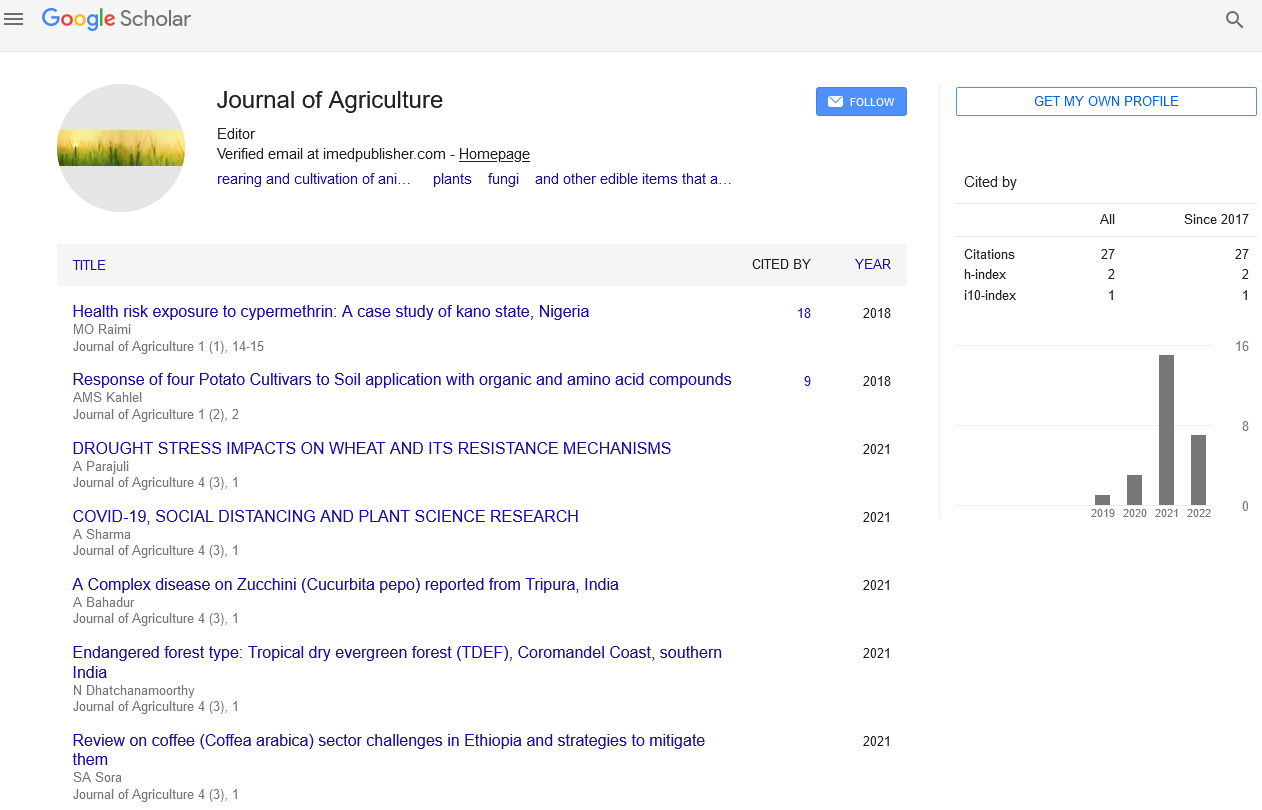
Editorial - Journal of Agriculture (2023) Volume 6, Issue 2
Aquaponic System's Compartmentalised Diversity of Microbes and the Connection To The Nitrogen Cycle
Clevo Wilsone*
Department of Fisheries and Aquaculture, Federal University Dustin-Ma, Nigeria
- *Corresponding Author:
- Clevo Wilsone
Department of Fisheries and Aquaculture, Federal University Dustin-Ma, Nigeria
E-mail: Wilsoneclevo45@gmail.com
Abstract
Recirculating aquaculture and hydroponic crop production combine inaquaponics. Eachcompartment in these systems—fish tank, bio filter, sump, horticulture table, radial flow settler, and anaerobic digester—has its own distinct environmental pressures that cause the formation of unique populations of bacteria. Triplicated the microbial community composition has been investigated in three cycles of growing lettuce using aquaponic systems. Using amp icon sequencing of the bacterial and archival 16S reran genes, ecosystem patterns were produced using the sampling of specific compartments. The presence of nitrifying bacteria in the hydroponic compartments indicates these compartments may be more important than initially thought in the nitrogen cycle of the system. More archival readings have been taken from sludge samples than from other sources, in addition to the temporal variations in community compositions within the anaerobic compartment.
Keywords
Aquaponics • Community analysis • bacteria • archaic • tilapia
Introduction
Important role knowledge of the microorganisms responsible for these nitrogen transformations remains incomplete organized in biofilms. Hitherto attempts to characterize the microbial community’s microbial populations developing in each individual compartment and flow rates. Therefore, environmental conditions will differ the development of unique, localized microbial communities directly or indirectly through their metabolites. Thus, understanding thecompartment-specific microbial communities in aquaponic systems becomesood safety bacterial and archival 16S rRNA genes [1].
