Research Article - Pharmaceutical Bioprocessing (2016) Volume 4, Issue 5
Adherence to antihypertensive medication in the older hypertensive patients: the role of blood pressure measurement assistance
- Corresponding Author:
- Alexander E.
Berezin
Internal Medicine Department, Medical University of Zaporozhye, Ukraine
E-mail: dr_berezin@mail.ru
Abstract
Aim: To evaluate the compliance of patients with hypertension retirement age recommended antihypertensive drug therapy, to develop methods of its increase in the outpatient treatment and medical supervision to ensure the effectiveness of treatment Methods: One hundred fourteen older persons with documented mild-to-moderate hypertension were enrolled in the study. Office blood pressure (BP) was measured at the visit of the patents in the office by physician using sphygmomanometer “OMRON” (Japan) accordingly conventional technique. Morisky Medication Adherence Scales (MMAS)-4 was used to measure the adherence to antihypertensive drugs at baseline and within 8 weeks. Education assistance regarding use of conventional methods for life style modification, selfmeasurement of blood pressure and regular exposure of antihypertensive remedies was driven within 6 weeks after baseline. All subjects have completed diary to control of BP at home within treatment course and they have opportunity to contact with the physician when needed. Results: All enrolled hypertensive subjects were predominantly female (51.8%) and have exhibited mean age equal 67.3 years. At least 26% of patient population were smokers, 25% of individuals were obese. Diabetes and metabolic syndrome were defined 14% and 14% respectively, and antidiabetic drug (metformin) was given 4% of the patients in the entire group. Accordingly Morisky Medication Adherence Scales 51.8% of the entire group patients have exhibited medium adherence to antihypertensive treatment and 48.2% have demonstrated low adherence to one. No any significant differences were found between both cohorts of the individuals at baseline. Morisky Medication Adherence Scale-4 at 8 week has significantly improved and exhibited the statistically significant increase of patient number with medium adherence to antihypertensive treatment and decrease of frequency of low adherence to remedy taking. Conclusion: we found that the assistance of the older patients to measure BP and analyse of the BP control state might improve sufficiently the adherence of the persons to antihypertensive treatment strategy.
Keywords
hypertension, antihypertensive drugs, adherence, efficacy
Introduction
The management of hypertension is addressed to decline of cardiovascular (CV) risk, prevention of CV complications, improve of well-being and quality-of-life through achieving full control of blood pressure elevation, attenuation of comorbidities’ development and diminish an effect of CV risk factors [1]. Although there are broad choosing of drugs for initial and maintenance treatment of hypertension, recent clinical guidelines indicate that up to 15-20% of the hypertensive patients are not controlled on dual antihypertensive fixed and none-fixed combinations [2,3]. Moreover, triple or more combinations of different antihypertensive drug classes are required to achieve adequate control in blood pressure [4]. On this way, adherence to antihypertensive therapy is crucial for clinical efficacy of the medical care [5]. Education level, cognitive function, side effect of the drugs, economic reasons are discussed as factors contributing to compliance of the hypertensive patients in short and long-term perspectives [6-8]. The aim of our study: to evaluate the compliance of patients with hypertension retirement age recommended antihypertensive drug therapy, to develop methods of its increase in the outpatient treatment and medical supervision to ensure the effectiveness of treatment.
Methods
The single site cross-sectional study has used a simple selection of 114 older (age higher 60 years) hypertensive persons from 236 older individuals who have admitted the ambulatory aimed the measurement of blood pressure. Subjects with normal office blood pressure (<140 / 90 mm Hg) beyond current any antihypertensive drugs’ exposure and patients with fully controlled hypertension were excluded Controlled hypertension was defined as a systolic BP of < 140 mmHg and a diastolic BP < 90 mmHg and uncontrolled hypertension as a systolic BP ≥140 mmHg and/or a diastolic BP ≥90 mmHg [9]. Cognitive impairment, unable to understand the study protocol, known cerebral and vascular diseases, severe and resistant hypertension were defined as criteria of excluding of the subjects in the study.
All enrolled patients were categorized as persons with mild hypertension (n=58) or moderate hypertension (n=56) accordingly current clinical guideline [9].
