Review Article - Interventional Cardiology (2022)
Aneurysmal coronary artery disease: A focus on percutaneous treatment modalities
- Corresponding Author:
- Fauzia Vendrametto
Division of Cardiology, Azienda Sanitaria Friuli Occidentale (ASFO), Ospedale Santa Maria degli Angeli, Pordenone, Italy,
E-mail: fauzia.v@tiscali.it
Received date: 18-Apr-2022, Manuscript No. FMIC-22-61125; Editor assigned: 20-Apr-2022, PreQC No. FMIC-22-61125 (PQ); Reviewed date: 09-May-2022, QC No. FMIC-22-61125; Revised date: 16-May-2022, Manuscript No. FMIC-22-61125 (R); Published date: 23-May-2022, DOI: 10.37532/1755-5310.2022.14(S10).252
Abstract
Aneurysmal Coronary Artery Disease (CAD) is an unsatisfactorily elucidated, multifaceted entity characterized by an abnormal enlargement of epicardial vessels. Unlike atherosclerotic coronary artery disease, the existing data regarding the optimal management and treatment options of aneurysmal CAD is poor and fragmentary due to the absence of large-scale clinical studies and cardiovascular society guideline recommendations. This paper is intended to provide a concise overview of definition, epidemiology, etiopathogenesis and clinical manifestations of aneurysmal CAD, with a special focus on contemporary percutaneous treatment modalities. In parallel, we briefly discuss two intriguing cases characterized by the superimposition of steno-occlusive and aneurysmal lesions, reflecting the challenges connected with the management of this entity in the elective and the emergency clinical setting, respectively.
Keywords
Coronary ectasia • Coronary aneurysm • Obstructive coronary artery disease • Percutaneous coronary intervention
Abbreviations
ACS: Acute Coronary Syndrome; ALCAPA: Anomalous origin of the Left Coronary Artery from the Pulmonary Artery; CAD: Coronary Artery Disease; CASS: Coronary Artery Surgery Study; CT: Computed Tomography; IVUS: Intravascular Ultrasound; LAD: Left Anterior Descending; MACE: Major Adverse Cardiovascular Event; MR: Magnetic Resonance; PCI: Percutaneous Coronary Intervention; PTFE: Polytetrafluoroethylene; RCA: Right Coronary Artery; TIMI: Thrombolysis In Myocardial Infarction; VKA: Vitamin K Antagonists
Introduction
Aneurysmal CAD is an heterogenous entity associated with different clinical presentations, ranging from asymptomatic condition to myocardial ischemia and infarction. Only recently its definition has been standardized and new light has been cast on natural history and underlying pathogenic mechanisms. In contrast, there is no consensus about the optimal treatment strategy, often leaving room for individualized and unconventional approaches. Future investigations in this complex field are needed to address these issues, especially as regards the most promising endovascular options.
Literature Review
Definition and epidemiologyAlthough various angiographic criteria have been proposed, aneurysmal CAD is currently defined as a dilation of an arterial segment to a diameter of at least 1.5 times the adjacent normal coronary artery [1]. More precisely, aneurysmal CAD encompasses two fundamental phenotypes according to lesion extension: The term ‘aneurysm’ is usually employed to describe a focal dilation, whereas the term ‘ectasia’ refers to a disease which involves 50% or more of the total length of the vessel [2]. Based on these assumptions, the recognition of aneurysmal CAD on coronary angiograms may not always be trivial, especially when coronary ectasia involves the entire length of the artery, resulting in the lack of a reference vessel diameter [3]. For similar reasons, the diagnosis of both aneurysms and ectasia can be insidious in the case of concomitant diffuse atherosclerotic lesions, potentially leading to an underestimation of aneurysmal CAD prevalence.
Although historically considered as rare anomalies, the real incidence of coronary aneurysms and ectasia remains unknown. Indeed, the aneur1ysmal CAD burden in the general population is widely variable across individual studies, ranging from 0.3% to 4.9%. Particularly, the latter reported incidence derives from the CASS (Coronary Artery Surgery Study) registry, in which 978 (4.9%) patients out of a total of 20087 hospitalized and undergoing coronary angiography were carriers of aneurysmal coronary dilatation [4]. Moreover, the detection rate of aneurysmal CAD is expected to increase over time in relation with the widespread of non-invasive advanced imaging modalities such as coronary Computed Tomography (CT) and Magnetic Resonance (MR) angiography [2]. Indeed, the growing availability of non-invasive cardiac imaging in various clinical settings could soon rewrite the epidemiology of aneurysmal artery disease, notwithstanding the fundamental role of intracoronary imaging such as Intravascular Ultrasound (IVUS) to assess the morphology of aneurysms and to distinguish between true aneurysms and pseudoaneurysms [5].
