Review Article - Imaging in Medicine (2013) Volume 5, Issue 6
Advances in CT for prediction of hematoma expansion in acute intracerebral hemorrhage
Thien J Huynh1, Sean P Symons1and Richard I Aviv*11Division of Neuroradiology, Department of Medical Imaging, Sunnybrook Health Sciences and University of Toronto, Toronto, Canada
- *Corresponding Author:
- Division of Neuroradiology
Department of Medical Imaging, Sunnybrook Health Sciences Centre
2075 Bayview Avenue, Room AG 31, Toronto
ON, M4N 3M5, Canada
Tel.: +1 416 480 4372
Fax: +1 416 480 5218
Email: richard.aviv@sunnybrook.ca
Abstract
Keywords
computed tomography angiography; computed tomography perfusion spot sign; hematoma expansion; intracerebral hemorrhage; stroke
Primary intracerebral hemorrhage (ICH) accounts for 10–15% of strokes and directly results from vessel rupture and bleeding within the brain parenchyma [1]. Worldwide incidence of ICH ranges from 10 to 30 per 100,000 population per year, in total affecting 2–3 million patients annually [1,2]. Despite advances in acute neurocritical care, ICH mortality and morbidity rates have remained high over the past 30 years, with 1-month case fatality ranging from 30–50% [3–6]. Only 20% of survivors remain functionally independent at 6 months, and the associated cost of care is high [7–10]. Promising innovative medical and surgical interventions are currently being studied; however, proven treatments for acute ICH remain limited to date [11–15].
Noncontrast CT imaging plays a critical role in acute ICH diagnosis, as clinical features are unable to reliably distinguish ischemic from hemorrhagic stroke [16]. For detection of acute hemorrhage, CT is considered the gold-standard; however CT and MRI have been found to be similar in accuracy [17]. CT is preferred over MR imaging due to reduced cost, rapid scan times, increased patient tolerability and increased accessibility in the emergency setting. It is important to note, however, that CT lacks sensitivity in identifying foci of chronic hemorrhage compared with gradient echo and T2* susceptibility- weighted MRI [16,18]. MR imaging may also provide additional information regarding the presence of cavernous malformations and characterizing perihematomal edema [19].
Hematoma expansion & its significance
Prospective serial CT studies in patients arriving within <3 h onset have demonstrated that hematoma expansion occurs in up to 73% of patients [20,21]. ICH expansion >33% from baseline volume at presentation occurs in 28–38% of patients by 24 h after symptom onset. Of those patients who experience >33% expansion, roughly 70% will experience the growth within the first hour after presentation [20–23]. A retrospective study by Kauzi et al. demonstrated that 36% of patients presenting within 3 h of onset had expansion with diminishing risk of 16%, 15% and 6% in patients presenting within 3–6 h, 6–12 h and 12–24 h after ictus, respectively [24].
Hematoma growth is associated with early neurological deterioration and poor long-term outcomes [20,25,26]. For each 10% increase in hematoma growth, there is a 5% increased hazard of death and 16% greater likelihood of worsening by 1 point on the modified Rankin scale (mRS) [20]. ICH volume is also a strong independent predictor of 30-day outcome [27,28]. Prevention of early expansion and reduction of final hematoma volume is thus a logical and attractive target for intervention [29]. As risk of expansion decreases with increasing time after onset [21,24,30], early imaging diagnosis prior to expansion is critical and emphasizes the important role of efficient prehospital triage and acute hospital investigation for ICH similar to ischemic stroke.
Investigational therapeutic strategies
The most promising medical interventions for prevention of hematoma expansion have been acute treatment with activated recombinant Factor VII (rFVIIa) within 4 h of symptom onset or acute intensive blood pressure (BP) reduction within 6 h onset [13,31]. Both rFVIIa and acute BP reduction treatments have demonstrated their ability to reduce absolute and relative hematoma growth; however, both treatment strategies have failed to translate effects of reduced expansion into improvements in 3-month clinical outcome [13,15,31]. Potential reasons for this are multifactorial; however, a central concern is potential dilution of treatment efficacy by inclusion of patients within trials that are unlikely to undergo expansion [32]. From the meta-analysis of prospective acute ICH serial CT studies by Davis et al., it was shown that up to 73% of patients have some degree of expansion, while approximately 32% of patients have expansion of >33% from their baseline scan [17]. Inclusion of patients expected to have relatively stable hematomas (∼27–68% of all patients presenting within 3 h onset) dilutes the expected treatment effect observed in those likely to undergo expansion [31]. Exclusion of patients unlikely to benefit from aggressive therapy would also ensure prevention of serious treatment side effects, including arterial thromboembolic events associated with rFVIIa [33], and also potentially decrease sample size needed for study enrolment [33]. Spot-positive patients are currently being specifically targeted for rFVIIa therapy in the Phase II Spot Sign for Predicting and Treating ICH Growth Study (STOP-IT) [34] and Spot Sign Selection of Intracerebral Hemorrhage to Guide Hemostatic Therapy (SPOTLIGHT) [35] trials. Tranexamic acid is also being studied as a hemostatic agent in spot-positive patients in the Phase II The Spot Sign and Tranexamic Acid On Preventing ICH Growth – Australasia Trial (STOP-AUST) trial [36].
Individual patient risk-stratification of hematoma expansion at presentation may also aid in identifying appropriate patients for surgical intervention [37]. Neurosurgical intervention in acute supratentorial ICH remains controversial; however, options currently being studied include surgical evacuation through craniotomy, endoscopic surgical evacuation, or minimally invasive stereotactic aspiration and drainage. In principle, surgical intervention in acute ICH is an attractive therapeutic option as it has the potential to directly remove the clot, relieve associated mass effect and raised intracranial pressure, and also prevent delayed secondary injury through perihematomal edema and inflammation [11]. A concern with acute surgical intervention, however, is the high risk of rebleeding postoperatively leading to poor outcomes [38]. Rates of rebleeding and associated high mortality are seen particularly when surgery is performed <4 h after onset [38]. Rebleeding postoperatively is likely a result of further bleeding from primary or secondary vessel injury, similar to the mechanisms causing initial hematoma expansion. Recent trials such as the Minimally-Invasive Surgery plus rtPA for Intracerebral Hemorrhage Evacuation (MISTIE) clinical trial have employed inclusion criteria that require imaging evidence of bleeding cessation as demonstrated by stable ICH volume on 6-h CT follow-up [39]. MISTIE has demonstrated promising preliminary results [39]; however, the need for stable follow-up hematoma volumes potentially excludes a third of acute ICH patients with expansion and potentially irreversible neurological decline, precisely the patients with greatest ICH volumes and worst outcomes in need of interventions. Baseline evaluation of hematoma expansion risk may thus allow patients at low risk of expansion to be selected for potential minimally invasive surgery, while those at higher risk may be suitable for hemostatic therapies and/or more aggressive surgical therapies. Direct evaluation of ICH bleeding has been reported and may benefit from direct hemostasis through craniotomy or endoscopic techniques; however, this requires further study [37].
Acute patient risk stratification for hematoma expansion is thus likely critical to identifying both efficacious medical and surgical therapies and guiding appropriate treatment in acute ICH [4,22,37,40]. Accordingly, identification of techniques to predict ICH expansion has been expressed as a research priority by the National Institute of Neurological Disorders [41] and the European Research Network on Intracerebral Hemorrhage [4]. In order to risk-stratify patients for hematoma expansion at presentation, accurate and reliable predictors of expansion available at baseline are urgently needed.