Socio-demographic data were obtained at the start of the study. Current smoking was defined as consumption of one cigarette daily for three months. Anthropometric measurements (body mass, waist circumference, weight, and waist-tohip ratio) were made using standard procedures.
MetS was diagnosed based on the National Cholesterol Education Program Adult Treatment Panel III criteria [10]. Patients were enrolled in the MetS cohort when at least three of the following components were defined: waist circumference ≥90 cm or ≥80 cm in men and women respectively; high density lipoprotein (HDL) cholesterol <1.03 mmol/L or <1.3 mmol/L in men and women respectively; triglycerides ≥1.7 mmol/L; blood pressure ≥130/85 mmHg or current exposure of antihypertensive drugs; fasting plasma glucose ≥5.6 mmol/L. Participants who had less than 2 criteria of metabolic syndrome were classified as metabolically healthy; those who had 2 or more criteria of metabolic syndrome were classified as metabolically abnormal.
T2DM was diagnosed with revised criteria (glycated hemoglobin [HbA1c] ≥6.5%; fasting plasma glucose ≥7 mmol/L; 2-h plasma glucose ≥11.1 mmol/L during an oral glucose tolerance test; a random plasma glucose ≥11.1 mmol/L; exposure of insulin or oral antidiabetic drugs; a previous diagnosis of T2DM) provided by American Diabetes Association [11].
Office blood pressure (BP) measurement
Office blood pressure (BP) was measured at the visit of the patents in the office by physician using sphygmomanometer “OMRON” (Japan) accordingly conventional technique [9].
Self-measured adherence to antihypertensive treatment
We used Morisky Medication Adherence Scales (MMAS)-4 to measure the adherence to antihypertensive drugs at baseline and within intervention period [12]. The MMAS-4 asks patients to respond with ‘‘yes” or ‘‘no” to a set of 4 questions. High adherence has determined when zero value of MMAS-4 score was found. Average MMAS-4 score between 1-2 values and 3-4 values has exhibited medium and low adherence respectively.
Intervention
Education assistance regarding use of conventional methods for life style modification, self-measurement of blood pressure and regular exposure of antihypertensive remedies was driven within 6 weeks after baseline [3,4]. All subjects have completed diary to control of BP at home within treatment course and they have opportunity to contact with the physician when needed.
Ethical declaration
The study protocol was approved by the Donetsk Medical University Ethnics committee review board. The study complied with the Declaration of Helsinki and voluntary informed written consent was obtained from all patients included in this study.
Statistical Analysis
Statistical analysis of the results obtained was performed in SPSS system for Windows, Version 22 (SPSS Inc, Chicago, IL, USA). The data were presented as mean (М) and error of mean (± EM) or 95% confidence interval (CI); median (Ме) and interquartile range (IQR). The hypothesis of normal distribution of the parameters analyzed was checked by means of Shapiro–Wilk test and Kolmogorov-Smirnov test.
To compare the main parameters of patients’ groups (subject to the type of distribution of the parameters analyzed), one-tailed Student t-test or Shapiro–Wilk U-test were used. To compare categorical variables between groups, Chi2 test (χ2) and Fisher F exact test were used. A calculated difference of P<0.05 was considered significant.
Results and Discussion
The basic patients’ characteristics are reported in the Table 1. As one can see, all enrolled hypertensive subjects were predominantly female (51.8%) and have exhibited mean age equal 67.3 years. At least 26% of patient population were smokers, 25% of individuals were obese. Therefore, T2DM and MetS were defined 14% and 14% respectively, and antidiabetic drug (metformin) was given 4% of the patients in the entire group.