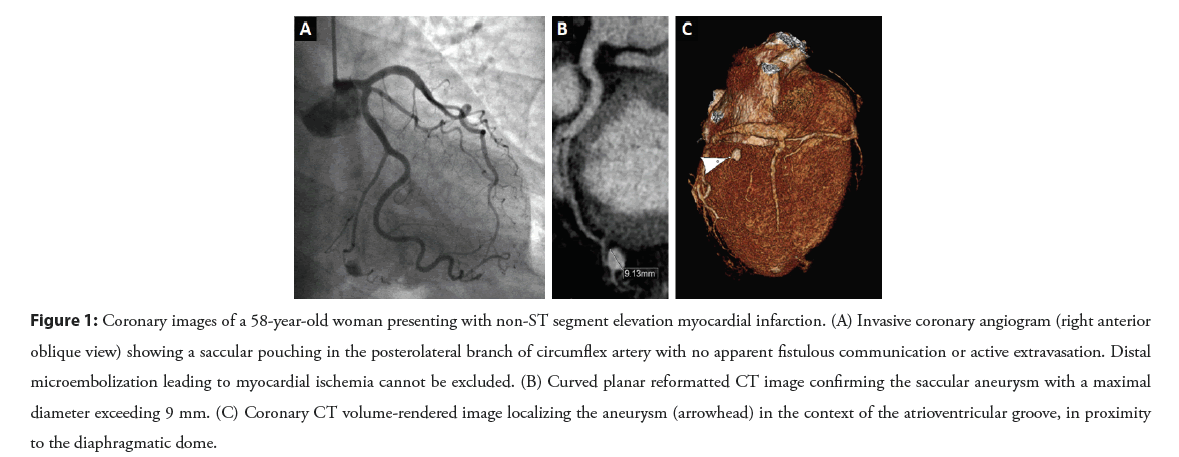
In terms of anatomical location, aneurysmal CAD can occur in any part of the coronary tree, although is most observed in the Right Coron1ary Artery (RCA). The only exception is represented by saccular aneurysms, which are more commonly placed in the Left Anterior Descending (LAD) coronary artery [1]. Figure 1 illustrates a rare finding of saccular aneurysm affecting the left circumflex artery.
Figure 1: Coronary images of a 58-year-old woman presenting with non-ST segment elevation myocardial infarction. (A) Invasive coronary angiogram (right anterior oblique view) showing a saccular pouching in the posterolateral branch of circumflex artery with no apparent fistulous communication or active extravasation. Distal microembolization leading to myocardial ischemia cannot be excluded. (B) Curved planar reformatted CT image confirming the saccular aneurysm with a maximal diameter exceeding 9 mm. (C) Coronary CT volume-rendered image localizing the aneurysm (arrowhead) in the context of the atrioventricular groove, in proximity to the diaphragmatic dome.
Etiopathogenesis
In the latest years, the progression of knowledge and research has shed light on some potential causes of aneurysmal CAD. A strong association has been demonstrated between aneurysmal CAD and some connective tissue disorders (Ehlers-Danlos and Marfan syndromes), as well as certain vasculitis and autoimmune diseases (Kawasaki disease, scleroderma, rheumatoid arthritis, systemic lupus erythematosus) [6-8]. Similarly, cocaine abuse has been noted to be an independent predicting factor for development of coronary ectasia, due to intermittent coronary spasms associated with severe endothelial injury [9]. Moreover, Staphylococcus aureus and Pseudomonas aeruginosa infections, syphilis and Lyme disease can be responsible for coronary aneurysm formation due to direct bacterial wall invasion and immune complex deposition [10,11]. In the era of interventional cardiology, a special mention goes to traumatic and iatrogenic aneurysms secondary to mechanical wall damage caused by Percutaneous Coronary Interventions (PCI) such as balloon angioplasty and stent deployment [12,13].
The potential role of the atherosclerotic process in the pathophysiology of aneurysmal CAD appears to be plausible: Previous studies have hypothesized that coronary ectasia may represent a variant of occlusive atherosclerosis [14], as well as a more recent investigation by Ovali and Morrad showed that the burden of coronary aneurysms is significantly higher in patients with angiographic coronary stenosis of >50% of the luminal diameter [15].