CTA spot sign
One of the most robust predictors of expansion to date has been visualization of intrahematoma iodinated contrast density on first-pass CT angiography (CTA), coined the ‘spot sign’ [42]. Spot sign identification can be performed by nonradiologists and is achieved by thorough review of noncontrast CT and multiplanar CTA images. The spot sign is specifically defined as contrast density within the margins of a parenchymal hematoma without connection to an outside vessel on CTA (Figure 1) [43]. Appearance may be serpiginous, spot-like or multi-focal. Although a maximum density roughly double to the background hematoma in Hounsfield units (HU) is previously proposed, the authors recommend inclusion of contrast density that stands out from background hematoma [43,44]. To enhance identification of spots relative to background hematoma, recommended CT viewing window width and level are 200 and 100 HU, respectively. Although the precise histopathology underlying the spot sign is poorly understood, it is thought that spot signs arise from a range of possible pathologies relating to extravasation from primary or secondary vessel injury, including parenchymal penetrating vessel dissection, pseudoaneurysm and/or vessel rupture [45–47]. Spots may be also related to pathology previously implicated in ICH formation and expansion, including Charcot–Bouchard aneurysms or fibrin globes [45–47]. There is evidence from case studies supporting the spot sign as the point of origin of contrast extravasation diffusing into surrounding nonopacified blood [48,49]. The eccentric location of the bleeding source is consistent with the observations of Fisher [45] and has recently been reproduced in an animal model [R Aviv. Pers. Comm.]. Only 50% of spot signs are actively extravasating at the time of contrast enhancement [42,50]. The presence of extravasated (i.e., dissection/pseudoaneurysm) but not extravasating (actively leaking) contrast in the remaining patients suggests a delayed potential for rebleeding in this group. The mechanism of subsequent bleeding is unknown, but may be post-primary or secondary due to vessel shearing, although this hypothesis remains to be proven [45,51].
Radiographic mimics of the spot sign have been described, and careful review of thin-section (0.625 mm) axial CTA source images and noncontrast CT is needed to exclude vascular and calcified nonvascular mimics, respectively [52]. As clinical factors have been shown to be variable in their association with expansion [53], the spot sign represents a significant advance in the ability to rapidly and reliably predict expansion at baseline.
Frequency & diagnostic performance for hematoma expansion prediction
From the original prospective study of 39 ICH patients presenting within 3 h of onset by Wada et al., overall frequency of CTA spot signs was 33% [42]. Diagnostic performance of the spot sign for hematoma expansion of >6 ml or >30% was 91% sensitivity, 89% specificity, 77% positive predictive value (PPV), and 96% negative predictive value (NPV), with overall accuracy of 90%. Multivariable regression demonstrated that the spot sign was independently associated with expansion (p < 0.001) controlling for the presence of anticoagulation, hyperglycemia and hypertension.
Other single-center studies have reported spot sign frequency ranging from 24 to 41% in patients presenting within 4 to 6 h onset [42,50,54–56]. A retrospective study examining patients presenting beyond the 6-h period reported spot frequency of 11% [57]. Studies including patients presenting at any time window or unknown time of onset have reported greater variation in spot frequency from 19 to 56% [57–60]. Diagnostic performance of the spot sign has also varied among studies. Retrospective and prospective studies have reported sensitivity to be 46–91%, while specificity has ranged from 84 to 100% [61]. Factors resulting in heterogeneity of spot sign frequency and performance for hematoma expansion prediction are protean and include differences in spot sign working definition, time window for patient inclusion, CTA technique, time to follow-up CT and criteria for defining hematoma expansion [61]. Presence of the spot sign is associated with larger baseline hematomas, lower Glasgow Coma Scale score (GCS) and greater National Institutes of Health Stroke Scale (NIHSS) score [59,62,63]. Shorter time from onset to baseline imaging, elevated mean arterial blood pressure, widened pulse pressure, impaired coagulation (international normalized ratio [INR] >1.5), antiplatelet therapy, hyperglycemia, intraventricular hemorrhage, apolipoprotein E ε2 genotype, and absence of microbleeds are also associated with increased frequency of spot presence; however, these findings are variable and/or require validation [56,59,61–66]. Individual study distribution of these factors may thus also affect heterogenous frequency of observed spots. Despite variation in frequency and predictive performance, all studies demonstrate significant independent statistical associations with expansion.
The spot sign was most recently prospectively validated in the multicenter Prediction of Hematoma Growth and Outcome in Patients with Intracerebral Hemorrhage Using the CT-Angiography Spot Sign (PREDICT) observational study [63]. PREDICT studied 228 acute ICH patient from 12 centres in six countries presenting within <6 h from symptom onset. All patients underwent standardized baseline noncontrast CT, CTA and 24-h follow-up noncontrast CT. Reflecting a pragmatic design, CTA protocols were performed according to routine institutional practice and not standardized across centers. CTA spot sign interpretation and hematoma volume measurements were performed by independent core lab investigators and blinded to clinical data. Overall frequency of spots signs was 27% and did not differ between patients presenting within 0–3 h and 3–6 h from onset (p = 0.29). For prediction of expansion >6 ml or >33%, spot sign sensitivity and specificity were 51% and 85%, respectively, while PPV, NPV and accuracy were 61%, 78% and 74%, respectively [63]. Presence of the spot sign was strongly associated with all definitions of hematoma expansion including absolute, relative and individual criterion growth (24-h hematoma expansion of >6 ml, >12.5, >33%, or >12.5 or 33%). Relative risk of hematoma expansion among patients with a spot sign was 2.3 (95% CI: 1.6–3.1).
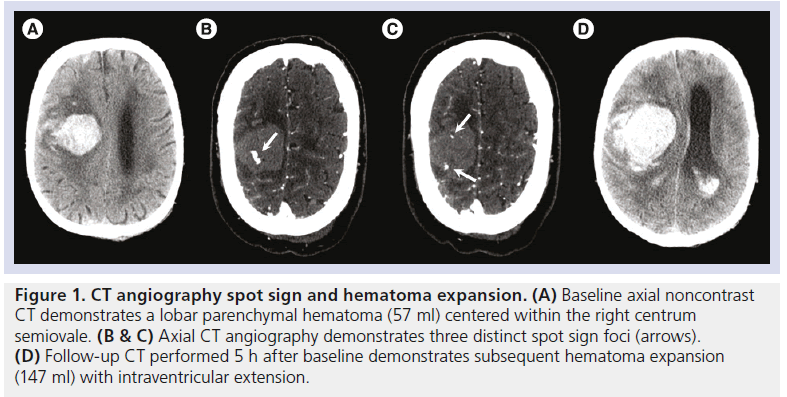
Figure 1.CT angiography spot sign and hematoma expansion. (A) Baseline axial noncontrast CT demonstrates a lobar parenchymal hematoma (57 ml) centered within the right centrum semiovale. (B & C) Axial CT angiography demonstrates three distinct spot sign foci (arrows). (D) Follow-up CT performed 5 h after baseline demonstrates subsequent hematoma expansion (147 ml) with intraventricular extension.
