Case Report - Interventional Cardiology (2022)
An unusual case of aortic regurgitation and LVOT obstruction secondary to valve fibromuscular metaplasia and leaflet delamination
- Corresponding Author:
- Daniela Palleri
Department of Cardio-Thoracic and Vascular Medicine,
IRCSS Azienda Ospedaliero-Universitaria di Bologna Policlinico Sant’Orsola Malpighi,
Bologna,
Italy,
E-mail: daniela.palleri2@unibo.it
Received date: 10-Oct-2022, Manuscript No. FMIC-22-76798; Editor assigned: 12-Oct-2022, PreQC No. FMIC-22-76798 (PQ); Reviewed date: 26-Oct-2022, QC No. FMIC-22-76798;Revised date: 02-Nov-2022, Manuscript No. FMIC-22-76798 (R);Published date: 09-Nov-2022, DOI: 10.37532/1755-5310.2022.14(S12).294
Abstract
Background: Aortic regurgitation and LVOT obstruction are two aspects of valvular and sub valvular apparatus disease which can seriously impair left ventricle function. An association between mutations in the TGF-β pathway and deformation in the geometry of the aortic wall is recognized and a role of TGF-β overexpression in the development of lens opacity in patients with congenital cataract is reported.
Case presentation: This case describes a peculiar aetiology of severe aortic regurgitation and LVOT obstruction in a young adult with bilateral congenital cataract and uncontrolled hypertension, who presented with heart failure and was found to have aortic valve degeneration with fibromuscular metaplasia and leaflet delamination.
Conclusion: An anomalous TGFβ pathway associated with congenital forms of cataract could explain the fibromuscular metaplasia of the aortic valve in presence of stress agents such as uncontrolled hypertension. More in vitro and in vivo studies are needed to confirm this theory.
Keywords
Aortic regurgitation • LVOT obstruction • Valve fibromuscular metaplasia • Aortic valve • Congenital cataract
Abbrevations
CT: Computer Tomography; LAVi: Left Atrial Volume index; LVEDVi: Left Ventricular End-Diastolic Volume index; LVOT: Left Ventricular Outflow Tract; TEE: Transoesophageal Echocardiography; TGF: Transforming Growth Factor; TTE: Transthoracic Echocardiography; VEC: Valve Endothelial Cells; VIC: Valve Interstitial Cells
Introduction
Aortic regurgitation is a valvular disease caused either by primary involvement of the aortic leaflets or by abnormalities in the dimension or morphology of the aortic root/ascending aorta. Degenerative alteration of the aortic valve leaflets is the most common aetiology for aortic regurgitation and for the combined defect of insufficiency and stenosis in high-income countries [1,2]. A recognized association between mutations in the TGF-β pathway and deformation of the geometry of the aortic wall exists [3], leading to aortic dilatation and type I aortic regurgitation [1]. Unfortunately, the effects of anomalies in the autocrine and paracrine TGF-β signaling on the valve structure are less known, although in vitro models have shown that a continuous communication between intra-ed extracellular environment can regulate the endothelial-to-mesenchymal transition process [4].
This case describes a male patient affected by congenital bilateral cataract, who presented progressive dyspnea and chest pain during exertion. Further examination revealed a fibromuscular metaplasia of the aortic valve structure and a delamination of the right coronary leaflet causing severe aortic regurgitation and Left Ventricular Outflow Tract (LVOT) obstruction. The aim of this case report was to raise concerns about the role of TGF-β pathway alterations in modifying not only the arterial wall but even valve’s structure in presence of a stressor such as uncontrolled hypertension.
Case Presentation
A 38-year-old obese man presented to our outpatients’ clinic for progressive dyspnea and chest pain during exertion. He denied weight gain, orthopnea or paroxysmal nocturnal dyspnea. The patient had a history of percutaneous patent foramen ovale closure, uncontrolled hypertension and congenital blindness secondary to bilateral cataract. His previous echocardiographic evaluation (2014) showed a mild aortic regurgitation with normal chamber volumes.