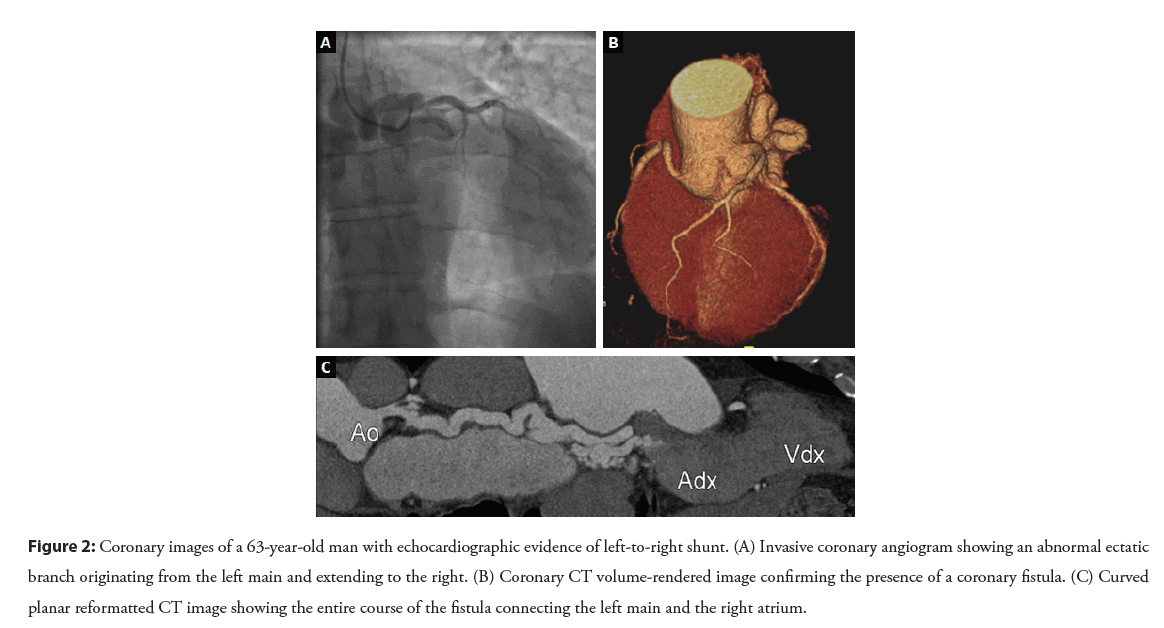
Lastly, in rare cases aneurysmal CAD can have congenital origin, occurring as an isolated disorder or in association with other cardiovascular defects such as coronary fistulas (Figure 2) [16] and abnormalities of coronary origin, particularly in the Anomalous origin of the Left Coronary Artery from the Pulmonary Artery (ALCAPA) or Bland-White-Garland syndrome [17].
Figure 2: Coronary images of a 63-year-old man with echocardiographic evidence of left-to-right shunt. (A) Invasive coronary angiogram showing an abnormal ectatic branch originating from the left main and extending to the right. (B) Coronary CT volume-rendered image confirming the presence of a coronary fistula. (C) Curved planar reformatted CT image showing the entire course of the fistula connecting the left main and the right atrium.
Clinical presentation
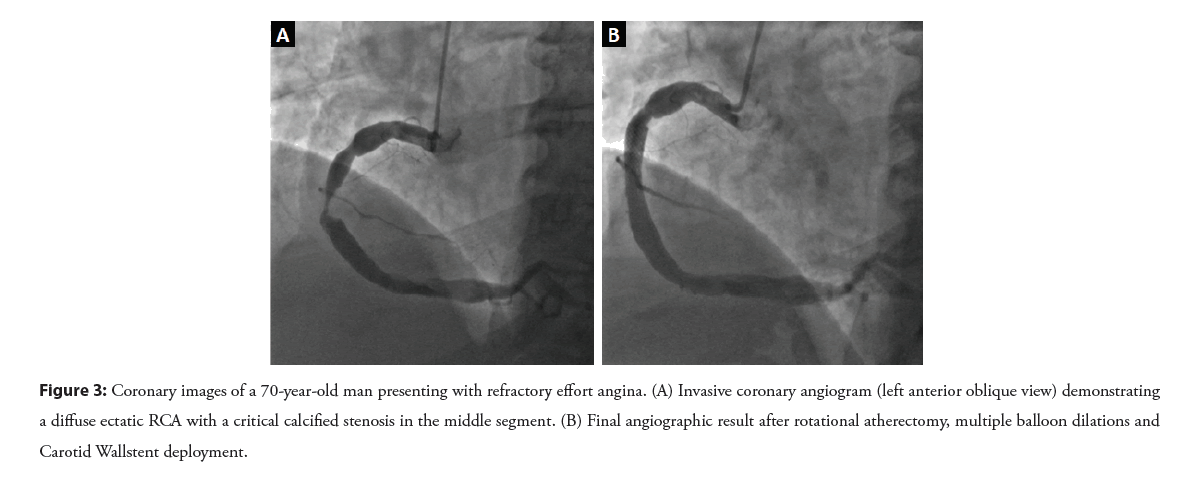
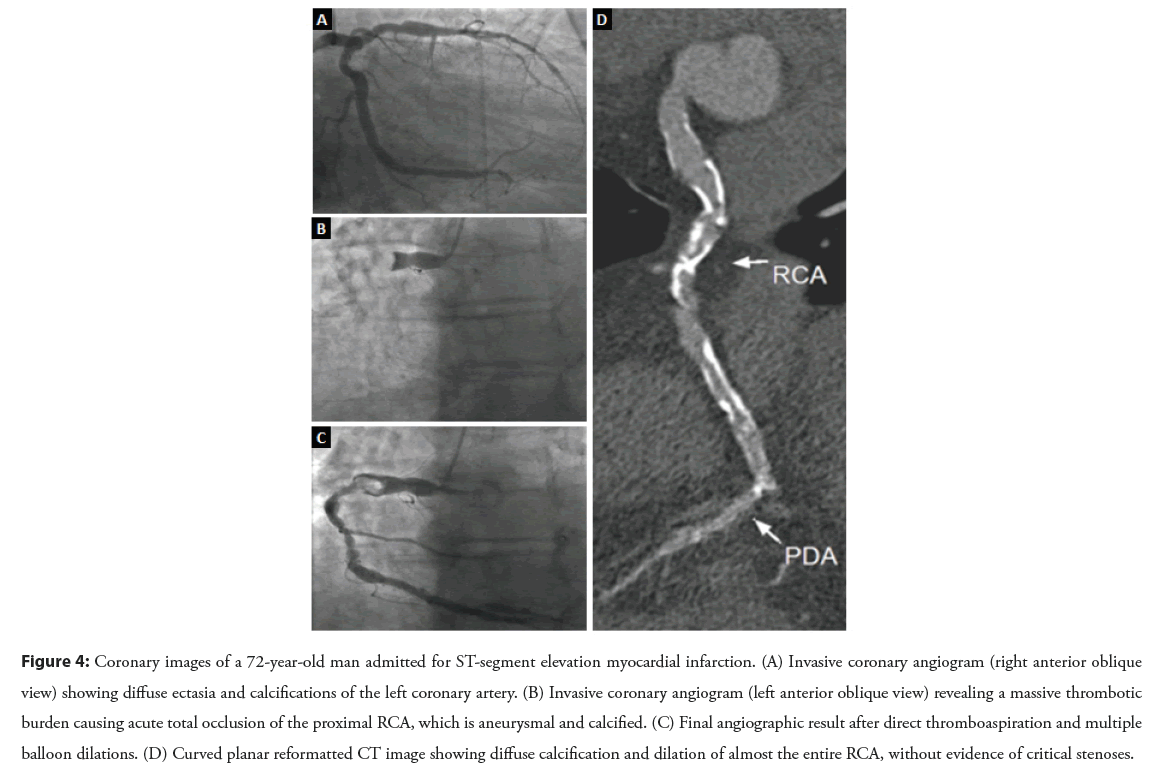
Coronary aneurysms and ectasia may have an heterogenous clinical course. Many patients remain asymptomatic until advanced age, therefore aneurysmal CAD could represent an occasional finding detected at coronary imaging or autopsy examination in these subjects [1-3]. However, this entity may also manifest with angina or angina-equivalent symptoms, especially in the presence of coexisting steno-occlusive atherosclerotic lesions or local thromboembolic phenomena due to blood stasis in the ectatic segment [18,19]. Particularly, in a recently