Research Article - Journal of Pharmaceutical Toxicology (2023) Volume 6, Issue 3
Advancements in Personalized Medicine: Revolutionizing the Pharmaceutical Industry
Nina Watson*
Oxford Suzhou Centre for Advanced Research (OSCAR), University of Oxford, Suzhou 215123, China
- *Corresponding Author:
- Nina Watson
Oxford Suzhou Centre for Advanced Research (OSCAR), University of Oxford, Suzhou 215123, China
E-mail: ninawatson56@edu.in
Abstract
Personalized medicine is an emerging field in the pharmaceutical industry that aims to tailor medical treatments to individual patients based on their genetic makeup, lifestyle, and environmental factors. This approach holds great promise for improving patient outcomes, reducing adverse reactions, and optimizing therapeutic efficacy. In recent years, significant advancements have been made in personalized medicine, driven by breakthroughs in genomics, bioinformatics, and data analytics. This article explores the latest developments in personalized medicine and their potential to revolutionize the pharmaceutical industry. Genomic medicine involves the study of an individual’s entire genetic makeup to understand disease susceptibility, predict treatment response, and guide drug development.
Keywords
Drug interactions • Side effects • Pharmacokinetics • Pharmacodynamics • Therapeutic index • Drug metabolism
Introduction Pharmacogenomics focuses on how genetic variations impact an individual’s response to medications. Researchers have identified specific genetic markers that influence drug metabolism, efficacy, and toxicity. By integrating genomic information into clinical practice, physicians can prescribe medications with greater precision, minimizing adverse effects and maximizing therapeutic benefits. Personalized medicine has paved the way for precision drug development, where therapies are designed to target specific molecular pathways involved in disease progression. This approach allows pharmaceutical companies to develop more effective and efficient drugs by identifying patient subgroups that are more likely to respond positively to a particular treatment. By employing biomarkers and genetic profiling, drug developers can stratify patient populations, conduct targeted clinical trials, and accelerate the approval process for new therapies [1-3].
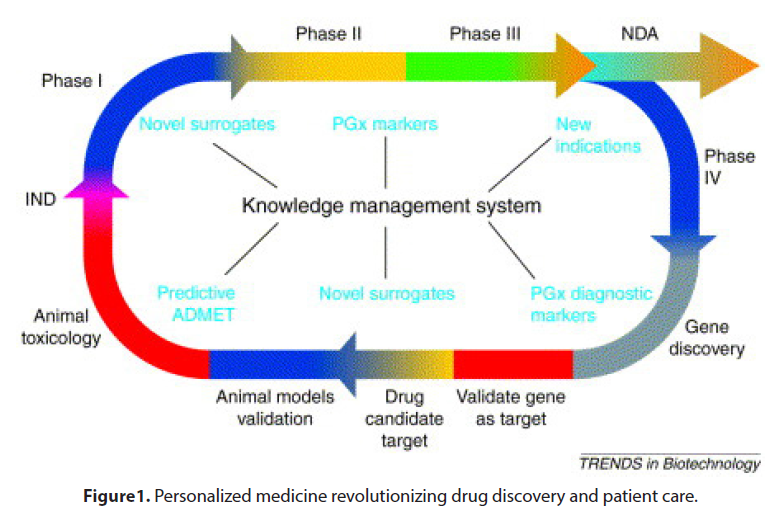
The advent of digital health technologies, such as wearable devices, mobile applications, and remote monitoring systems, has enabled the collection of real-time patient data. This data includes vital signs, activity levels, medication adherence, and patient-reported outcomes. Pharmaceutical companies are leveraging this wealth of information to gain insights into treatment effectiveness, identify potential side effects, and optimize therapy regimens. The integration of digital health technologies with personalized medicine has the potential to revolutionize patient care by enabling continuous monitoring and personalized interventions (Figure 1).