| Variable | Entire group (n=114) | Cohort 1 (n=58) | Cohort 2 (n=56). | P value between both cohorts |
|---|---|---|---|---|
| Age, years | 67.3 (63 – 71) | 67.6 (63 – 73) | 66.9 (63 – 71) | NS |
| Male, n (%) | 55 (48.2%) | 28 (48.3%) | 27 (48.2%) | NS |
| Systolic BP, mm Hg | 158.5 ± 8.8 | 144.3 ± 4.1 | 169.1 ± 3.8 | NS |
| Diastolic BP, mm Hg | 101.2 ± 6.5 | 98.0 ± 2.7 | 105.0 ± 2.4 | NS |
| Smoking, n (%) | 30 (26.3%) | 11 (18.9%) | 19 (33.9%) | <0.01 |
| T2DM, n (%) | 16 (14.0%) | 4 (6.9%) | 12 (21.4%) | <0.001 |
| MetS, n (%) | 16 (14.0%) | 5 (8.6%) | 11 (19.6%) | <0.001 |
| Obesity, n (%) | 29 (25.4%) | 10 (17.2%) | 19 (33.9%) | <0.001 |
| Thiazide-like diuretics, n (%) | 72 (63.2%) | 21 (36.2%) | 51 (91.1%) | <0.001 |
| ACE inhibitors, n (%) | 92 (80.7%) | 42 (72.4%) | 50 (89.3%) | NS |
| ARBs, n (%) | 22 (19.3%) | 16 (27.6%) | 6 (10.7%) | <0.001 |
| CCBs, n (%) | 18 (15.8%) | 5 (8.6%) | 13 (23.2%) | <0.001 |
| Metformin, n (%) | 5 (4.4%) | 0 | 5 (8.9%) | <0.001 |
Note: Cohort 1, Mild hypertension cohort; cohort 2, Moderate hypertension
Table 1: Basic characteristics of the study patient population.
The treatment strategy of the patients has based on life style modification and orally used antihypertensive drugs taking. Thiazide-like diuretics and ACE inhibitors have prescribed frequently (63.2% and 80.7% respectively). ARBs and CCBs were used rarely, whereas non fixed or fixed combination based on ARBs / CCBs using were determined at least 50% of all patients enrolled in the study. There are not sufficient differences between both cohorts of the patients in age, sex, CV risk factors, and remedies.
The results of Morisky Medication Adherence Scale-4 in patients study population at baseline are reported in the Table 2. Accordingly scales of the questioner 51.8% of the entire group patients have exhibited medium adherence to antihypertensive treatment and 48.2% have demonstrated low adherence to one. No any significant differences were found between both cohorts of the individuals at baseline.
| Variables | Entire group (n=114) | Mild hypertension cohort (n=58) | Moderate hypertension (n=56). | P value between both cohorts |
|---|---|---|---|---|
| Questions | ||||
| Do you ever forget to take your medicine? | 111 (97.4%) | 57 (98.3%) | 54 (96.4%) | NS |
| Are you careless at times about taking your medicine? | 54 (47.4%) | 25 (43.1%) | 29 (51.8%) | NS |
| Sometimes if you feel worse when you take the medicine, do you stop taking it? | 15 (13.2%) | 8 (13.8%) | 7 (12.5%) | NS |
| When you feel better do you sometimes stop taking your medicine? | 8 (7.0%) | 4 (6.9%) | 4 (7.1%) | NS |
| Adherence scale | ||||
| High Adherence, n (%) | 0 | 0 | 0 | - |
| Medium Adherence, n (%) | 59 (51.8%) | 30 (51.7%) | 29 (51.8%) | NS |
| Low Adherence, n (%) | 36 (48.2%) | 28 (48.3%) | 27 (48.2%) | NS |
Table 2: Morisky Medication Adherence Scale-4 in patients study population at baseline.