published practical experience [20], We reported the case of an ectatic RCA with a focal critical calcified stenosis Figures 3A and 3B in a 70-year-old man presenting with chronic stable angina despite optimal medical therapy. On the other hand, Figure 4 illustrates an emblematic case of three-vessel aneurysmal CAD in a 72-year-old male patient suffering from acute myocardial infarction. The reperfusion strategy of each case is described and argued in the Discussion section.
Figure 3: Coronary images of a 70-year-old man presenting with refractory effort angina. (A) Invasive coronary angiogram (left anterior oblique view) demonstrating a diffuse ectatic RCA with a critical calcified stenosis in the middle segment. (B) Final angiographic result after rotational atherectomy, multiple balloon dilations and Carotid Wallstent deployment.
Figure 4: Coronary images of a 72-year-old man admitted for ST-segment elevation myocardial infarction. (A) Invasive coronary angiogram (right anterior oblique view) showing diffuse ectasia and calcifications of the left coronary artery. (B) Invasive coronary angiogram (left anterior oblique view) revealing a massive thrombotic burden causing acute total occlusion of the proximal RCA, which is aneurysmal and calcified. (C) Final angiographic result after direct thromboaspiration and multiple balloon dilations. (D) Curved planar reformatted CT image showing diffuse calcification and dilation of almost the entire RCA, without evidence of critical stenoses.
