with a history of paroxysmal atrial fibrillation, in addition to hypertension and mitral valve prolapse. She developed atrial fibrillation 3 days after vaccination. In some cases, AF occurs as a sign of myocarditis/ pericarditis or other autoinflammatory disorders. For example, He described a case of an elderly female who presented atrial fibrillation among the signs of acute myocarditis that occurred after receiving the mRNA vaccine Comirnaty. Subsequently, the patient was diagnosed with post-vaccination Adult-Onset Still’s Disease (AOSD), a rare autoinflammatory disease, complicated with myocarditis and heart failure. In the same way, a case report described a new-onset AF in a healthy 49-year-old male who had received a second dose of mRNA-1273 (Moderna), to whom subsequently it was diagnosed a pericarditis related to SARS CoV- 2 vaccination.
They described the case of a previously healthy 22-year-old female in South Korea, who developed Multisystem Inflammatory Syndrome after the vaccination with the ChAdOx1 COVID-19 vaccine. On hospital day 4, she developed AF, requiring 2 treatments with cardioversion. He described the case of a 31-year-old man, who was diagnosed with Marfan syndrome at 24 years. He showed AF with a rapid ventricular rate and occasional premature ventricular beats following SARS-CoV-2 vaccination with the inactivated vaccine Vero Cell.
Moreover, some analyses of pharmacovigilance databases focused on AF as a suspected Adverse Event Following Vaccination (AEFI) related to COVID-19 vaccines. The reporting of Atrial Fibrillation (AF) associated with COVID-19 vaccines emerged both in the European and the US pharmacovigilance databases. In particular, we found three analyses of international pharmacovigilance databases, of which two used the US database (Vaccine Adverse Event Reporting System, VAERS) and one the EU one (Eudravigilance, EV). In our recent pharmacovigilance study, more than 6,000 reports describing AF as AEFI with anti-COVID-19 vaccines were described and analyzed as collected in the EV database from 2020- 2022. AF reports mainly referred to adults (>65 years old), with an equal distribution in sex. From our study, a higher reporting frequency of AF emerges with mRNA vaccines compared to viral- vector vaccines These European AF reports were more than double those collected in the US pharmacovigilance VAERS database during an almost overlapping study period. From post-marketing analyses emerged that COVID-19 vaccination has been associated with both new-onset as well as exacerbated AF. Extracting data from VAERS until January 7, 2022, Kumar et al. found a total of 2,611 events of AF reported as AEFI after COVID- 19 vaccination, of which 315 were new-onset AF. These latter were mainly related to mRNA vaccines. Considering the COVID-19 vaccine doses administered until January 7, 2022, an AF reporting rate of around 5 per million COVID-19 vaccine doses administered emerged from their analysis. The reported AF were equally distributed following the first and the second dose. Over the 1–28 days postexposure, Patone et coll. showed an increased risk of atrial fibrillation or flutter arrhythmia at 15–21 days following a first dose of the Spikevax vaccine. According to the disproportionality analysis conducted by Choi et al. on VAERS data, a signal of AF emerged as serious AEFI for the two mRNA vaccines. Finally, Marques et al. published the results of an anonymous online survey posted on closed Facebook groups of patients and parents with systemic Juvenile Idiopathic Arthritis (sJIA) and Adult-Onset Still’s Disease (AOSD). From a total of 167 obtained responses, only one report of AF emerged as a cardiac side effect reported by a 63-year-old male patient following the administration of the Oxford/Astra Zeneca viral vector vaccine.
Results and Discussion
To date, AF is not included in any COVID-19 vaccines’ Summary of Product Characteristics (SmPCs) or their Risk Management Plans (RMPs). Neither signals have currently emerged in this regard from medicines regulatory agencies. It is important to highlight that it could represent a very rare event for which the possible relationship with COVID-19 remains unclear. According to Kumar et al. results, the AF reporting rate was around 5 per million COVID-19 vaccine doses administered. Even if in the literature some studies described AF as suspected AEFI related to COVID-19 immunization, data are scarce and conflicting. According to the results of a meta-analysis conducted to evaluate the cardiovascular safety of COVID-19 vaccines in the real world, no significant increase in the risk of arrhythmia events was observed following anti-COVID-19 vaccination.