At clinical evaluation a new 3/6 ejection murmur on the aortic area with no carotid radiation was revealed. He had neither lung crackles, nor hepatomegaly, or extremity edema. His vital signs were: blood pressure 170/75 mm Hg, heart rate 80 beats/min and oxygen saturation 98% on room air. Electrocardiogram showed a first-degree atrio-ventricular block with signs of left ventricular hypertrophy. A Transthoracic Echocardiography (TTE) showed a slightly reduced left ventricular ejection fraction (about 50%), with a severe left ventricle dilatation and mild left atrial dilatation (LVEDVi 133 ml/m2, LAVi 40 ml/m2), severe aortic regurgitation with Vena Contracta>8 mm and a mobile ill-defined subaortic structure prolapsing into the Left Ventricular Outflow Tract (LVOT) causing a severe LVOT obstruction (peak/mean gradient 129/73 mmHg).
Identification of a mobile structure in the LVOT gave rise to concern for vegetation, aortic leaflet flail, subaortic membrane, redundant mitral valve chordae, primary or secondary cardiac tumors, and accessory mitral valve tissue.
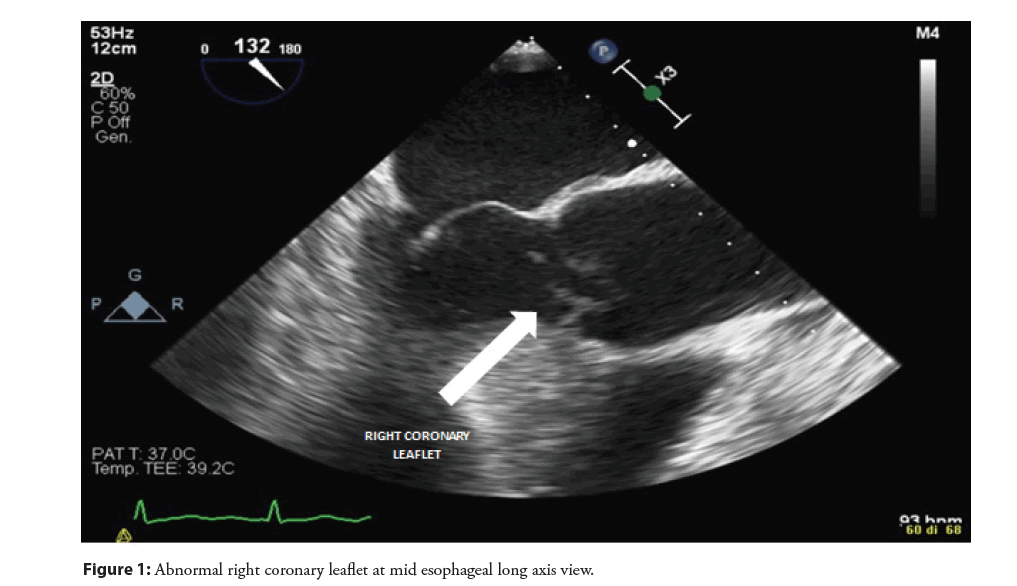
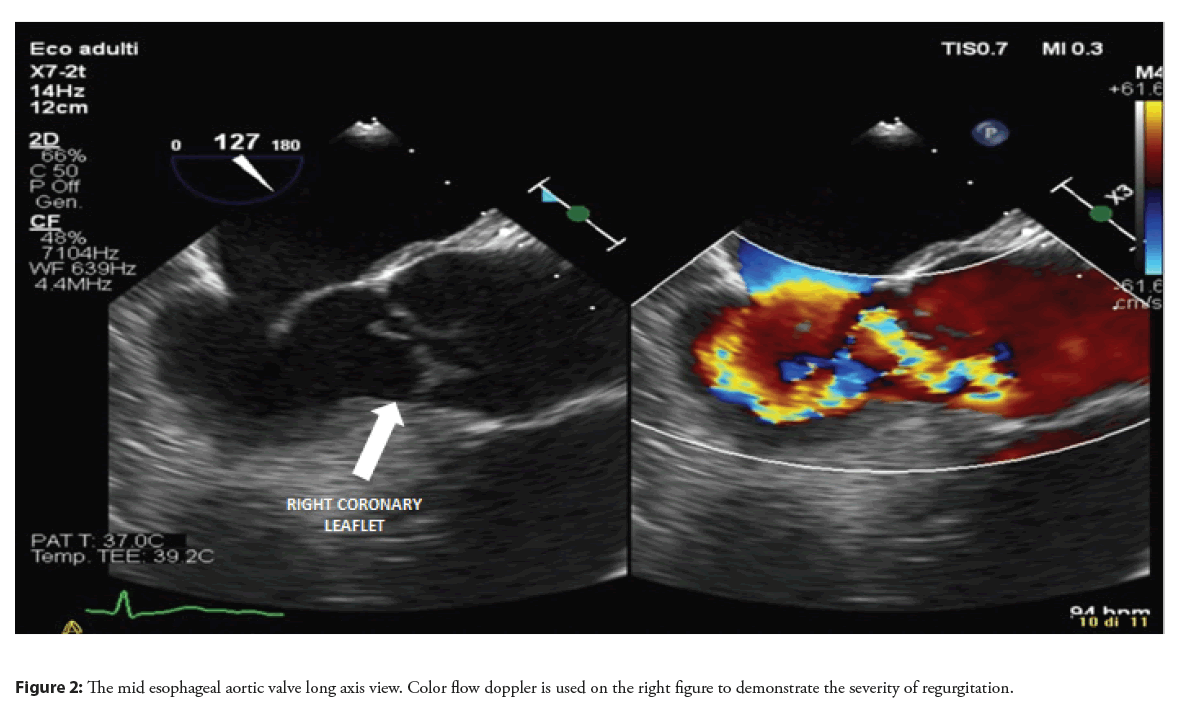
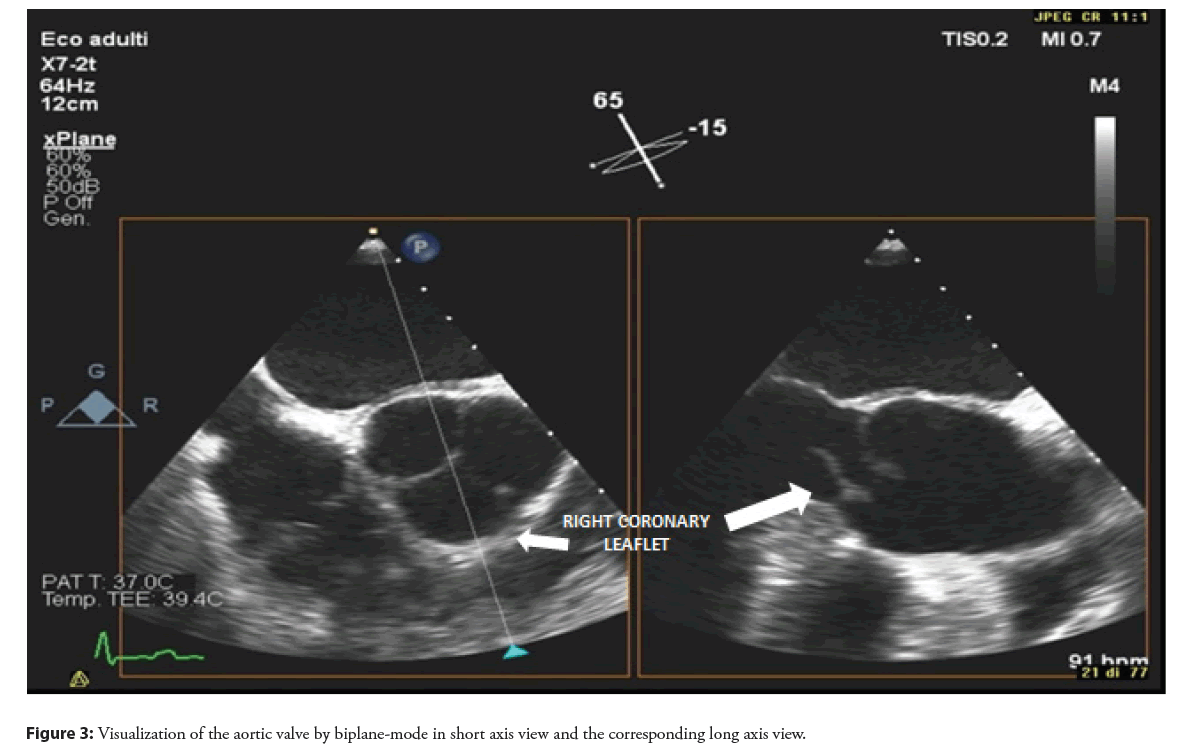
Therefore, in order to better characterize the lesion in the LVOT and to define the cause of aortic regurgitation, a Transesophageal Echocardiography (TEE) was performed. At standard mid-esophageal long axis view, an abnormal movement of the right coronary aortic leaflet, losing the coaptation point and everting totally in the LVOT was revealed and confirmed on 3D reconstruction (Figures 1-3, Video 1). This finding was interpreted as a right coronary leaflet flail, which was consistent with the severe aortic regurgitation but not with the LVOT obstruction. Therefore, a CT angiography was performed in order to exclude