Other well-documented cardiovascular complications of aneurysmal CAD are external compression of adjacent structures [21] and aneurysm rupture with high risk of hemopericardium and cardiac tamponade [22]. At last, although less common, malignant arrhythmias and sudden cardiac death can be associated with expansion in size or rupture of coronary aneurysms [19].
Management strategies
Lacking evidence in controlled clinical studies and specific guidelines poses significant challenges both for the medical and the interventional approach in patients with aneurysmal CAD. Nevertheless, the type of clinical presentation (essentially asymptomatic, chronic effort angina or acute coronary syndrome) and the imaging aspects (especially in terms of aneurysm maximal diameter and expansion rate) are key to guide the therapeutic choices. As stated by Kawsara, et al. the decision-making process in patients without an Acute Coronary Syndrome (ACS) is based on risk profile and the interventional treatment is usually considered in case of refractory ischemic symptoms or alarming anatomic features [23]. Apart from surgical techniques such as aneurysm resection, ligation, and bypass grafting [24], various transcatheter strategies have emerged over time for the treatment of coronary aneurysms: More than twenty years have passed since the publication of the first positive results with the use of covered stents, particularly the Polytetrafluoroethylene (PTFE)-covered stent (Abbott Vascular) [25,26]. Thereafter, rapid advances in stent technology have improved the management of aneurysmal CAD: In 2015, Lattuca, et al. reported the first successful experience with a new polyurethane covered stent (Biotronik Papyrus) for the treatment of a large pseudoaneurysm formed after a bare metal stent implantation [27]; a couple of years later, Kim, et al. described a complex PCI performed by inserting an off-label Viabahn (W. L. Gore and Associates) covered stent in a giant RCA aneurysm [28]. In a recently published study involving 81 patients presenting with aneurysmal CAD in a non-emergency setting, the elective treatment with covered stents has demonstrated to be effective and safe, although a non-negligible percentage of target lesion revascularization was documented at follow-up [29]. Nevertheless, this drawback could be potentially prevented by “burying” covered coronary stents under drug-eluting stents to minimize the neointimal proliferation phenomena, as suggested by Bossard, et al. [30]. Moreover, covered stent delivery has a high failure/complication rate in tortuous and calcified vessels, besides the increased risk of side branch loss. More recently, to overcome these limitations, coil embolization technique has been successfully translated from neuroradiology, particularly in bulky wide-neck aneurysms. This strategy seeks to seal the aneurysm by placing soft platinum microcoils through a catheter inside the aneurysm sac, thus excluding it from coronary circulation [31]. A further refinement of this technique was provided by Gasparini, et al. who described an interesting case of stent-assisted coronary aneurysm coil embolization using a Stentys X-position S (Stentys S.A.) self-expanding stent [32]. This ingenious method intends to avoid coil protrusion into the main vessel, thereby reducing the risk of thromboembolic complications and artery occlusion [33].
Discussion
The implementation of devices in the armamentarium of the interventional cardiologist as well as increased experience have led operators to adopt multiple endovascular strategies. Covered stenting and coil occlusion have demonstrated satisfactory safety and efficacy profiles for a large portion of coronary aneurysms, excepting for those with prohibitive anatomical features [24]. However, the coexistence of significant obstructive CAD raises further technical challenges to manage these uncommon and complex lesions. The following case-based discussion is aimed to explore the current therapeutic approach in this specific setting, according to clinical and angiographic information: The first paragraph deals with some procedural issues related to the close association between stenotic and aneurysmal lesions in chronic coronary syndromes, while the second examines the situation in which an acute thrombotic event complicates an ectatic vessel.