Analyzing the possible relationship between AF and COVID-19 vaccination should be also considered some possible confounding factors, including concomitant and pre-existing diseases, concomitant therapies as well as COVID-19 itself. It is important to highlight that in a global evaluation of the risk/benefit ratio of COVID-19 vaccines, the risk of AF following COVID-19 vaccination should be compared with that related to COVID-19 infection, and in the general population, balancing the risks and benefits.
Overall, AF is highly prevalent in males than females. In particular, hormonal fluctuations in women, including the increase in estrogen levels, might offer a protective effect against AF. Studies showed an increasing global prevalence of AF, with more than 59 million individuals living with AF in 2019, with an expected further growth both in Europe and America. Undoubtedly, globally longer average life expectancy as well as increased screening and awareness of AF had a significant contribution. As well known, AF is an age-related disease. Hypertension, diabetes mellitus, obesity, chronic kidney disease, and inflammatory diseases, are some of the primary risk factors that play a pivotal role in AF etiopathogenesis.
This cardiac arrhythmia may present with palpitations, fatigue, dizziness, and dyspnea or be asymptomatic. AF has an important impact on the incidence of other serious cardiac and neurological conditions. In particular, its occurrence increases the risks of myocardial infarction (2-fold), stroke and heart failure, dementia, and cognitive decline (5-fold). The lifetime risk of AF consists of about 1 in 3–5 individuals after the age of 45 years and is associated with substantial morbidity and mortality.
AF represents a frequent complication that can occur in COVID-19 patients. The possible cardiac damage associated with SARS-CoV-2 is supported by several published works. In the United States, mortality related to AF increased during the COVID-19 emergency. The risk of new-onset AF was estimated to increase within the first 60 days after COVID-19 infection in the general population. SARS-CoV-2 can directly invade the atrial cardiomyocytes with cellular death, necrosis, and fibrosis of atrial tissue or indirectly induce alterations in ionic currents (Ca2+, K+), subsequential to cor pulmonale, pulmonary embolism, hypoxia, and/or inflammation, leading to atrial stretch. Considering the AF as an adverse event induced by COVID-19 immunization, the possible underlying mechanisms remain unclear. Aberrant inflammatory responses as well as activation and/or dysregulation of the immune system could be involved in the biological plausibility of AF induced by COVID-19 immunization. Spike(S) protein of SARS-CoV-2 could be represented as the common ground, triggering these processes. Regardless of the used technology, all vaccines ensure that the S protein is presented to the immune system to stimulate it. Beyond the inflammatory processes, also sympathetic hyperactivation emerged as a shared process, occurring both in COVID-19 infection and following vaccination. Angiotensin-Converting Enzyme 2 (ACE2) plays a critical role in the regulation of the Renin-Angiotensin-Aldosterone System (RAAS) by converting Angiotensin I (Ang I) and angiotensin II (Ang II) into Ang (1–9) and Ang (1–7), respectively. These peptides have counter-regulatory effects that balance the classical RAAS axis, promoting cardioprotective actions. During SARS- CoV-2 infection, the S protein enters and infects various host cells, including cardiomyocytes, pericytes, pneumocytes, endothelial cells, and macrophages through the transmembrane form of ACE2, which acts as a receptor and serves as a “gateway” for viral infection. This interaction results in a reduction of ACE2 on the cell surface, suppressing the degradation of Ang II to Ang (1–7) and increasing the Ang II/Ang (1–7) ratio. This shift favors the Ang II pro-inflammatory effects. Increasing adhesion molecules, cytokines, and chemokines, Ang II exerts its effects on leucocytes, endothelial cells, and vascular smooth muscle cells, leading to adverse effects such as cardiac hypertrophy, vasoconstriction, tissue fibrosis, and oxidative stress. Additionally, there is an increase in pro-inflammatory cytokines (IL1, IL-6, TNF-α, MMP) can be related to the cytokine release syndrome, which can occur in the secondary stage of COVID-19 disease. In the same way, following the administration of COVID-19 vaccines have been observed transient hyper-inflammatory states. COVID-19 vaccines, especially mRNA vaccines, can induce a robust immune response that includes the production of inflammatory cytokines. This heightened inflammatory state may contribute to the development of cardiac arrhythmias, including AF.