Advancements in digital health technologies have opened up new possibilities for personalized medicine. Wearable devices, smartphone applications, and remote monitoring systems allow for real-time tracking of patients’ health parameters, such as heart rate, blood pressure, glucose levels, and medication adherence. This data can provide valuable insights into an individual’s health status and response to treatment, enabling healthcare professionals to make informed decisions and adjust therapies as needed. Artificial intelligence (AI) and machine learning (ML) are revolutionizing drug discovery, clinical trials, and patient care in the pharmaceutical industry. AI algorithms can analyze vast amounts of patient data, including electronic health records, genomics, and medical imaging, to identify patterns and correlations. This knowledge helps researchers and pharmaceutical companies develop targeted therapies, predict drug responses, optimize dosages, and improve patient stratification for clinical trials. AI-powered tools also assist in identifying potential side effects and drug-drug interactions, enhancing drug safety [4-6].
Material & Methods
Artificial intelligence (AI) and machine learning (ML) algorithms are playing a pivotal role in personalized medicine. These technologies can analyze vast amounts of patient data, including genetic information, medical records, and clinical trial results, to identify patterns and make predictions. AI-powered tools can assist in drug discovery, optimize treatment algorithms, and predict patient responses to therapies. Additionally, ML algorithms can aid in the identification of novel drug targets and the repurposing of existing drugs for new indications, significantly reducing the time and cost of drug development.
Despite the tremendous potential of personalized medicine, several ethical and regulatory challenges need to be addressed. Issues related to patient privacy, data security, informed consent, and equitable access to personalized treatments require careful consideration. Regulatory bodies must adapt to the rapid advancements in personalized medicine to ensure safety, efficacy, and ethical standards are maintained. The rapid progress in personalized medicine is revolutionizing the pharmaceutical industry, ushering in an era of targeted therapies and improved patient care. Genomic medicine, precision drug development, digital health technologies, and AI-driven approaches are driving this transformation. However, it is crucial to address the ethical and regulatory challenges associated with personalized medicine to ensure its widespread adoption and benefit for all patients. As personalized medicine continues to evolve, it holds the potential to revolutionize disease prevention, diagnosis, and treatment strategies, ultimately leading to better health outcomes for individuals around the world.
Results
In recent years, there has been a remarkable surge in the development of targeted drug delivery systems, aiming to improve the efficacy and safety profiles of pharmaceutical therapies. These systems offer the potential to selectively deliver therapeutic agents to specific tissues or cells, thereby minimizing off-target effects and enhancing the therapeutic outcome. This article highlights the recent advances in targeted drug delivery systems and their impact on the field of pharmaceutical research and development. Nanoparticles have emerged as promising carriers for targeted drug delivery due to their unique physicochemical properties and ability to encapsulate a wide range of therapeutic agents. Various types of nanoparticles, including liposomes, polymeric nanoparticles, and inorganic nanoparticles, have been extensively explored. These nanoparticles can be surfacemodified with ligands or antibodies to target specific receptors or biomarkers overexpressed on diseased cells, enabling efficient drug delivery and enhanced therapeutic efficacy.
ADCs represent a sophisticated class of targeted drug delivery systems that combine the specificity of monoclonal antibodies with the potency of cytotoxic drugs. By attaching a cytotoxic drug to an antibody that recognizes a specific antigen on cancer cells, ADCs can selectively deliver the drug to tumor cells, minimizing systemic toxicity. Recent advancements in ADC technology, including the design of novel linkers and payloads, have improved their stability, specificity, and therapeutic index, leading to several successful clinical outcomes.
Discussion
Stimuli-responsive drug delivery systems have gained significant attention in recent years. These systems employ external stimuli or endogenous triggers to release drugs at specific sites or in response to particular physiological conditions. Examples include pH-responsive systems for targeted delivery to acidic tumor environments and temperature-responsive systems that release drugs upon mild hyperthermia. Such strategies allow precise control over drug release, optimizing therapeutic efficacy and minimizing side effects.