Association with neurological deterioration, functional outcome & mortality
The PREDICT study also demonstrated significant associations with spot sign and 24-h neurological deterioration and 3-month poor clinical outcome [63]. At total of 32% of spotpositive patients experienced 4-point worsening in NIHSS score at 24-h, while spot-negative patients had deterioration in 14% (p = 0.006). Median (range) mRS and 3-month mortality was 5 (0–6) and 43% for spot-positive patients and 3 (0–6) and 20% for spot-negative patients (p ≤ 0.001 for both comparisons). These findings are concordant with several prior singlecenter studies examining association between spot and outcome [54,56,59,61,62,65,67]. In 1999, Becker et al. originally reported a significant independent association between in-hospital mortality and presence of contrast extravasation (64% vs 16%, respectively; p = 0.011) [62]. More recently from 2011, a prospective study (n = 160) by Li et al. from Beijing, China, demonstrated that spot-positive patients had greater in-hospital mortality (20% vs 4%; p = 0.008), poor outcome (mRS 3–6) at discharge (90% vs 70%; p = 0.025), 3-month mortality (27% vs 7%; p = 0.009), and 3-month poor outcome (87% vs 42%; p < 0.001) [56]. From a single center in Barcelona, Spain (n = 114), Rodriguez-Luna et al. prospectively demonstrated significant association between spot sign presence and 24-h neurological deterioration (4-point decrease in NIHSS; 53% vs 12%, p < 0.001) and 3-month mortality (35% vs 14%; p = 0.026) [64].
Patterns of contrast extravasation & implications for expansion prediction & risk stratification
In addition to first-pass CTA, studies examining use of delayed CTA and post-contrast CT (PCT) in acute ICH, typically acquired within 1–4 min after contrast-bolus injection, have also been found to reveal additional foci of intrahematoma contrast enhancement occurring with and without the associated spatial presence of a spot sign visualized on CTA, illustrated in Figures 2 & 3 [50,65,68–70]. This important finding has provided insights into methods to improve sensitivity of expansion prediction, in addition to providing details regarding potential differential rates of expansion based on the contrast enhancement pattern. Clarification of the patterns of contrast enhancement, however, is needed. The term ‘spot sign’ has typically been reserved for the appearance of intrahematoma contrast on CTA source images, typically acquired within 20–30 s after bolus injection. When contrast enhancement is noted on PCT, comparison with the CTA is needed to determine whether the contrast enhancement is spatially located with the presence of a spot on CTA. Findings of enhancement on PCT with the presence of an associated spot sign on CTA has been termed true ‘active contrast extravasation’, and often the PCT enhancement will be larger than the original spot, indicating active diffusion and/or pooling of contrast within the hematoma. Conversely, when a focus of PCT enhancement is noted without the presence of a spot sign on CTA, this appearance is coined post-contrast leakage (PCL). It is important to note that spot signs can also be identified without active extravasation on PCT, but retains high predictive ability for hematoma expansion (Figure 4) [42].
Ederies et al. retrospectively studied 61 patients undergoing baseline CTA and PCT, and found that in addition to the 21/61 (34%) patients with the presence of a spot sign, an additional 5/61 (8%) patients had presence of PCL in the absence of a spot [50]. Importantly, presence of a spot sign or PCL, found in 26/61 (43%) patients, compared to spot sign alone increased hematoma expansion sensitivity and NPV from 78% and 90%, respectively, to 94% and 97%, respectively. There were only modest associated reductions in specificity from 84% to 79% and PPV from 64% to 62%. Among patients without spot signs, patients with PCL vs no PCL demonstrated greater absolute (5.2 ± 5.7 vs -1.5 ± 6.4; p = 0.020) and relative expansion (26 ± 17% vs -0.1 ± 24%; p = 0.02). Patients with PCL with and without spots also demonstrated significant differences in absolute growth (14.5 ± 8.4 ml vs 5.2 ± 5.7 ml, respectively; p = 0.030). Overall, patients with the presence of spot sign and PCL, spot sign alone, and PCL alone were at risk of clinically significant hematoma expansion (>6 ml or 33%; 67%, 67% and 60% respectively). This highlights the ability of PCT to detect more foci of enhancement increasing prediction of hematoma expansion. This finding was confirmed prospectively by Hallevi et al. in 28 patients undergoing baseline CTA and PCT studies [54]. Addition of spot and PCL increased sensitivity of hematoma expansion prediction of >20% baseline volume from 73% for spot alone to 100% with inclusion of PCL, while specificity was maintained at 100% [54]. Kim et al. demonstrated an additional 5/56 (9%) of patients who had evidence of PCL [65]. Presence of enhancement on either CTA or PCT was the only independent predictor of 30-day mortality (odds ratio 4.7; 95% CI: 1.31–16.9; p = 0.017) on backwards stepwise multivariable regression (including variables such as initial hematoma size, presence of intraventricular hemorrhage, GCS, INR and age). Delgado Almandoz et al. retrospectively analyzed 75 ICH patients with both first-pass and delayed CTA (of 367 with first-pass CTA studies available) and found that an additional 6/75 (8%) patients had enhancement only on the delayed study, while 18/75 (24%) demonstrated enhancement both on early and delayed CTA [58]. Compared with spots detected only on first-pass CTA, enhancement on delayed CTA had similar PPV for expansion >6 ml or >30% (69% vs 67%, respectively).
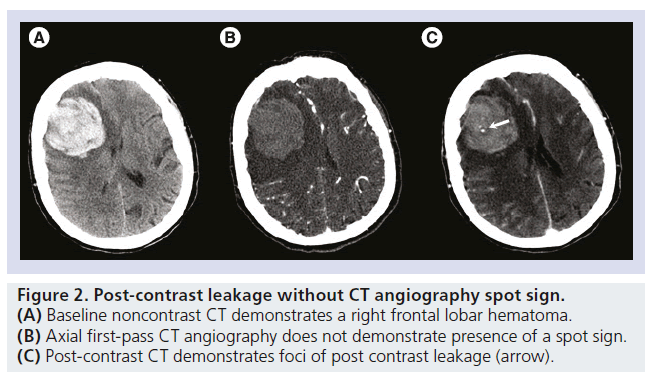
Figure 2.Post-contrast leakage without CT angiography spot sign. (A) Baseline noncontrast CT demonstrates a right frontal lobar hematoma. (B) Axial first-pass CT angiography does not demonstrate presence of a spot sign. (C) Post-contrast CT demonstrates foci of post contrast leakage (arrow).
Qualitative analysis of CTA spot sign characteristics have also provided additional means of expansion risk stratification. Delgado Almandoz et al. systematically characterized spot sign number, maximum axial size, and maximum attenuation in 367 ICH patients with first-pass CTA and found that increasing spot number, spot size and spot attenuation demonstrated increasing risk of hematoma expansion [58]. Based on these spot features, a Spot Sign Score was developed and calculated as follows: 1–2 spot signs, 1 point; ≥3 spot signs, 2 points; spot sign maximum axial dimension ≥5 mm, 1 point; spot sign maximum attenuation ≥180 HU, 1 point [58]. The developed Spot Sign Score demonstrated incremental risk of expansion for scores 0 to 4, with observed expansion occurring in 2%, 33%, 50%, 94% and 100% of cases, respectively [32,58,71]. Sensitivity of Spot Sign Score from dichotomization at scores of 1 to 4 was 88%, 77%, 61% and 30%, respectively [71]. Multivariable regression also demonstrated that the Spot Sign Score was the only predictor of expansion, independent of time of onset to imaging [32]. The Spot Sign Score also provided prediction and risk-stratification for in-hospital mortality and 3-month poor outcome (mRS 4–6) [67].