Concomitant aneurysmal and obstructive CAD: Coping with vessel diameter mismatch
In patients with coexisting aneurysmal and obstructive CAD, the interventional treatment is usually tailored to the underlying coronary artery stenosis [2,3]. However, the presence of aneurysmatic segments can represent a significant technical problem with respect to revascularization planning and optimal stent sizing, which is crucial to prevent stent dislodgment and embolization. Regarding this aspect, Figure 3A shows an ectatic RCA with a severe lumen narrowing affecting a 70-year-old male patient hospitalized for chronic refractory angina. This angiographic scenario represented a challenging situation where none of the current dedicated percutaneous techniques (covered stent and coil embolization) was appropriate and the diameter mismatch between the stenotic lesion and the dilated portion of the RCA (approximately 7.5-8 mm) was very troublesome. Consequently, the case was discussed during the multidisciplinary meeting involving interventional radiologists and various treatment strategies were assessed. The aforementioned Stentys X-position S (Stentys S.A.), a self-positioning sirolimus-eluting stent, is a reliable option when an important vessel variance is present, but its maximum achievable diameter is about 6 mm [34]. The peripheral Herculink Elite stent (Abbott Vascular) was also considered, although its 18 mm maximum length appeared insufficient to obtain a complete lesion coverage, unless an overlap stenting was performed. Finally, the Carotid Wallstent (Boston Scientific) was selected both for its caliber, with a reference vessel diameter within the range of 4.0 and 9.0 mm at the target lesion, and for its 30 mm length, without needing an overlap stenting. In this case, an IVUS-guided revascularization procedure was successfully performed by using an off-label Carotid Wallstent (Figure 3B), highlighting the frequent need for individualized approaches in patients affected by aneurysmal CAD. Another controversial issue concerns the potential role of anticoagulant drugs in this setting: While some evidence suggests a possible advantage from Vitamin K Antagonists (VKA) in patients with coronary ectasia and ACS [35], the role of long-term anticoagulation in patients with chronic stable angina is not clear. Therefore, for what concerns this patient, a dual antiplatelet regimen was chosen with no recurrence of chest pain after an 18-month clinical follow-up.
Emergency clinical setting: Still room for improvement?
Based on the available literature, primary PCI of an ectatic culprit artery in the setting of ACS often provides suboptimal results, with high rates of no-reflow and adverse ventricular remodeling [23,36]. The negative prognostic impact of aneurysmal CAD in the emergency setting of ACS is basically attributable to the larger thrombus load and the higher risk of distal embolization of clot fragments in comparison with non-ectatic infarct-related arteries [23,37]. These aspects are witnessed by a more extensive use of glycoprotein IIb/IIIa antagonists and thrombus aspiration in patients treated with primary PCI of an aneurysmal culprit vessel [38]. Furthermore, lower rates of stent implantation and more aggressive anticoagulant regimens can be observed in this subset of patients [35,39]. Anecdotal experiences reporting a beneficial effect from rheolytic thrombectomy with the AngioJet device (Boston Scientific) in aneurysmal infarct-related vessels are mentioned in contemporary literature [40,41]. Apart from procedural complexity, some evidence suggests a high incidence of Major Adverse Cardiovascular Event (MACE) at long-term follow-up in patients with aneurysmal CAD presenting with ACS. Notably, a recent retrospective investigation from Wang, et al. has shown that the presence of coronary ectasia on a total of 4788 patients suffering from acute myocardial infarction significantly increased the composite risk of cardiac death, stroke, myocardial infarction, and repeated coronary revascularization at a median follow-up of 4 years [39].
Figure 4 shows a paradigmatic case of multivessel aneurysmal CAD in a 72-year-old man presenting with chest pain and ST- segment elevation in the inferior leads on his electrocardiogram. The emergency coronary angiography revealed an acute proximal RCA occlusion due to a huge thrombotic burden. Therefore, the patient was treated with intravenous abciximab and thrombus aspiration, followed by sequential dilations with compliant balloons of increasing sizes. Post-procedural Thrombolysis In Myocardial Infarction (TIMI) grade 2 flow suggested partial myocardial reperfusion, and intravenous unfractionated heparin infusion was administrated. Lastly, the patient was discharged home on oral anticoagulation with VKA, and the coronary CT angiography performed at 1-month follow-up showed no critical RCA narrowings. Although the patient remains symptom-free at 2-years follow-up, inferior wall motion abnormalities persist on echocardiogram despite a timely primary PCI. In accordance with the available literature [42,43], this case substantially reflects the need to perfect current reperfusion strategies for aneurysmal infarct-related vessels and to establish more effective and standardized approaches to this type of lesions.
Conclusion
Although numerous dilemmas and uncertainties around aneurysmal CAD have now been elucidated, there is a long way to go yet, especially with respect to the optimal therapeutic strategies in relation to the various clinical and anatomical contexts. As regards the interventional treatment, different percutaneous techniques and stent models have evolved considerably over the last twenty years, thereby improving the management of aneurysmal CAD. Particularly, treatment options have progressed including two popular endovascular procedures with proven efficacy for a conspicuous portion of coronary aneurysms: Covered stenting and stent-assisted coil insertion. Nonetheless, comprehensive multicenter registries are required to have adequate numbers of patients to address clinical questions, as well as dedicated randomized trials would be useful to compare different treatment modalities and their outcomes. Lastly, the development of new endovascular tools, specifically designed for the treatment of aneurysmal CAD and customized on the individual anatomy, would be vital to optimize procedural results.