Targeted drug delivery is not limited to small molecule drugs but also extends to gene-based therapies. Viral vectors, non-viral vectors, and lipid-based systems have been developed to deliver therapeutic genes to specific cell types. Targeting ligands or antibodies can be incorporated into these systems to enhance cellular uptake and gene expression in the desired tissues. The advent of gene editing technologies like CRISPR-Cas9 has further accelerated the development of targeted gene delivery systems. Advances in targeted drug delivery systems have revolutionized the field of pharmaceutical research and development. These systems offer the potential to overcome longstanding challenges associated with drug efficacy and toxicity. Through nanoparticlebased formulations, antibody-drug conjugates, stimuli-responsive systems, and gene delivery approaches, targeted drug delivery has opened up new avenues for personalized medicine and improved patient outcomes. As the field continues to evolve, it holds immense promise for the future of pharmaceutical therapeutics [7- 9].
The field of pharmaceuticals has witnessed significant advancements in recent years, particularly in the realm of personalized medicine. Traditional approaches to drug development and treatment have often followed a one-size-fitsall model, but with the advent of personalized medicine, the industry is undergoing a profound transformation. This article explores the key developments in personalized medicine and their implications for the pharmaceutical industry.
The mapping of the human genome and subsequent advances in genomics has revolutionized the understanding of genetic factors in disease susceptibility, drug response, and treatment outcomes. Pharmacogenomics, a branch of personalized medicine, focuses on studying how an individual’s genetic makeup influences their response to drugs. Pharmaceutical companies are leveraging this knowledge to develop targeted therapies tailored to specific patient populations, resulting in improved efficacy and reduced adverse effects. Biomarkers play a crucial role in personalized medicine by identifying specific molecular indicators that can predict disease progression or response to treatment. Pharmaceutical companies are investing in research to identify and validate biomarkers that can guide therapy selection and monitoring. Diagnostic tests, including genetic tests and liquid biopsies, are being developed to detect biomarkers, enabling clinicians to make informed treatment decisions based on individual patient profiles.
Personalized medicine has prompted a shift in the traditional drug development paradigm. Pharmaceutical companies are increasingly focusing on developing drugs that target specific molecular pathways or genetic mutations associated with particular diseases. By identifying subgroups of patients who are likely to respond well to a specific drug, pharmaceutical companies can optimize clinical trial designs, streamline drug development timelines, and enhance overall success rates. The proliferation of digital health technologies, including wearable devices, smartphone applications, and remote monitoring tools, has opened new avenues for personalized medicine. These technologies enable continuous monitoring of patients’ health parameters, collection of real-time data, and early detection of disease progression. Pharmaceutical companies are exploring partnerships and collaborations with technology companies to integrate digital health technologies into their drug development processes and improve patient outcomes [10-13].
The rise of personalized medicine presents unique regulatory and ethical challenges for the pharmaceutical industry. Regulatory bodies are adapting to the evolving landscape by developing guidelines for the approval and commercialization of personalized therapies. Ethical considerations related to data privacy, consent, and equitable access to personalized treatments also needs to be addressed to ensure the responsible and ethical implementation of personalized medicine. Personalized medicine is transforming the pharmaceutical industry by shifting the focus from a one-size-fits-all approach to individualized patient care. Genomics, biomarkers, precision drug development, digital health technologies, and evolving regulatory frameworks are driving this paradigm shift.
As personalized medicine continues to evolve, pharmaceutical companies have the opportunity to develop innovative therapies with higher efficacy and safety profiles, ultimately improving patient outcomes and revolutionizing healthcare delivery. In recent years, significant progress has been made in the field of targeted drug delivery systems, revolutionizing the pharmaceutical industry. These innovative approaches aim to enhance drug efficacy, reduce side effects, and improve patient outcomes. By precisely delivering therapeutic agents to specific cells or tissues, targeted drug delivery systems have the potential to transform the treatment of various diseases, ranging from cancer to chronic conditions like diabetes and cardiovascular disorders. This article explores some of the latest advancements in this exciting field and their implications for the future of medicine.