an associated aortic dissection and to evaluate the epicardial coronary arteries before surgery: no signs of aortic dissection or obstructive coronary artery disease were observed.
Video 1: 3D Reconstruction of aortic valve movement.
Patient’s symptoms together with the echocardiographic evidence of significant LVOT obstruction and severe aortic regurgitation made the surgical solution recommended.
Results
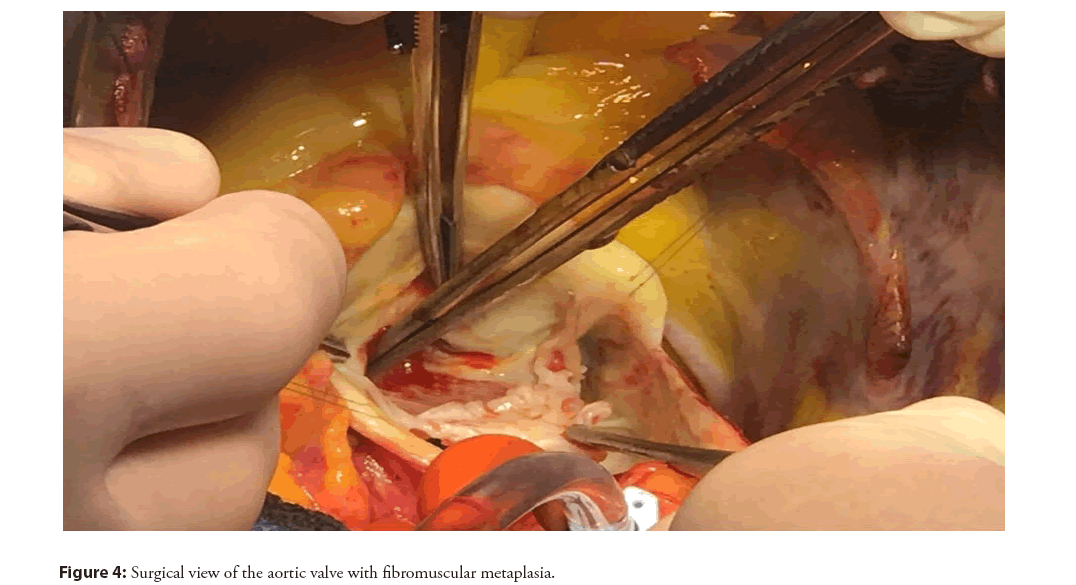
Intraoperative inspection of the aortic valve showed a tricuspid aortic valve with a fibromuscular metaplasia of the leaflets structure and a delamination of the right coronary leaflet, splitting in two layers: one layer adherent to the valvular hinge and the other protruding into the LVOT (Figure 4). The native aortic valve was then removed and a mechanical prosthesis was implanted. The patient had a regular peri-operative course without complications.