Conflict of Interest
All authors have no conflicts of interest to declare.
Authors’ Contributions
F. Vendrametto and A. Pierri contributed equally
Funding
All authors disclose no funding sources.
References
- Kawsara A, Núñez Gil IJ, Alqahtani F, et al. Management of coronary artery aneurysms. JACC Cardiovasc Interv. 11(13): 1211-1223 (2018).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Díaz-Zamudio M, Bacilio-Pérez U, Herrera-Zarza MC, et al. Coronary artery aneurysms and ectasia: Role of coronary CT angiography. RadioGraphics. 29(7): 1939-1954 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Dendramis G, Paleologo C, Lo Presti A, et al. Coronary artery ectasia: Etiopathogenesis, diagnosis and treatment. G Ital Cardiol. 15(3): 161-169 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Swaye PS, Fisher LD, Litwin P, et al. Aneurysmal coronary artery disease. Circulation. 67(1): 134-138 (1983).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Maehara A, Mintz GS, Ahmed JM, et al. An intravascular ultrasound classification of angiographic coronary artery aneurysms. Am J Cardiol. 88(4): 365-70 (2001).
[CrossRef] [Google Scholar] [PubMed]
- Gelb BD. Marfan's syndrome and related disorders-more tightly connected than we thought. N Engl J Med. 355: 841-844 (2006).
[CrossRef] [Google Scholar] [PubMed]
- Thangathurai J, Kalashnikova M, Takahashi M, et al. Coronary artery aneurysm in Kawasaki disease: Coronary CT angiography through the lens of pathophysiology and differential diagnosis. Radiol Cardiothorac Imaging. 3(5): e200550 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Van Stijn D, Korbee JM, Netea SA, et al. Treatment and coronary artery aneurysm formation in Kawasaki disease: A per-day risk analysis. J Pediatr. 243: 167-172.e1 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Satran A, Bart BA, Henry CR, et al. Increased prevalence of coronary artery aneurysms among cocaine users. Circulation. 111(19): 2424-2429 (2005).
[CrossRef] [Google Scholar] [PubMed]
- Ford SR, Rao A, Kochilas L. Giant coronary artery aneurysm formation following meningococcal septicaemia. Pediatr Cardiol. 28(4): 300-302 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Singh H, Singh C, Aggarwal N, et al. Mycotic aneurysm of left anterior descending artery after sirolimus-eluting stent implantation: A case report. Catheter Cardiovasc Interv. 65(2): 282-285 (2005).
[CrossRef] [Google Scholar] [PubMed]
- Hill JA, Margolis JR, Feldman RL, et al. Coronary arterial aneurysm formation after balloon angioplasty. Am J Cardiol. 52(3): 261-264 (1983).
[CrossRef] [Google Scholar] [PubMed]
- Aoki J, Kirtane A, Leon MB, et al. Coronary artery aneurysms after drug-eluting stent implantation. JACC Cardiovasc Interv. 1(1): 14-21 (2008).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Swanton RH, Thomas MC, Coltart DJ, et al. Coronary artery ectasia-a variant of occlusive coronary arteriosclerosis. Br Heart J. 40(4): 393-400 (1978).
[CrossRef] [Google Scholar] [PubMed]
- Ovali C, Morrad B. Associations between coronary artery disease, aneurysm and ectasia. Kardiochir Torakochirurgia Pol. 14: 158-163 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Qureshi SA. Coronary arterial fistulas. Orphanet J Rare Dis. 1: 51 (2006).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Peña E, Nguyen ET, Merchant N, et al. ALCAPA syndrome: Not just a pediatric disease. RadioGraphics. 29(2): 553-565 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Shaikh AH, Hanif B, Hassan K, et al. Coronary aneurysm complicated by acute myocardial infarction. J Pak Med Assoc. 62(8): 854-856 (2012).
[Google Scholar] (All versions) [PubMed]
- Chrissoheris MP, Donohue TJ, Young RS, et al. Coronary artery aneurysms. Cardiol Rev. 16(3): 116-123 (2008).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Vendrametto F, Pierri A, Mancinelli P, et al. An attractive endovascular strategy for combined-aneurysmal and stenotic-coronary artery disease. J Cardiovasc Med (Hagerstown). 22(12): e32-e34 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Ebina T, Ishikawa Y, Uchida K, et al. A case of giant coronary artery aneurysm and literature review. J Cardiol. 53(2): 293-300 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Ramirez JL, Kratz JR, Wieselthaler GM. Giant right coronary artery aneurysm presenting as cardiac tamponade. Interact Cardiovasc Thorac Surg. 27(5): 787-789 (2018).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Kawsara A, Núñez Gil IJ, Alqahtani F, et al. Management of Coronary Artery Aneurysms. JACC Cardiovasc Interv. 11(13): 1211-1223 (2018).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- ElGuindy MS, ElGuindy AM. Aneurysmal coronary artery disease: An overview. Glob Cardiol Sci Pract. 2017(3): e201726 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Szalat A, Durst R, Cohen A, et al. Use of polytetrafluoroethylene-covered stent for treatment of coronary artery aneurysm. Catheter Cardiovasc Interv. 66: 203-208 (2005).