Conclusion
In conclusion, while COVID-19 vaccines have been crucial in controlling the pandemic, rare cardiac adverse events, including Atrial Fibrillation (AF), were reported in the literature. These rare events appear to be more frequently associated with mRNA vaccines. The possible mechanisms behind vaccine-induced AF could involve the RAAS system with a shift in favor of Ang II, heightened inflammatory responses, and immune system activation. These changes can affect cardiac electrophysiology. However, it is essential to balance these risks against the substantial benefits of vaccination, particularly as COVID-19 infection itself is associated with significant cardiovascular risks, including newonset AF. Ongoing pharmacovigilance and further research are necessary to better understand this hypothetical association with COVID-19 vaccines. Despite the reported cases, AF remains a very rare event post-vaccination, and current regulatory assessments do not indicate a clear causal relationship. Moreover, considering the important impact of this arrhythmia in terms of hospitalization, morbidity, and mortality, data analysis and insights on the possible relationship between AF and COVID-19 vaccination represent an important topic for further research.
References
- Filip R, Gheorghita Puscaselu R, Anchidin-Norocel L, et al. Global challenges to public health care systems during the COVID-19 pandemic: A review of pandemic measures and problems. J Pers Med. 12(8):1295 (2022).
[Crossref] [Google Scholar] [PubMed]
- Zinzi A, Gaio M, Liguori V, et al. Safety monitoring of mRNA COVID-19 vaccines in children aged 5 to 11 years by using eudravigilance pharmacovigilance database: The CoVax child study. Vaccines. 11(2):401 (2023).
[Crossref] [Google Scholar] [PubMed]
- Rudolph A, Mitchell J, Barrett J, et al. Global safety monitoring of COVID-19 vaccines: How pharmacovigilance rose to the challenge. Ther Adv Drug Saf. 13:20420986221118972 (2022).
[Crossref] [Google Scholar] [PubMed]
- Younus MM, Al-Jumaili AA. An overview of COVID-19 vaccine safety and post-marketing surveillance systems. Innov Pharm. 12(4):10 (2021).
[Crossref] [Google Scholar] [PubMed]
- Alzarea AI, Khan YH, Alatawi AD, et al. Surveillance of post-vaccination side effects of COVID-19 vaccines among Saudi population: A real-world estimation of safety profile. Vaccines. 10(6):924 (2022).
[Crossref] [Google Scholar] [PubMed]
- Bernardi FF, Mascolo A, Sarno M, et al. Thromboembolic events after COVID-19 vaccination: An Italian retrospective real-world safety study. Vaccines. 11(10):1575 (2023).
[Crossref] [Google Scholar] [PubMed]
- Liguori V, Zinzi A, Gaio M, et al. Multisystem inflammatory syndrome in children following COVID-19 vaccination: A sex-stratified analysis of the VAERS database using Brighton Collaboration Criteria. Pharmaceuticals. 16(29):1231 (2023).
[Crossref] [Google Scholar] [PubMed]
- Ruggiero R, Balzano N, Di Napoli R, et al. Capillary leak syndrome following COVID-19 vaccination: Data from the European pharmacovigilance database Eudravigilance. Front Immunol. 13:956825 (2022).
[Crossref] [Google Scholar] [PubMed]
- di Mauro G, Mascolo A, Longo M, et al. European safety analysis of mRNA and viral vector COVID-19 vaccines on glucose metabolism events. Pharmaceuticals. 15(6):677 (2022).
[Crossref] [Google Scholar] [PubMed]
- Husby A, Kober L. COVID-19 mRNA vaccination and myocarditis or pericarditis. Lancet. 399(10342):2168-2169 (2022).
[Crossref] [Google Scholar] [PubMed]