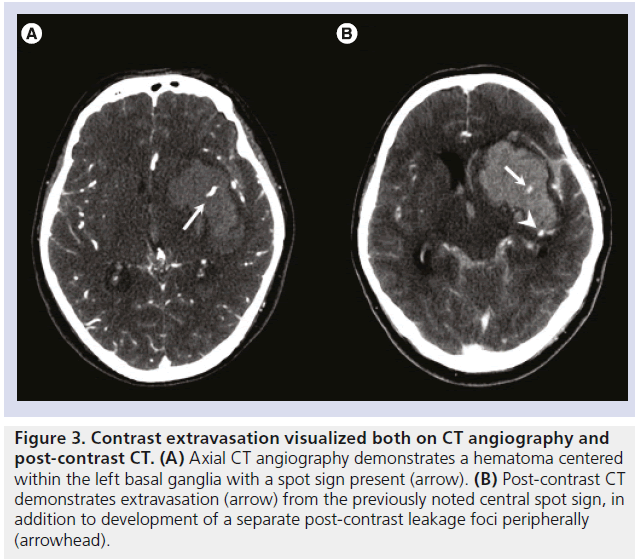
Figure 3.Contrast extravasation visualized both on CT angiography and post-contrast CT. (A) Axial CT angiography demonstrates a hematoma centered within the left basal ganglia with a spot sign present (arrow). (B) Post-contrast CT demonstrates extravasation (arrow) from the previously noted central spot sign, in addition to development of a separate post-contrast leakage foci peripherally (arrowhead).
Recently, the Spot Sign Score was externally evaluated using the first-pass CTA data from the prospective multicenter PREDICT study. Results from this post hoc analysis demonstrated that only spot sign number provided additional risk stratification for hematoma expansion over spot sign alone, and that spot sign number was similar to Spot Sign Score for hematoma expansion discrimination [44]. Tests for trend in absolute, relative and criterion growth of >6 ml or >33% demonstrated significant results for spot number, but not for Spot Sign Score. Based on spot number alone, risk of expansion with 0, 1, 2–3 and ≥4 spots were 22%, 53%, 65% and 100%, respectively. Inability to observe an association between spot density and size with expansion, despite potential biological plausibility, likely relates to variations between institutional CTA protocols and technique, resulting in heterogeneity of spot characteristics [72]. Despite these differences, spot number remains a significant predictive factor for incremental risk stratification and may be an important method to reliably predict expansion in the future.
Dynamic CT characterization of contrast extravasation
A limitation regarding spot sign characterization using static imaging, including CTA and PCT, is that they represent single snapshots in time and may inadequately characterize the transient and dynamic process of contrast extravasation. This was demonstrated in a case report by Chakraborty et al. in which a patient with a deep right-sided ICH was acutely imaged using a dynamic CTA acquisition protocol with temporal resolution of up to 1 s over 60 s [48]. Dynamic CTA source images demonstrated gradual development of multiple extravasation foci within the hematoma, with peak enhancement at 49 s after bolus injection. All enhancement foci, however, were not initially seen during the arterial phase of the study, the typical time of CTA acquisition. Furthermore, all foci had faded in attenuation by the end of the study at 60 s, well before typical PCT acquisition. A subsequent acute ICH case report by Dowlatshahi et al. similarly demonstrated extensive confluent accumulation and growth of contrast extravasation volume during dynamic first-pass CTA associated with rapid early neurological deterioration [49].
Single-acquisition first-pass CTA likely underestimates the true number of patients with spot signs, and data from CTP and PCT studies helps explain why up to 22% apparently spotnegative patients undergo hematoma expansion [63]. Optimal acquisition timing of CTA and PCT for extravasation detection and characterization is also uncertain. A large number of potential physiologic processes may affect spot sign visualization. Although standardization of contrast injection rate, volume and timing may help improve spot visualization, variation in patient blood pressure, circulation time and perihematomal intracranial pressure gradients may also influence timing to spot visualization and make a single optimal time for CTA/ PCT acquisition unlikely [72]. Further variation between centers in a multicenter setting may further increase heterogeneity [44].
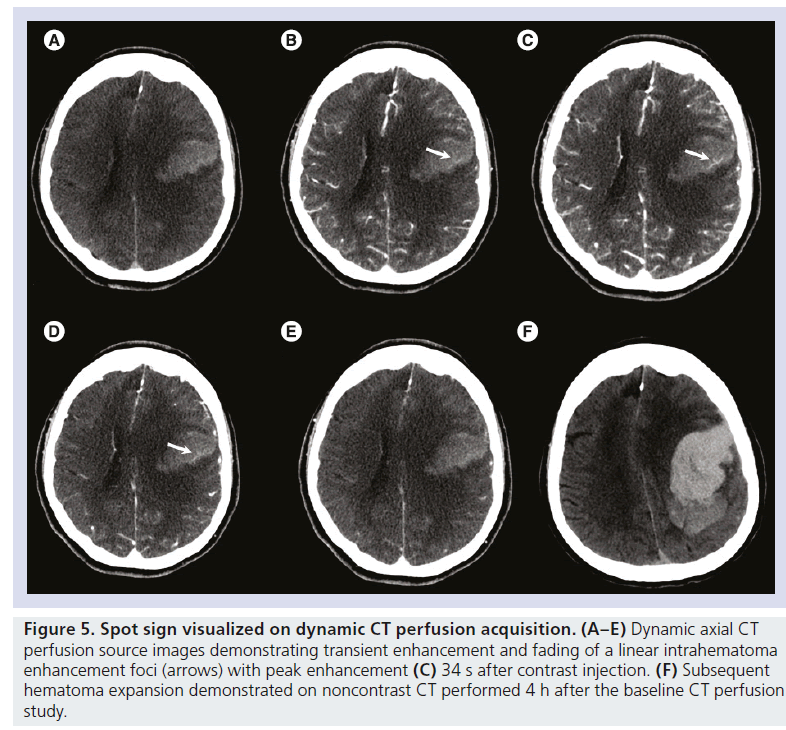
Similar to dynamic CTA, CT perfusion (CTP) is a dynamic study that tracks a contrast bolus through the intracranial circulation, typically for 60–120 s with modern bi-phasic techniques, and is already commonly used in the evaluation of ischemic stroke [73]. CTP circumvents the need for early and late acquisitions and may show contrast extravasation not present on either CTA or PCT (Figure 5) [69,70]. Peak attenuation and maximum extravasation size of the spot sign may also be better characterized on CTP and provide the opportunity to improve the accuracy of the spot sign score for expansion, outcome prediction and risk-stratification, particularly in a multicenter setting [70].
To characterize the utility of CTP source images for spot sign detection, characterization and prediction of outcome, Koculym et al. studied 28 acute ICH patients presenting < 6 h onset undergoing baseline CTA, CTP and PCT [70]. Total number of spots, maximum spot attenuation and axial dimensions were examined for all patients on each of the CTA, CTP and PCT source images. Location of each spot was cross-referenced to determine whether each was present on the other study types. A combined primary outcome of hematoma expansion, need for emergent surgical drainage, or in-hospital mortality was used. From this study, it was found that CTP detected the greatest number of patients with spots compared to CTA and PCT (50% vs 29% and 32%, respectively). Sensitivity for outcome prediction was significantly greater for CTP (78%) compared with CTA (44%) or PCT (50%). CTP also was able to detect the greater number of individual spot foci found on all modalities and demonstrated that maximum spot density was found at a median time of 50 s (interquartile range: 34–63 s) after contrast injection.