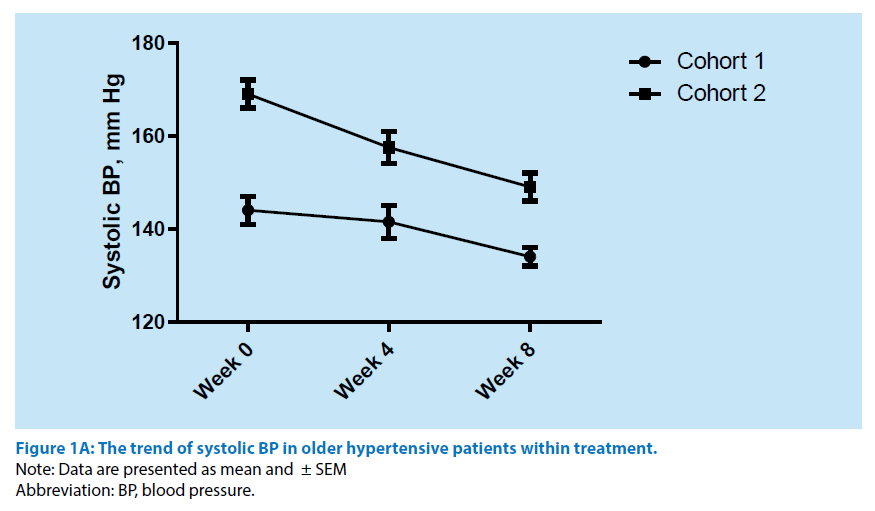
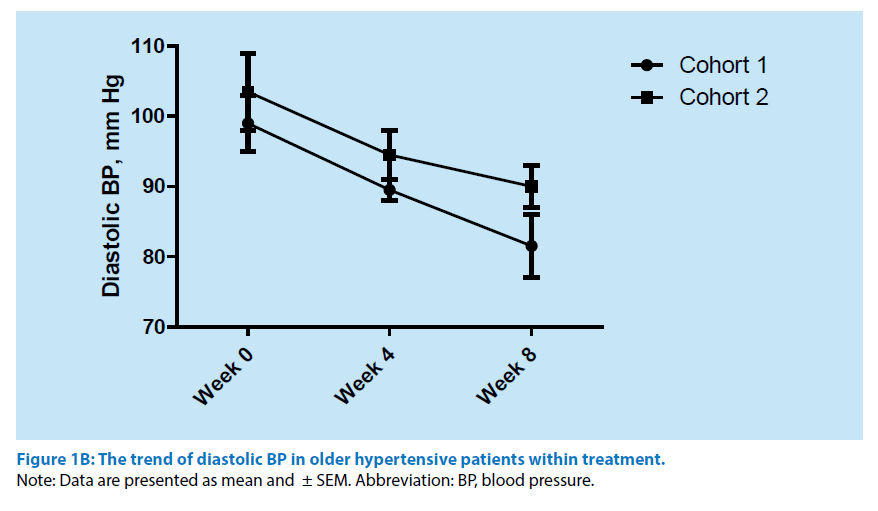
Within 8 weeks of the treatment course the evaluation of office BP trend was determined (Figure 1A and 1B). All persons included in the cohort 1 have exhibited a full control hypertension. In contrast, at least 23 patients (41.1%) from the cohort 2 were found to be associated with full control hypertensive state. However, the BP assistance leaded to improving office BP level in short term perspective. Furthermore, Morisky Medication Adherence Scale-4 at 8 week was analysed also. The results of the test have shown the statistically significant increase of patient number with medium adherence to antihypertensive treatment and decrease of frequency of low adherence to remedy taking (Table 3).
| Variables | Cohorts | At baseline | AT the study end | P value |
|---|---|---|---|---|
| High Adherence, n (%) | 1 (n=58) | 0 | 2 (3.4%) | <0.001 |
| 2 (n=56) | 0 | 0 | - | |
| Medium Adherence, n (%) | 1 (n=58) | 30 (51.7%) | 38 (65.5%) | 0.046 |
| 2 (n=56) | 29 (51.8%) | 39 (69.6%) | 0,044 | |
| Low Adherence, n (%) | 1 (n=58) | 28 (48.3%) | 18 (31.1%) | 0.048 |
| 2 (n=56) | 27 (48.2%) | 17 (30.4%) | 0.046 |
Table 3: Morisky Medication Adherence Scale-4 in patients study population within treatment.
Figure 1A: The trend of systolic BP in older hypertensive patients within treatment.
Note: Data are presented as mean and ± SEM
Abbreviation: BP, blood pressure.
Figure 1B: The trend of diastolic BP in older hypertensive patients within treatment.
Note: Data are presented as mean and ± SEM. Abbreviation: BP, blood pressure.
Recently it has been found that improving medication adherence may lower visit-to-visit variability of BP [13] and, however, increase clinical efficacy of hypertension treatment [14]. We suggested that BP measurement assist could be a simple and affordable method toward merging adherence to antihypertensive treatment. The results of the study have clarified that older hypertensive individuals are candidates to optimize the response to physician prescribing via self-measured BP evaluation. Self-completed diary presented by patient to physician at the visit to the office might help to consolidate efforts of both sides on the treatment strategy: patient and physician. Moreover, in order to achieve optimal BP control and reduce co-occurring conditions associated with BP elevation adherence to antihypertensive drugs are validated an essential tool [15]. Whether similar approach would be effective to reduce urgent admission in older persons is not fully understand that requires more investigations.