After three months, a clinical evaluation was planned. The patient referred no cardiovascular symptoms and the TTE showed no residual LVOT obstruction, a good performance of the mechanical prosthesis and normalization of left ventricle volume and ejection fraction.
Discussion
In human and animal models, valve integrity is actively maintained by paracrine interactions between Valve Interstitial Cells (VIC) and Valve Endothelial Cells (VEC). The disruption of this homeostasis of signals may underlie the endothelial-to-mesenchymal transition [4]. In response to stress factors (shear stress, bending forces, load and strain forces), the valve structure can change in size, shape and stiffness due to the interaction between cells and extracellular matrix [5].
In condition of excessive shear stress and anomalous autocrine and paracrine signaling, this fine balance may be affected determining valve metaplasia and disruption. Several studies demonstrated the role of TGFβ1 and TGFβ2 overexpression in the development of lens opacity in patients with congenital cataract [6,7]. In VIC and VEC environment these ligands can activate SMAD-dependent transcriptional cascades through TGFβ/ALK receptors and they can stimulate mesenchymal signaling programs (including migratory, contractile, and proliferative) possibly inducing the disassembly of the tight junctions and digestion of the basement membrane substances [5].
In canine valve colture the combination of overexposure to TGFβ1 and cyclic strain is able to induce myofibroblastic differentiation in VIC, revealing how mechanical and biochemical interactions can regulate VIC phenotype in an interdependent way, promoting cellular differentiation [8].
Uncontrolled hypertension is a cause of excessive shear stress and together with an anomalous autocrine and paracrine signaling, such as the hyperactivation of the TGFβ cascade, may result in valve metaplasia and valve disruption.
These mechanical and biochemical mechanisms could explain the singular finding of the aortic valve fibromuscular metaplasia with leaflet delamination causing severe valve regurgitation and LVOT obstruction.
Conclusion
We described the peculiar case of a young adult with congenital cataract and arterial hypertension, which developed a fibromuscular degeneration of the aortic valve producing severe regurgitation and stenosis, with the need for surgical replacement. Echocardiography played an important role for working diagnosis, but surgical evaluation was essential to characterize this unusual condition. We speculated that the fibromuscular metaplasia of aortic valve could be associated with an anomalous TGFβ pathway in a patient affected by congenital cataract. Anyway, more in vitro and in vivo studies are needed to confirm this theory.
Conflicts of Interest
The authors have no example conflicts of interest to disclose.
Funding
No funding was secured for this paper.
References
- Alec V, Friedhelm B, Fabien P, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 43(7): 561-632 (2022).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Iung B, Delgado V, Rosenhek R, et al. Contemporary presentation and management of valvular heart disease: The EURObservational research programme valvular heart disease II survey. Circulation. 140(14): 11561169 (2019).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Jondeau G, Ropers J, Regalado E, et al. International registry of patients carrying TGFBR1 or TGFBR2 mutations: Results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet. 9(6): 548-558 (2016).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Shapero K, Wylie-Sears J, Levine RA, et al. Reciprocal interactions between mitral valve endothelial and interstitial cells reduce endothelial-to-mesenchymal transition and myofibroblastic activation. J Mol Cell Cardiol. 80: 175-85 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Chester AH, El-Hamamsy I, Butcher JT, et al. The living aortic valve: From molecules to function. Glob Cardiol Sci Pract. 2014(1): 11 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Waxman AS, Kornreich BG, Gould RA, et al. Interactions between TGFβ1 and cyclic strain in modulation of myofibroblastic differentiation of canine mitral valve interstitial cells in 3D culture. J Vet Cardiol. 14(1): 211-21 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Banasiak P, Strzalka-Mrozik B, Forminska-Kapuscik M, et al. Quantitative relationships between transforming growth factor beta mRNA isoforms in congenital and traumatic cataracts. Mol Vis. 17: 3025-33 (2011).
[Google Scholar] [PubMed]
- Nakamura H, Siddiqui SS, Shen X, et al. RNA interference targeting transforming growth factor-beta type II receptor suppresses ocular inflammation and fibrosis. Mol Vis. 10: 703-11 (2004).
[Google Scholar] [PubMed]