In recent years, significant progress has been made in the field of targeted drug delivery systems, revolutionizing the pharmaceutical industry. These innovative approaches aim to enhance drug efficacy, reduce side effects, and improve patient outcomes. By precisely delivering therapeutic agents to specific cells or tissues, targeted drug delivery systems have the potential to transform the treatment of various diseases, ranging from cancer to chronic conditions like diabetes and cardiovascular disorders. This article explores some of the latest advancements in this exciting field and their implications for the future of medicine [14-16].
Nanotechnology has emerged as a promising tool in pharmaceutical research, offering precise drug delivery and controlled release mechanisms. Nanoparticles, liposomes, and polymeric micelles are examples of nanoscale carriers that can encapsulate drugs and navigate through biological barriers to reach targeted sites. By optimizing the physicochemical properties of these carriers, researchers have achieved improved drug stability, prolonged circulation times, and enhanced tissue penetration. Moreover, surface modifications allow for targeted binding to specific cells or tissues, enabling site-specific drug release and minimizing off-target effects. Antibody-drug conjugates (ADCs) represent a groundbreaking approach in cancer therapy. ADCs consist of monoclonal antibodies designed to recognize specific tumor antigens, linked to potent cytotoxic drugs. This strategy allows for targeted delivery of highly toxic compounds directly to cancer cells while sparing healthy tissues. Recent advancements in ADC technology have led to the development of novel linkers, improved antibody design, and enhanced payload potency, resulting in improved therapeutic efficacy and reduced systemic toxicity.
MRNA-based vaccines have gained tremendous attention during the COVID-19 pandemic, with the successful development and deployment of vaccines against the SARS-CoV-2 virus. These vaccines utilize messenger RNA (mRNA) to instruct cells to produce viral proteins that trigger an immune response. The mRNA is encapsulated in lipid nanoparticles, enabling efficient delivery into cells. This groundbreaking technology not only allows for rapid vaccine development but also holds promise for the treatment of other infectious diseases, as well as cancer immunotherapy.
Advancements in genomics and personalized medicine have transformed the field of pharmacology. Pharmacogenomics involves studying how an individual’s genetic makeup influences their response to drugs. By identifying genetic markers and variations associated with drug metabolism and efficacy, healthcare providers can tailor treatments to a patient’s specific genetic profile. This approach minimizes adverse drug reactions, optimizes therapeutic outcomes, and enhances patient safety.
Targeted drug delivery systems have opened up new avenues in pharmaceutical research and development, enabling the delivery of therapeutic agents with unprecedented precision. Nanotechnology, antibody-drug conjugates, mRNA-based vaccines, and personalized medicine are just a few examples of the transformative advancements in this field. As researchers continue to innovate and refine these technologies, we can expect more effective treatments with fewer side effects, revolutionizing patient care and improving health outcomes in the years to come.
Personalized medicine, also known as precision medicine, is revolutionizing the field of healthcare and transforming the pharmaceutical industry. This innovative approach tailors medical treatment and drug therapies to an individual’s specific characteristics, including their genetic makeup, lifestyle, and environmental factors. By moving away from the traditional one-size-fits-all model, personalized medicine holds the promise of improving patient outcomes, enhancing drug efficacy, and reducing adverse reactions. In this article, we will explore the key advancements in personalized medicine and their impact on the pharmaceutical landscape [17-19].
The emergence of genomics has paved the way for personalized medicine. Genomic sequencing technologies enable the analysis of an individual’s DNA, identifying genetic variations that may influence drug responses. Pharmacogenomics, a subset of genomics, focuses on studying how genes affect a person’s response to medications. By understanding these genetic variations, pharmaceutical companies can develop drugs that target specific molecular pathways and deliver optimal therapeutic outcomes for patients. Biomarkers play a crucial role in personalized medicine by serving as indicators of disease presence, progression, or response to treatment. They can be genetic, molecular, or cellular in nature. Pharmaceutical companies are increasingly utilizing biomarkers to develop companion diagnostics, which help identify patients who are likely to respond to a particular therapy. These diagnostic tools enable healthcare providers to prescribe medications that have a higher probability of success, thereby avoiding ineffective treatments and minimizing side effects [20].
Conclusion
Recent breakthroughs in gene editing technologies, such as CRISPR-Cas9, have the potential to revolutionize the treatment of genetic disorders. Gene therapies involve modifying or replacing defective genes to restore normal cellular functions. Pharmaceutical companies are investing in gene therapies that can provide longlasting or permanent solutions to previously untreatable conditions. Personalized gene therapies hold immense promise for diseases with a strong genetic component, including certain cancers, inherited disorders, and rare diseases. The advent of personalized medicine represents a paradigm shift in the pharmaceutical industry. By harnessing the power of genomics, biomarkers, digital health, AI, and gene editing, pharmaceutical companies can develop targeted therapies, reduce healthcare costs, and improve patient outcomes. As personalized medicine continues to evolve, it is expected to reshape drug discovery, clinical practice, and healthcare delivery, ultimately leading to a more patientcentric and effective approach to treating diseases.
Acknowledgement
None
Conflict of Interest
None
References
- Van den Anker J, Reed MD, Allegaert K et al. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J Clin Pharmacol. 58, 10-25 (2018).
- Aagaard L. Off-Label and Unlicensed Prescribing of Medicines in Paediatric Populations: Occurrence and Safety Aspects. Clin Pharmacol Toxicol. 117, 215–218(2015).
- Gore R, Chugh PK, Tripathi CD. Pediatric Off-Label and Unlicensed Drug Use and Its Implications. Curr Clin Pharmacol.12, 18–25 (2018).
- Sketris, IS. American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 67, 674–694 (2019).
- Hill-Taylor B, Walsh KA, Stewart S et al. Effectiveness of the STOPP/START (Screening Tool of Older Persons’ potentially inappropriate Prescriptions/Screening Tool to Alert doctors to the Right Treatment) criteria: Systematic review and meta-analysis of randomized controlled studies. JClin PharmTher. 41, 158–169 (2016).
- Tommelein E, Mehuys E, Petrovic M et al. Potentially inappropriate prescribing in community-dwelling older people across Europe: A systematic literature review. Eur J Clin Pharmacol. 71, 1415–1427.
- Prot-Labarthe S, Weil T, Angoulvant F et al. POPI (Pediatrics: Omission of Prescriptions and Inappropriate prescriptions): Development of a tool to identify inappropriate prescribing. PLoS ONE. 9,25-68.
- Corrick F, Conroy S, Sammons H et al. Paediatric Rational Prescribing: A Systematic Review of Assessment Tools. Int J Environ Res Public Health. 17, 1473-1496 (2015).
- Sadozai L, Sable S, Le E Roux et al. International consensus validation of the POPI tool (Pediatrics: Omission of Prescriptions and Inappropriate prescriptions) to identify inappropriate prescribing in pediatrics. PLoS ONE .15, 47-72 (2018).
- Barry E, Moriarty F, Boland F et al. The PIPc Study-application of indicators of potentially inappropriate prescribing in children (PIPc) to a national prescribing database in Ireland: A cross-sectional prevalence study. BMJ Open. 8, 69-556 (2019).
Indexed at, Google Scholar, Cross ref
Indexed at, Google Scholar, Cross ref
Indexed at, Google Scholar, Cross ref
Indexed at, Google Scholar, Cross ref
Indexed at, Google Scholar, Cross ref
Indexed at, Google Scholar, Cross ref