In conclusion, we found that the assistance of the older patients to measure BP and analyse of the BP control state might improve sufficiently the adherence of the persons to antihypertensive treatment strategy.
Acknowledgement
We thank all patients for their participation in the investigation, staff of the Regional Donetsk Hospital (Ukraine) and the doctors, nurses, and administrative staff in University Hospital (Donetsk, Ukraine), general practices, and site-managed organizations that assisted with the study.
Authors’ contributions
Elena N Nalotova initiated the hypothesis and designed the study protocol, contributed to analyze and interpret the data, and wrote the manuscript. Michail M Alesinskiy contributed to enroll the patients, collected and analyzed the data, checked clinical events, reviewed the source documents, and performed statistical analysis; Alexander E. Berezin interpreted the obtained results. Sergey V Nalotov contributed to enroll the patients in the study and analyzed the results of examinations.
Note
This research received no specific grant from any funding agency in the public, commercial, or not-forprofit sectors.
References
- Pagliaro B, Santolamazza C, Rubattu S, Volpe M. New therapies for arterial hypertension. Panminerva. Med. 58(1), 34-47 (2016).
- Van Camp G, Pasquet A, Sinnaeve P, et al. Summary 2015 ESC guidelines. Acta. Cardiol. 71(1), 7-13 (2016).
- Daskalopoulou SS, Khan NA, Quinn RR, Ruzicka M, McKay DW, Hackam DG, et al. The 2013 Canadian hypertension education program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention and treatment of hypertension. Can. J. Cardiol. 28, 270-287 (2012).
- Kim Y, Kong KA. Do Hypertensive Individuals Who Are Aware of Their Disease Follow Lifestyle Recommendations Better than Those Who Are Not Aware? PLoS One. 10(8), e0136858 (2015).
- Wannasirikul P, Termsirikulchai L, Sujirarat D, Benjakul S, Tanasugarn C. Health literacy, medication adherence, and blood pressure level among hypertensive older adults treated at primary health care centers. Southeast. Asian. J. Trop. Med. Public. Health. 47(1), 109-20 (2016).
- Doering BK, Szécsi J, Bárdos G, Köteles F. Somatosensory Amplification Is a Predictor of Self-Reported Side Effects in the Treatment of Primary Hypertension: a Pilot Study. Int. J. Behav. Med. 23(3), 327-332 (2016).
- Conn VS, Ruppar TM, Chase JA, Enriquez M, Cooper PS. Interventions to Improve Medication Adherence in Hypertensive Patients: Systematic Review and Meta-analysis. Curr. Hypertens. Rep. 17(12), 94 (2015).
- Krevolin L, Ilagan J. Blood pressure control. Improving medication compliance among ESRD patients. Nephrol. News. Issues. 29(9), 33-36 (2015).
- Mancia G, Fagard R, Narkiewicz K, et al. Task Force Members, 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertension. 311, 281-357 (2013).
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report, Circulation. 106, 3143-421 (2002).
- American Diabetes Association. Classification and Diagnosis of Diabetes. Diabetes. Care. 39 Suppl 1, S13-22 (2016).
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med. Care. 24, 67-74 (1986).
- Kronish IM, Lynch AI, Oparil S, et al. The Association Between Antihypertensive Medication Nonadherence and Visit-to-Visit Variability of Blood Pressure: Findings From the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Hypertension (2016).
- Meinema JG, van Dijk N, Beune EJAJ, Jaarsma DADC, et al. Determinants of Adherence to Treatment in Hypertensive Patients of African Descent and the Role of Culturally Appropriate Education. PloS. ONE. 10(8): e0133560 (2015).
- Li YT, Wang HH, Liu KQ, et al. Medication Adherence and Blood Pressure Control Among Hypertensive Patients With Coexisting Long-Term Conditions in Primary Care Settings: A Cross-Sectional Analysis. Medicine (Baltimore). 95(20): e3572 (2016).