In a recent prospective study of 112 patients presenting < 6 h of onset undergoing CTP and CTA at baseline, Sun et al. demonstrated improved sensitivity for hematoma expansion (>6 ml or >30%) prediction compared with CTA alone while maintaining excellent specificity [69]. Specifically, sensitivity was increased from 61% to 89%, while specificity was maintained at 92% to 94% for CTA compared with CTP detected spots, respectively. The overall frequency of patients with CTA and CTP spots was 21% and 27%, respectively. Interestingly, the authors noted a variation in the duration of enhancement of CTP-detected spots, ranging from 2 to 30 s, emphasizing the transient nature of spot sign appearance. Sixty-three percent of CTP-detected foci of enhancement were shown only from arterial to the capillary phase, while 37% cases demonstrated enhancement from arterial to venous or venous sinus phase. Of the 30 cases with a positive spot on CTP, 37% demonstrated progressive enlargement of the enhancement foci.
The differences in enhancement duration and size of spot appearance likely reflect differential rates of contrast extravasation, which may be further quantified using CTP permeability analysis. In 2011, D’Esterre et al. characterized the rate of contrast extravasation from individual foci of contrast extravasation using CTP-derived permeability surface product [74]. Perfusionderived permeability surface product is a novel method of objectively measuring rate of contrast extravasation from the intra- to extra-vascular compartment [75]. In 16 ICH patients presenting within 6 h onset undergoing baseline CTA, CTP and PCT, regions of interest were placed at the location of spot signs, PCL lesions, background hematoma and contralateral normal brain parenchyma. From this study it was found that average permeability surface product for spot sign foci was 6.5 ± 1.6 ml/min/100 g, while PCL foci and background hematoma demonstrated values of 1.0 ± 0.4 and 0.1 ± 0.4, respectively. Visualization of permeability maps enabled identification of all spot and PCL foci. Based on this data, it may possible to distinguish spot sign foci and PCL in addition to objectively characterize rate of contrast leakage using a single CTP study, precluding need for multiple single CTA and PCT acquisitions.
A limitation of CTP performed on 16- or 64-row detector CT is lack of full head spatial coverage [69,70]. Current CTP protocols typically acquire ∼4 cm z-axis coverage from the basal ganglia to the lateral ventricles. In the study by Sun et al., this resulted in inadequate spatial coverage of the hematoma in 44/112 (39%) of cases [69]. Based on the preliminary data, the clinical significance of this lack of coverage appears minimal, as Sun et al. demonstrated only one false-negative CTP case (of 112 studied patients) that was detected by CTA. Similarly Koculym et al. found only one false-negative case (of 28 studied patients) demonstrating a spot sign on CTA [70]. In both studies, overall sensitivity for spot detection was significantly improved with CTP. The ideal method of dynamic spot imaging may be provided with modern 320-row detector CT, as demonstrated by a recent case report by Dowlatshahi et al. [49]. With 320-row detector CT, full-head acquisition for CTA and CTP data may be obtained simultaneously using a single acquisition and contrast bolus injection protocol. The same protocol may be used in cases of ischemic stroke and may provide a standardized acute stroke protocol. Scan coverage for 16- and 64-row detector CT may also be extended to 80 mm with the use of table toggling techniques [76].
A concern with CTP use is patient radiation dose. Reported radiation dose of CTP (∼1.9–3.5 mSv), however, is similar to that in a noncontrast head CT (∼2.6–3.3 mSv) and can be further reduced with modern low-dose protocols [69,70,77]. CTP is also able to avoid radiosensitive structures such as the thyroid and orbits. Contrast-induced nephropathy (CIN), potentially leading to life-threatening acute renal failure, may also be a potential concern for utilization of iodinated contrast in acute stroke multimodal CT imaging. This issue is further exacerbated in the emergent stroke setting as there is often lack of patient serum creatinine level available prior to CT in order to exclude patients with reduced glomerular filtration rate. Several investigators have examined the incidence of CIN among patients undergoing CTA or CTP for acute stroke evaluation and have found low incidence (2–5%) of CIN in patients without history of prior chronic renal disease, even when baseline creatinine levels were not measured prior to the scan [78–81]. In these studies, few patients who developed CIN required in-hospital dialysis (0.2%) and none developed chronic kidney disease. It has thus been recommended that emergent advanced CT imaging in acute stroke does not require a serum creatinine level in patients without existing history of chronic renal disease and that emergent imaging should take priority over ‘relatively weak’ contraindications to contrast administration [82,83].
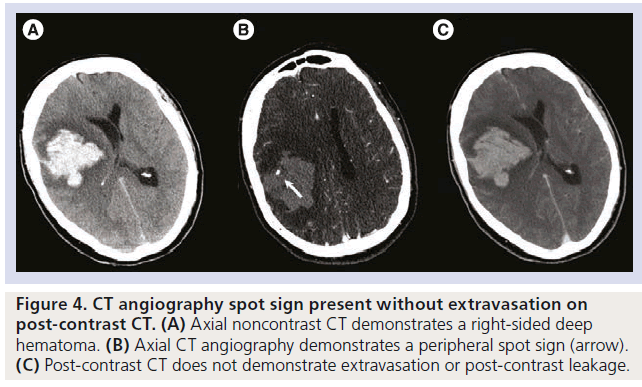
Figure 4.CT angiography spot sign present without extravasation on post-contrast CT. (A) Axial noncontrast CT demonstrates a right-sided deep hematoma. (B) Axial CT angiography demonstrates a peripheral spot sign (arrow). (C) Post-contrast CT does not demonstrate extravasation or post-contrast leakage.s
Contrast extravasation interobserver agreement
Interobserver agreement of the spot sign has varied from substantial agreement (kappa statistic [κ] 0.72) among untrained observers in PREDICT [63] to near perfect (κ = 0.92) among experienced neuroradiologists [42,58,69]. Online educational spot sign identification training and imaging certification prior to patient enrolment for trials may help improve reader agreement and accuracy. Spot sign imaging certification has already be incorporated as a requirement for patient enrolment for the STOP-IT [84], SPOTLIGHT [85] and STOP-AUST [86] studies [4,87]. Interobserver agreement for contrast extravasation detection may also potentially be improved with delayed or dynamic imaging. Delgado Almandoz et al. reported first-pass CTA spot sign agreement ranged from κ = 0.88 to 0.92 and was increased for delayed CTA detected spots (K= 0.93–0.97) amongst three readers. Similarly Hallevi et al., also with three independent readers, demonstrated improved interrater reliability from 0.812 to 0.952 for enhancement on CTA and PCT respectively. Sun et al. also reported modest improvement in agreement using CTP compared with CTA with κ = 0.92 on CTA and κ = 0.94 on CTP, citing greater ease of identifying growing contrast extravasation rather than static density. Correlation of extravasation foci between modalities and with CTP permeability maps can also potentially improve confidence of extravasation identification [70,74]. Thus, delayed or dynamic imaging may not only aid in improving prediction and risk-stratifying hematoma expansion, but may also provide opportunity to improve diagnostic confidence and agreement of spots identification.
Figure 5.Spot sign visualized on dynamic CT perfusion acquisition. (A–E) Dynamic axial CT perfusion source images demonstrating transient enhancement and fading of a linear intrahematoma enhancement foci (arrows) with peak enhancement (C) 34 s after contrast injection. (F) Subsequent hematoma expansion demonstrated on noncontrast CT performed 4 h after the baseline CT perfusion study.
Other radiographic predictors of expansion
Although the spot sign represents one of the most validated and robust imaging predictors of expansion to date, several other radiographic predictors have been described. Baseline ICH volume has also been associated with subsequent expansion from multiple studies [30,88,89]. Based on a large multicenter cohort, Dowlatshahi et al. described baseline ICH volume <10 ml were at much lower odds of experiencing hematoma expansion (categorically defined as >6 ml or >12 ml, separately), early neurological deterioration and 3-month mortality compared with hematoma with volume >30 ml. Specifically, patients with <10 ml baseline ICH volume had an odds ratio of 0.1 (95% CI: 0.0–0.2) of experiencing subsequent >6 ml expansion compared with patients with ICH volumes of >30 ml [88]. Broderick et al. studied 399 patients from the Phase IIb rFVIIa study and identified that greater ICH volume was also significantly associated with increased proportion of patients with categorical hematoma growth (defined as meeting >12.5 ml or >33% growth) [30]. As time to presentation has also been associated with hematoma expansion, Rodriguez-Luna et al. evaluated whether adjustment of ICH volume based on time of symptom onset could provide predictive benefit for hematoma expansion [64]. The adjusted value, coined ultra-early hematoma growth (uHG) rate and defined as the baseline hematoma volume (in ml) divided by the time from onset-to-imaging (in hours), was found to be a strongly associated with expansion (>6 ml or >33%), early neurological deterioration and 3-month mortality [64]. A uHG rate of >10.2 ml/h demonstrated an odds ratio of 3.6 (95% CI: 1.4–9.1) for expansion.
Barras et al. studied the potential association between hematoma contour irregularity, density heterogeneity and hematoma expansion by examining the baseline CT studies of the placebo- arm patients from the Phase IIb rFVIIa trial [89]. Theoretically, hematomas that arise from multiple bleeding foci at the margins of hematoma may produce an irregular margin and result in larger hematomas as opposed to more rounded and regular hematomas that arise from a single focus [90]. Heterogenous density may result from a hematoma with portions of unclotted active hemorrhage, coined the ‘swirl sign’ [65,91]. Barras et al. demonstrated that heterogenous density, as characterized by a novel grading system based on visual assessment, was independently associated with absolute hematoma expansion (p = 0.046), while irregular margin was not. A follow-up study utilizing systematic quantitative CT densitometry in the same cohort revealed that the coefficient of variation of hematoma attenuation (defined as the standard deviation of the hematoma density divided by the mean attenuation) was the best predictor of absolute expansion compared with baseline ICH volume, time from onset-to-imaging and other mathematical characteristics of hematoma density [92]. A multivariable model incorporating the coefficient of variation, baseline ICH volume, and time from onset-to-imaging provided the best predictive model in their dataset. Further validation and methods to incorporate these findings into potential clinical practice is required [92].
Recently, a study by Sorimachi et al. described the association between presence of visualized intrahematoma lenticulostriate arteries on CTA in patients with putaminal ICH and subsequent acute deterioration (defined as hematoma expansion >12.5 ml or >33% and need for surgical evacuation or 1-day mortality) [60]. Coined the ‘tail sign’, the presence of the lenticulostriate artery and the spot sign were independent predictors of deterioration and provided increased sensitivity for outcome prediction. External validation of the tail sign is, however, required.
Future perspective
ICH remains a devastating disease with significant associated morbidity and mortality, particularly among patients with hematoma expansion and early neurological deterioration. As novel medical and surgical interventions for acute ICH patients emerge, there will be increasing emphasis on ensuring appropriate selection of patients based on hematoma expansion risk in order to identify and improve treatment efficacy and prevent serious treatment side effects. Hematoma risk stratification by the spot sign and other radiographic methods offer the best means for accurate and reliable risk stratification. This is reflected in the latest American Heart Association and American Stroke Association ICH management guidelines that recommend that “CTA and contrast-enhanced CT may be considered to help identify patients at risk for hematoma expansion” [16]. The STOP-IT, SPOTLIGHT and STOP-AUST trials are currently evaluating spot sign image-guided therapy as a feasible and effective therapeutic strategy for prevention of expansion. Advanced multimodal CT imaging including delayed CTA, CTP and PCT, already commonly used in the evaluation of patients with ischemic stroke, may be incorporated into the evaluation of acute ICH patients to further improve accuracy of hematoma expansion and interobserver agreement. Noncontrast CT is no longer sufficient to guide appropriate ICH patient care [82]. Further multicenter collaborative effort is required to identify and characterize the utility of advanced CT imaging in improving patient outcomes in acute ICH. A novel animal model is also under development that will permit real-time in vivo study of contrast extravasation, hematoma expansion and the impact of established and novel therapies on contrast extravasation-induced hematoma.
Financial & competing interests disclosure
TJ Huynh is supported by a Canadian Institutes of Health Research Master's Award and Physician Services Incorporated Resident Grant. RI Aviv is supported by a Heart and Stroke Foundation of Ontario grant. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as:
*of interest
**of considerable interest
References
- Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet 373(9675), 1632–1644 (2009).
- Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nat. Rev. Neurol. 6(11), 593–601 (2010).
- Van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 9(2), 167–176 (2010).
- Steiner T, Petersson J, Al-Shahi Salman R et al. European research priorities for intracerebral haemorrhage. Cerebrovasc. Dis. 32(5), 409–419 (2011).
- Rincon F, Mayer SA. The epidemiology of intracerebral haemorrhage in the United States from 1979 to 2008. Neurocrit. Care 19(1), 95–102 (2013).
- Andaluz N, Zuccarello M. Recent trends in the treatment of spontaneous intracerebral hemorrhage: analysis of a nationwide inpatient database. J. Neurosurg. 110(3), 403–410 (2009).
- Hemphill JC, Farrant M, Neill TA. Prospective validation of the ICH Score for 12-month functional outcome. Neurology 73(14), 1088–1094 (2009).
- Broderick JP, Brott T, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J. Neurosurg. 78(2), 188–191 (1993).
- Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 344(19), 1450–1460 (2001).
- Russell MW, Boulanger L, Joshi A, Neumann PJ, Menzin J. The economic burden of intracerebral hemorrhage: evidence from managed care. Manag. Care Interface 19(6), 24–28, 34 (2006).
- Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. 11(1), 101–118 (2012).
- Rincon F, Mayer SA. Intracerebral hemorrhage: getting ready for effective treatments. Curr. Opin. Neurol. 23(1), 59–64 (2010).
- Mayer SA, Brun NC, Begtrup K et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N. Engl. J. Med. 358(20), 2127–2137 (2008).
- Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 6736, 1–12 (2013).
- Anderson CS, Heeley E, Huang Y et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N. Engl. J. Med. 368(25), 2355–2365 (2013).
- Morgenstern LB, Hemphill JC, Anderson C et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 41(9), 2108–2129 (2010).
- Kidwell CS, Chalela JA, Saver JL et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 292(15), 1823–1830 (2004).
- Ginde AA, Foianini A, Renner DM, Valley M, Camargo CA. Availability and quality of computed tomography and magnetic resonance imaging equipment in U.S. emergency departments. Acad. Emerg. Med. 15(8), 780–783 (2008).
- Kidwell CS, Wintermark M. Imaging of intracranial haemorrhage. Lancet Neurol. 7(3), 256–267 (2008).
- Davis SM, Broderick J, Hennerici M et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 66(8), 1175–1181 (2006). & Meta-analysis demonstrating up to 73% of patients presenting within 3 h of onset have expansion at 24-h follow-up. Significant associations between expansion and mortality and functional outcome were demonstrated.
- Brott T, Broderick J, Kothari R et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 28(1), 1–5 (1997).
- Steiner T, Bösel J. Options to restrict hematoma expansion after spontaneous intracerebral hemorrhage. Stroke 41(2), 402–409 (2010).
- Josephson CB, Frantzias J, Samarasekera N, Salman Al-Shahi R. The persisting burden of intracerebral haemorrhage: can effective treatments be found? PLoS Med. 7(10), e1000353 (2010).
- Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke 27(10), 1783–1787 (1996).
- Leira R, Dávalos A, Silva Y et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology 63(3), 461–467(2004). & Multicenter study demonstrating predictors of early neurological deterioration including hematoma growth, intraventricular hemorrhage and high systolic blood pressure.
- Dowlatshahi D, Demchuka M, Flaherty ML, Ali M, Lyden PL, Smith EE. Defining hematoma expansion in intracerebral hemorrhage: Relationship with patient outcomes. Neurology 76(14), 1238– 1244(2011). & International multicenter study using data from the Virtual International Stroke Trials Archive, demonstrating a robust association between hematoma expansion and poor outcome regardless of growth or outcome definition.
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 24(7), 987–993 (1993).
- Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC, Tuhrim S. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage editorial comment: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32(4), 891 (2001).
- Mayer SA, Rincon F. Treatment of intracerebral haemorrhage. Lancet Neurol. 4(10), 662–672 (2005). & Phase III activated recombinant Factor VII study demonstrating ability of acute hemostatic therapy to limit hematoma volumes. Improvement in 3-month clinical outcome, however, was not demonstrated.
- Broderick JP, Diringer MN, Hill MD et al. Determinants of intracerebral hemorrhage growth: an exploratory analysis. Stroke 38(3), 1072–1075 (2007).
- Anderson CS, Huang Y, Wang JG et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 7(5), 391–399 (2008).
- Becker K, Tirschwell D. Stroke. “Spotting” patients at the highest risk of hematoma growth. Nat. Rev. Neurol. 5(10), 526–528 (2009).
- Diringer MN, Skolnick BE, Mayer SA et al. Thromboembolic events with recombinant activated factor VII in spontaneous intracerebral hemorrhage: results from the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke 41(1), 48–53 (2010).
- Flaherty ML, Jauch EC. The Spot Sign for Predicting and Treating ICH Growth Study (STOP-IT). http://clinicaltrials.gov/show/NCT00810888
- Gladstone DJ. “Spot Sign” Selection of Intracerebral Hemorrhage to Guide Hemostatic Therapy (SPOTLIGHT): randomized controlled trial. ISRCTN29749408. www.controlled-trials.com
- Davis SM, Donnan GA. STOP-AUST: The Spot Sign and Tranexamic Acid On Preventing ICH Growth– AUStralasia Trial. http://clinicaltrials.gov/show/ NCT01702636
- Nagasaka T, Inao S, Wakabayashi T. What does the CT angiography “spot sign” of intracerebral hemorrhage mean in modern neurosurgical settings with minimally invasive endoscopic techniques? Neurosurg. Rev. 36(3), 341–348 (2013).
- Morgenstern LB, Demchuk AM, Kim DH Frankowski RF, Grotta JC. Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology 56(10), 1294–1299 (2001).
- Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D. Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochir. Suppl. 105, 147–151 (2008).
- Mayer SA, Davis SM, Skolnick BE et al. Can a subset of intracerebral hemorrhage patients benefit from hemostatic therapy with recombinant activated factor VII? Stroke 40(3), 833–840 (2009).
- NINDS ICH Workshop Participants. Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke 36(3), e23–e41 (2013).
- Wada R, Aviv RI, Fox AJ et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke 38(4), 1257–1262 (2007). & Original prospective study demonstrating association between CT angiography (CTA) spot sign and hematoma expansion.
- Thompson AL, Kosior JC, Gladstone DJ et al. Defining the CT Angiography “Spot Sign”in Primary Intracerebral Hemorrhage. Can. J. Neurol. Sci. 36(4), 456–461 (2009).
- Huynh TJ Demchuk AM Dowlatshahi D et al. Spot sign number is the most important spot sign characteristic for predicting hematoma expansion using first-pass computed tomography angiography: analysis from the PREDICT study. Stroke 44(4), 972–977 (2013). & External validation of the spot sign score demonstrates that spot sign number is the most important spot characteristic for prediction and risk-stratification of expansion.
- Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J. Neuropathol. Exp. Neurol. 30(3), 536–550 (1971).
- Fisher CM. Hypertensive cerebral hemorrhage. Demonstration of the source of bleeding. J. Neuropathol. Exp. Neurol. 62(1), 104–107 (2003).
- Huynh TJ, Julia K, Aviv RI. Histopathological characteristics of the 'spot sign' in spontaneous intracerebral hemorrhage. Arch. Neurol. 69(12), 1654–1655 (2012).
- Chakraborty S, Stotts G, Rush C, Hogan MJ, Dowlatshahi D. Dynamic “spot sign” resolution following INR correction in a patient with warfarin-associated intracerebral hemorrhage. Case Rep. Neurol. 3(2), 154–159 (2011).
- Dowlatshahi D, Hogan MJ, Sharma M, Stotts G, Blacquiere D, Chakraborty S. Ongoing bleeding in acute intracerebral haemorrhage. Lancet 6736(C), 60829 (2012).
- Ederies A, Demchuk A, Chia T et al. Postcontrast CT extravasation is associated with hematoma expansion in CTA spot negative patients. Stroke 40(5), 1672–1676 (2009). & Utilization of post-contrast CT in acute intracerebral hemorrhage (ICH) is able to identify an additional 8% of patients with intrahematoma contrast enhancement compared to CTA.
- Greenberg CH, Frosch MP, Goldstein JN, Rosand J, Greenberg SM. Modeling intracerebral hemorrhage growth and response to anticoagulation. PLoS One 7(10), e48458 (2012).
- Gazzola S, Aviv RI, Gladstone DJ et al. Vascular and nonvascular mimics of the CT angiography “spot sign” in patients with secondary intracerebral hemorrhage. Stroke 39(4), 1177–1183(2008).
- Jauch EC, Lindsell CJ, Adeoye O et al. Lack of evidence for an association between hemodynamic variables and hematoma growth in spontaneous intracerebral hemorrhage. Stroke 37(8), 2061–2065 (2006).
- Hallevi H, Abraham AT, Barreto AD, Grotta JC, Savitz SI. The spot sign in intracerebral hemorrhage: the importance of looking for contrast extravasation. Cerebrovasc. Dis. 29(3), 217–220 (2010).
- Wang Y-H, Fan J-Y, Luo G-D et al. Hematoma volume affects the accuracy of computed tomographic angiography “spot sign” in predicting hematoma expansion after acute intracerebral hemorrhage. Eur. Neurol. 65(3), 150–155 (2011).
- Li N, Wang Y, Wang W. Contrast extravasation on computed tomography angiography predicts clinical outcome in primary intracerebral hemorrhage: a prospective study of 139 cases. Stroke 42(12), 3441–3446 (2011).
- Brouwers HB, Falcone GJ, McNamara KA et al. CTA spot sign predicts hematoma expansion in patients with delayed presentation after intracerebral hemorrhage. Neurocrit. Care 17(3), 421–428 (2012).
- Delgado Almandoz JE, Yoo AJ, Stone MJ et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke 40(9), 2994–3000 (2009).
- Goldstein JN, Fazen LE, Snider R et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology 68(12), 889–894 (2007).
- Sorimachi T, Osada T, Baba T et al. The striate artery, hematoma, and spot sign on coronal images of computed tomography angiography in putaminal intracerebral hemorrhage. Stroke 44(7), 1830–1832 (2013).
- Brouwers HB, Goldstein JN, Romero JM, Rosand J. Clinical applications of the computed tomography angiography spot sign in acute intracerebral hemorrhage:a review. Stroke 43(12), 3427–3432 (2012).
- Becker KJ, Baxter AB, Bybee HM, Tirschwell DL, Abouelsaad T, Cohen WA. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke 30(10), 2025–2032 (1999).
- Demchuk AM, Dowlatshahi D, Rodriguez- Luna D et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 11(4), 307–314 (2012). & Multicenter prospective validation of the spot sign for prediction of hematoma expansion, early neurological deterioration and 3-month poor clinical outcome.
- Rodriguez-Luna D, Rubiera M, Ribo M et al. Ultraearly hematoma growth predicts poor outcome after acute intracerebral hemorrhage. Neurology 77(17), 1599–1604 (2011).
- Kim J, Smith A, Hemphill JC. Contrast extravasation on CT predicts mortality in primary intracerebral hemorrhage. AJNR Am. J. Neuroradiol. 29(3), 520–525(2008).
- Evans A, Demchuk A, Symons SP et al. The spot sign is more common in the absence of multiple prior microbleeds. Stroke 41(10), 2210–2217 (2010).
- Delgado Almandoz JE, Yoo AJ, Stone MJ et al. The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke 41(1), 54–60 (2010).
- Aviv RI, Gladstone D, Goldstein J, Flaherty M, Broderick J, Demchuk A. Contrast extravasation predicts hematoma growth: where to now? AJNR Am. J. Neuroradiol. 29(9), E80(2008).
- Sun S-J, Gao P-Y, Sui B-B et al. “Dynamic spot sign” on CT perfusion source images predicts haematoma expansion in acute intracerebral haemorrhage. Eur. Radiol. 23(7), 1846–1854 (2013).
- Koculym A, Huynh TJ, Jakubovic R, Zhang L, Aviv RI. CT perfusion spot sign improves sensitivity for prediction of outcome compared with CTA and postcontrast CT. AJNR Am. J. Neuroradiol. 34(5), 965–970 (2013). & Use of CT perfusion in acute ICH allows dynamic visualization of contrast extravasation increasing sensitivity for spot sign detection and prediction of outcome.
- Delgado Almandoz JE, Yoo AJ, Stone MJ et al. Extended analysis of the spot sign score's performance. Nat. Rev. Neurol. 6(6), 1–1 (2010).
- Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 256(1), 32–61 (2010).
- Aviv RI, D’Esterre CD, Murphy BD et al. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology 250(3), 867–877 (2009).
- d’Esterre CD, Chia TL, Jairath A, Lee TY, Symons SP, Aviv RI. Early rate of contrast extravasation in patients with intracerebral hemorrhage. AJNR Am. J. Neuroradiol. 32(10), 1879–1884 (2011).
- St Lawrence KS, Lee TY. An adiabatic approximation to the tissue homogeneity model for water exchange in the brain: II. Experimental validation. J. Cereb. Blood Flow Metab. 18(12), 1378–1385 (1998).
- Suzuki K, Tanaka N, Morita S, Machida H, Ueno E, Kasuya H. Active bleeding in acute subarachnoid hemorrhage observed by multiphase dynamic-enhanced CT. AJNR Am. J. Neuroradiol. 33(7), 1374–1379 (2012).
- Latchaw RE, Alberts MJ, Lev MH. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke 40(11), 3646–3678 (2009).
- Hopyan JJ, Gladstone DJ, Mallia G. Renal safety of CT angiography and perfusion imaging in the emergency evaluation of acute stroke. AJNR Am. J. Neuroradiol. 29(10), 1826–1830 (2008).
- Lima FO, Lev MH, Levy RA. Functional contrast-enhanced CT for evaluation of acute ischemic stroke does not increase the risk of contrast-induced nephropathy. AJNR Am. J. Neuroradiol. 31(5), 817–821 (2010).
- Krol AL, Dzialowski I, Roy J et al. Incidence of radiocontrast nephropathy in patients undergoing acute stroke computed tomography angiography. Stroke 38(8), 2364–2366 (2007).
- Josephson SA, Dillon WP, Smith WS. Incidence of contrast nephropathy from cerebral CT angiography and CT perfusion imaging. Neurology 64(10), 1805–1806 (2005).
- Khosravani H, Mayer SA, Demchuk A et al. Emergency noninvasive angiography for acute intracerebral hemorrhage. AJNR Am. J. Neuroradiol. 34(8), 34–38 (2012).
- Benko A, Fraser-Hill M, Magner P et al. Canadian Association of Radiologists: consensus guidelines for the prevention of contrast-induced nephropathy. Can. Assoc. Radiol. J. 58(2), 79–87 (2007).
- STOP-IT. The Spot Sign for Predicting and Treating ICH Growth Study. www.stopitstudy.org/
- "Spot Sign" Selection of Intracerebral Hemorrhage to Guide Hemostatic Therapy (SPOTLIGHT): A Randomized Controlled Trial. www.spotlightstudy.com
- STOP-AUST. The Spot sign and Tranexamic acid On Preventing ICH growth– AUStralasia Trial: Imaging Module. www.stopauststudy.com
- Havsteen I, Christensen A, Nielsen JK, Christensen L, Krieger DW, Christensen H. E-learn computed tomographic angiography: a proposed educational tool for computed tomographic angiography in acute stroke. J. Stroke Cerebrovasc. Dis. 21(8), 684–688 (2012).
- Dowlatshahi D, Smith EE, Flaherty ML, Ali M, Lyden P, Demchuk AM. Small intracerebral haemorrhages are associated with less haematoma expansion and better outcomes. Int. J. Stroke. 6(3), 201–206 (2011).
- Barras CD, Tress BM, Christensen S et al. Density and shape as CT predictors of intracerebral hemorrhage growth. Stroke 40(4), 1325–1331 (2009).
- Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke 29(6), 1160–1166 (1998).
- Al-nakshabandi NA. The swirl sign. Radiology 218(2), 433–433 (2001).
- Barras CD, Tress BM, Christensen S. Quantitative CT densitometry for predicting intracerebral hemorrhage growth. AJNR Am. J. Neuroradiol. 34(6), 1139–1144 (2013).