[CrossRef] [Google Scholar] [PubMed]
- Briguori C, Sarais C, Sivieri G, et al. Polytetrafluoroethylene-covered stent and coronary artery aneurysms. Catheter Cardiovasc Interv. 55(3): 326-330 (2002).
[CrossRef] [Google Scholar] [PubMed]
- Lattuca B, Schmutz L, Cornillet L, et al. New polyurethane covered stent with low profile for treatment of a large aneurysm after left anterior descending artery stenting: First experience. Int J Cardiol. 201: 208-209 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Kim TH, Marfatia R, Lee J, et al. Giant coronary aneurysm management with Viabahn covered stent. Cardiovasc Revasc Med. 18(S1): 56-59 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Will M, Kwok CS, Nagaraja V, et al. Outcomes of patients who undergo elective covered stent treatment for coronary artery aneurysms. Cardiovasc Revasc Med. 36: 91-96 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Bossard M, Cioffi GM, Yildirim M, et al. “Burying” covered coronary stents under drug-eluting stents: A novel approach to ensure long-term stent patency. Cardiol J. (2021).
[CrossRef] [Google Scholar] [PubMed]
- Saccà S, Pacchioni A, Nikas D. Coil embolization for distal left main aneurysm: A new approach to coronary artery aneurysm treatment. Catheter Cardiovasc Interv. 79(6): 1000-1003 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Gasparini GL, Oreglia JA, Reimers B. Self-apposing stent-assisted coil embolization for the treatment of coronary artery aneurysm. Catheter Cardiovasc Interv. 91(3): 470-474 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Abou Sherif S, Ozden Tok O, Taşköylü Ö, et al. Coronary artery aneurysms: A review of the epidemiology, pathophysiology, diagnosis, and treatment. Front Cardiovasc Med. 4: 24 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Golino M, Nuzzo S, Briguori C. STENTYS coronary system: Current status and future direction. Minerva Cardiol Angiol. 69(2): 201-214 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Doi T, Kataoka Y, Noguchi T, et al. Coronary artery ectasia predicts future cardiac events in patients with acute myocardial infarction. Arterioscler Thromb Vasc Biol. 37(12): 2350-2355 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Ipek G, Gungor B, Karatas MB, et al. Risk factors and outcomes in patients with ectatic infarct-related artery who underwent primary percutaneous coronary intervention after ST elevated myocardial infarction. Catheter Cardiovasc Interv. 88(5): 748-753 (2016).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Shanmugam VB, Psaltis PJ, Wong DTL, et al. Outcomes after primary percutaneous coronary intervention for st-elevation myocardial infarction caused by ectatic infarct related arteries. Heart Lung Circ. 26(10): 1059-1068 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Baldi C, Silverio A, Esposito L, et al. Clinical outcome of patients with ST-elevation myocardial infarction and angiographic evidence of coronary artery ectasia. Catheter Cardiovasc Interv. 99(2): 340-347 (2022).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Wang X, Montero-Cabezas JM, Mandurino-Mirizzi A, et al. Prevalence and long-term outcomes of patients with coronary artery ectasia presenting with acute myocardial infarction. Am J Cardiol. 156: 9-15 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Giombolini C, Notaristefano S, Santucci S, et al. AngioJet thrombectomy for the treatment of coronary artery aneurysm after failed thrombolysis in acute myocardial infarction. Heart Int. 2(2): 94 (2006).
[CrossRef] [Google Scholar] [PubMed]
- Lee MS, Nero T, Makkar RR, et al. Treatment of coronary aneurysm in acute myocardial infarction with AngioJet thrombectomy and JoStent coronary stent graft. J Invasive Cardiol. 16(5): 294-296 (2004).
[Google Scholar] [PubMed]
- Joo HJ, Woong Yu C, et al. Clinical outcomes of patients with coronary artery aneurysm after the first generation drug-eluting stent implantation. Catheter Cardiovasc Interv. 92(3): E235–45 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Mir T, Uddin M, Changal K, et al. Mortality outcomes and 30-day readmissions associated with coronary artery aneurysms; a National Database Study. Int J Cardiol. 356: 6-11 (2022).
[CrossRef] [Google Scholar] [PubMed]