Materials and Methods
In color and the chemistry sampling points (DFW, drum filter outflow water; BFO, bio filter outflow water; FTW, fish tank water; HPI, inflow into hydroponic part of the sludge; DS, digested sludge; SS, supernatant of digested sludge returned back to the system) are marked in white rectangle . Experiments were conducted with the authorization [2]. Sampling procedure approx. 100 cm2 surface areas. The movingbed bio filter material bio carrier media, 10 × 10 mm, surface of approx. 800 m2 per m3 fish from each system digested sludge returned to the system (RSS), 1.5 mL of each was collected (Supplementary file: Table S2).
Microbial sample analysis
Using the primers 27F (5′-AGAGTTTGATCCTGGCTCAG3′) and534R (5′ATTACCGCGGCTGCTGG3′), which have already been used in previous16S rRNA study and the primers Arch 516F (5′TGYCAGCCGCCGCGGTAAHACCVGC3′) andUniv806R (5′GGACTACHVGGGTWTCTAAT- 3′) were used for the archival 16S rRNA both primer sets used their respective adapters (Supplementary file: Table S4). After a first targeted amplification and clean-up, the samples were uniquely barcoded using dualindexing with a Nextera XT Index kit v2 [3].
Results and Discussion
Cycles (Supplementary file: Table S1). During one cycle, the fish gained on average 4.4 kg per system and 11.6 kg of lettuce biomass was produced. Bacterial and archival communities were analysed using 16S rRNA gene amp icon sequencing A total of3, 165,652 (average per sample: 20556 ± 6744) bacterial and 843,060 (average per sample: 6435 ± 7639) [4].
Microbial diversity parameters
Based on rarefaction plots both primer sets were for the FEC samples. When comparing the bacterial and archival datasets, there were 10 times as many ZOTUs observed in the formed taxa using an archival primer set. Unchanged. Aquaponic systems represented a unique microenvironment. The microbialcommunity compositions were clustered by compartment in the archival present 35 % of the bacterial population relative abundance of, Hyphomicrobiaceae (8.6 %). A bacterium commonly found in biofilms of human-made aquatic systems, Pedomicrobium was also identified [5]. Euryarchaeota, Methanobacteriaceae (67.1 %), Methanosarcinacea from the order Thermoplasmatales Increate Sides(5.8 %), commonly found in water systems. maples, with Fusobacteria, Bacteroidetes and Formicates representing >97% of all of the assigned bacterial reads. A substantial proportion of sequences belonged to the genus Cetobacterium (50.1 %), a common bacterium found in the fish gut [6]. Furthermore, undescribed members of the families were also observed. Rachael community counts were 3.2.4. Hydroponic compartments (HPS and HTS) and root samples (ROT) ROT mainly contained the same microbial communities [7]. However, the relative abundances of bacteria differed. In total, >44 % of the reads originated from the phylum Proteobacteria. Surface samples from the HTS and PS were dominated by the bacterial groups Planctomycetaceae and Methylobacteriaceae (>5.7 % of the bacterial population), both commonly be assigned to a family. (5.7 %), and 3 % of the reads could not be assigned. The ROT samples were genus taxa was also present In contrast to found in the bio filter [8].
Conclusion
Higher micro biome diversity was observed in the aerobic loop of the system. Their activity. Aquaponic systems. Credit authorship contribution statementZala Schmautz: Conceptualization, Data duration, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft; Jean- Claude Walker [9]. Data duration, Formal analysis, Visualization, Writing – review & editing; Carlos Investigation, Methodology, Visuaediting; Ranke Jungle: Conceptualization, Funding acquisition, Conceptualization, Data duration, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – originaldraft, Writing – review & editing. Data availability Data will be made available on request.Declaration of competing interest work reported in this paper [10].
References
- Bergmann EJ, Raupp MJ. Efficacies of Common Ready to Use Insecticides AgainstHalyomorpha halys(Hemiptera: Pentatomidae). Florida Entomol. 97(2), 791-800 (2014).
- Dian Dhika A, Nasution U. Effect Of Extraction Method Of Tithonia Diversifolia Leaves Against Spodoptera Frugiperda Larva Mortality. J Pharm Life Sci. 8(10), 22-25 (2022).
- Islam MS, Morshed A. Study on Homemade Bio-Pesticides and Organic Pest Management in Organic Farming. Int J Eng Sci. 2(7), 18-25 (2013).
- Nyirenda SP, Sileshi GW, Belmain SR et al. Farmers’ ethno-ecological knowledge of vegetable pests and pesticidal plant use in Northern Malawi and Eastern Zambia. Afr J Agri Res. 6, 2 (2011).
- Benineza GG, Rwabudandi I, Nyiransabimana MJ. Landslides hazards assessment using geographic information system and remote sensing: Gakenke District. Earth and Environ Science. 389(1), 012015 (2019).
- Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 51, 45-66 (2006).
- Amoabeng BW, Gurr GM, Gitau CW et al. Cost:benefit analysis of botanical insecticide use in cabbage: Implications for smallholder farmers in developing countries. Crop Protection. 57, 71-76 (2014).
- Belmain S, Stevenson P. Ethnobotanicals in Ghana: reviving and modernising age-old farmer practice. Pesticide Outlook. 12(6), 233-238 (2001).
- Antonious GF, Meyer JE, Snyder JC. Toxicity and repellency of hot pepper extracts to spider mite, Tetranychus urticae Koch. J Environ Sci Health B. 41(8), 1383-1391(2006).
- Isman MB. Botanical insecticides: for richer, for poorer. Pest Manag Sci. 64(1), 8-11 (2008).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref