Case Report - International Journal of Clinical Rheumatology (2018) Volume 13, Issue 4
A rare case of Sjögren's Syndrome associated with Intracranial Hemorrhage- case report and literature review
- *Corresponding Author:
- Diana M. Girnita
Department of Rheumatology
Trihealth Physician Partners
Cincinnati, Ohio, USA
E-mail: Diana_girnita@trihealth.com
Abstract
Sjögren Syndrome (SS) is an autoimmune rheumatic disease, whose hallmark is ocular and mouth dryness secondary to autoimmune exocrinopathy. Rarely, SS can affect both peripheral nervous system (PNS) as well as central nervous system (CNS). When CNS is involved, SS is usually diagnosed after patients present with neurologic symptoms. Both ischemic and hemorrhagic stroke can present as first manifestation of primary SS. Stroke is described in literature as a rare entity associated with primary SS, and only few cases were reported. In some cases, CNS involvement was thought to be related to CNS vasculitis, but most of the times remains questionable. We present a rare case of intracranial hemorrhage as first manifestation of primary SS and a review of the pertinent literature.
Introduction
Sjögren Syndrome (SS) is an autoimmune rheumatic disease, whose hallmark is ocular and mouth dryness secondary to autoimmune exocrinopathy [1]. These symptoms are due to excessive lymphocyte infiltration. SS typically affects women after 40 years of age, but it has also been reported in young adults and adolescents. Serologic markers are positive Ro/ SSA, La/SSB and antinuclear (ANA) antibodies. SS can also affect extra glandular systems such as neurologic system. While Peripheral Nervous System (PNS) involvement is often described, CNS manifestations are controversial and extremely rare [2]. The incidence of stroke in patients with primary SS is about 2% [3]. Based on a PubMed search, only 15 cases of stroke were described in primary SS since 1982. Out of these cases, five patients presented with subarachnoid hemorrhage and two patients with intracranial hemorrhage (ICH). Here we report a case of ICH as first manifestation of primary SS.
Case presentation
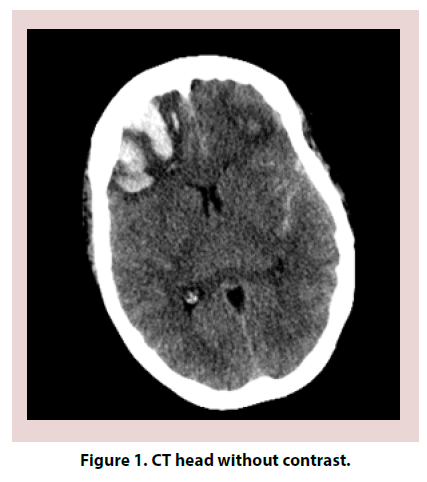
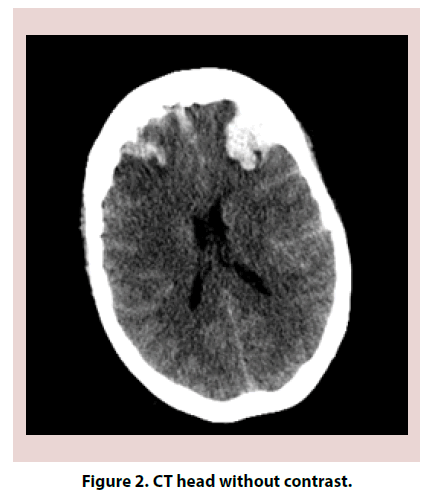
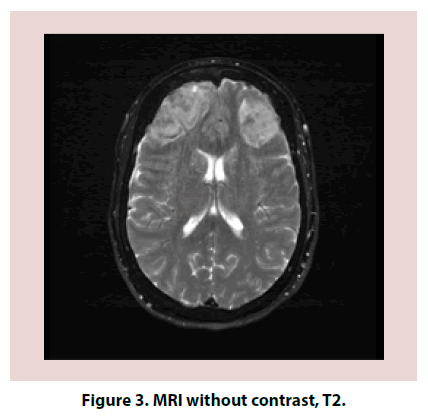
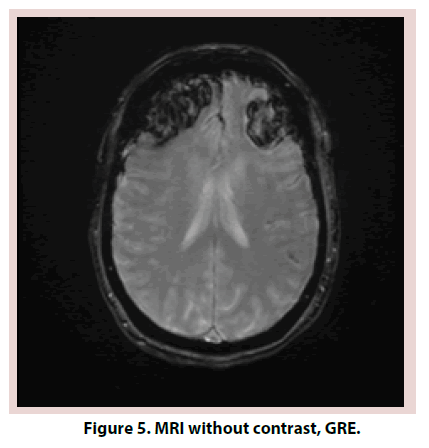
A 61-year-old Caucasian female with a past medical history significant for Hashimoto thyroiditis, cervical cancer, osteoarthritis and migraines presented in the emergency room for worsening headaches. Patient was treated for one year with sumatriptan and topiramate for migraines. Within the previous year, she had both Computed Tomography (CT) head and magnetic resonance imaging MRI brain that were reportedly normal. Despite treatment, headaches worsened and interfered with her work as an accountant. On the day of admission, she also complained of dizziness, nausea and vomiting, photophobia. On examination, patient was confused, lethargic and requiring repeated stimulation to answer questions. Neurologic exam revealed 5/5 strength in all extremities, no pronator drift, no change in sensation, patellar deep tendon reflexes (DTR) 2+ bilaterally and negative bilateral Babinski. Electroencephalogram (EEG) was negative for acute seizures. CT head without contrast revealed subarachnoid hemorrhage (SAH) (Figures 1 and 2). Brain MRI was obtained and was consistent with SAH in left posterior fossa, bifrontal intracranial hemorrhage (ICH) and no evidence of midline shift (Figures 3-5). Neurosurgery was consulted, and cerebral angiogram was performed which revealed normal four vessel cerebral angiogram. MR Venogram of brain was negative for thrombosis. Neurosurgery performed right craniotomy and evacuation of the right frontal hematoma with cortical and dural biopsies. Following that, a detailed review of systems revealed long history of eye dryness (she had laser surgery and was applying lubricant drops in her eyes for many years), mouth dryness (using very frequent mint drops) and occasionally parotid gland swelling, along with multiple tooth decays, cavities, and halitosis. Over the past two to three months, the patient also noticed an unintentional 20-pound weight loss, decreased appetite, night sweats, jaw and axilla pain and short term memory loss.
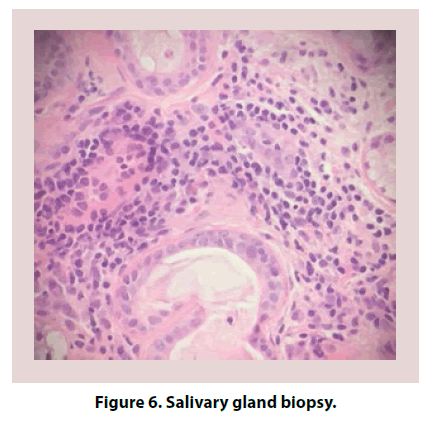
Due to concern of CNS vasculitis process, an autoimmune work up was performed and revealed positive ANA (titer 1: 160, speckled pattern) and SSA. All other autoantibodies were negative (Table 1). Complement levels were normal. Both Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP) were elevated. Salivary gland biopsy confirmed lymphocytic infiltration consistent with a diagnosis of primary SS (Figure 6). Given the sudden onset of intracranial hemorrhage and newly diagnosed primary SS, the suspicion of associated CNS vasculitis persisted. Further work up with brain and leptomeningeal biopsy was negative for vasculitis, amyloid deposition or neoplasia.
| Lab | Result | Reference Value |
|---|---|---|
| CRP | 34 | 3-24 mg/L |
| ESR | 54 | 0-30 mm/h |
| ANA | Positive, titers 1: 160 homogenous | Negative |
| Anti SSA/Ro antibody | 73 | 0-40 AU/mL |
| Anti SSB/La antibody | 2 | 0-40 AU/mL |
| Anti dsDNA antibody | Negative | Negative |
| Anti-Smith antibody | 0 | 0-40 AU/mL |
| Rheumatoid factor | <20 | <20 IU/mL |
| Anti CCP antibody | 14 | 0-19U |
| Anti Scl antibody | 2 | 0-40 AU/mL |
| Anti-centromere antibody | 1 | 0-40 AU/mL |
| Ant phospholipid antibodies | negative | negative |
| Anti RNP antibody | 1 | 0-40 AU/mL |
| ANCA | <1: 20 | <1: 20 |
| Complement C3 | 93 | 79-152 mg/dL |
| Complement C4 | 18 | 16-38 mg/dL |
CRP= C-reactive protein; ESR=erythrocyte sedimentation rate, ANA=antinuclear antibody, dsDNA= double stranded DNA, CCP=cyclic citrullinated peptide, RNP= ribonucleic protein, ANCA= antineutrophilic cytoplasmic antibodies
Table 1. Autoimmune work up.
During this time, patient was started on high dose IV methylprednisolone for three days, followed prednisone 60 mg daily. Within the first week, the patient mentation improved significantly. She was discharged to acute rehabilitation on prednisone taper, levetiracetam and all previous home medications. Repeat cerebral angiogram was performed in one month after discharge from the hospital and was also normal with no evidence for primary CNS vasculitis.
Discussion
Neurologic and psychiatric involvement in primary SS varies between 0%-70% according to reports and depends on their clinics, but generally affects 20% of the patients [1]. PNS involvement can manifest as axonal polyneuropathies, sensory ganglioneuronopathy, motor neuropathy, small-fiber neuropathy, multiple mononeuritis, trigeminal and other cranial neuropathies, autonomic neuropathies, or demyelinating polyradiculoneuropathy [1].
CNS involvement can be divided into focal (seizures, movement disorders, cerebellar syndrome, optic neuropathy, etc.), multifocal (cognitive impairment, encephalopathy, dementia, aseptic meningoencephalitis), spinal cord dysfunctions, MS-like syndrome and CNS vasculitis [1].
CNS involvement occurs mostly in women, with a mean age of 40-50-years-old. It usually manifests after 10-11 years of disease. The focal manifestation is the most common CNS manifestation [1]. The initial work-up that should be done when neurologic primary SS is suspected consists of autoimmune serologies. Anti-Ro antibodies are associated with severity of disease as well as abnormal findings suggestive of small vessel angiitis seen on cerebral angiography [4].
Lumbar puncture may be considered, however is nonspecific. Cerebrospinal fluid (CSF) analysis may show increased lymphocytosis, oligoclonal bands number and increased IgG index [5-7].
EEG has limited value and is more useful in detecting subclinical abnormalities that precede the development of clinical primary SS with CNS manifestations [5].
In terms of imaging studies, MRI is more sensitive than CT scan. MRI findings include anatomical abnormalities, increased signal intensity (T2) predominantly in subcortical and periventricular white matter, cerebral venous thrombosis or transverse myelitis.
MRI is also useful for detecting focal CNS involvement, more than diffuse CNS disease [5,8,9]. Cerebral angiography is helpful to exclude other causes of CNS disease however only about 45% of patients with primary SS and CNS disease have findings suggestive of small vessel vasculitis.
Stroke is an extremely rare manifestation of primary SS It can be ischemic or hemorrhagic. Since 1982, fifteen cases have been reported including ours [3,10-21]. Out of these, six patients presented with SAH, three patients with cerebral infarction, four patients with venous sinus thrombosis, two patients with ICH and one of them with thalamic hemorrhage [3]. In all these reported cases, patients were females (Tables 2-5).
| Lab | Result | Reference Value |
|---|---|---|
| WBC | 15.8 | 3.60 -10.50 thou/mcL |
| RBC | 4.32 | 3.80-5.20 thou/mcL |
| Hemoglobin | 12.8 | 12.0-15.2 g/dL |
| Hematocrit | 38 | 36%-46% |
| MCV | 88 | 82-97 fL |
| Platelet | 273 | 140-375 thou/mcL |
| Absolute neutrophil count | 14.60 | 1.80-7.70 thou/mcL |
| Absolute lymphocyte count | 0.90 | 1.00-4.00 thou/mcL |
| Absolute monocyte count | 0.40 | 0.20-0.90 thou/mcL |
| Absolute eosinophil count | 0.00 | 0.03-0.45 thou/mcL |
| Absolute basophil count | 0.00 | 0.00-0.20 thou/mcL |
WBC= white blood cell count, RBC= red blood cell count, MCV=mean corpuscular volume
Table 2. Complete blood count with differential.
| Lab | Result | Reference Value |
|---|---|---|
| Sodium | 134 | 135-145 mEq/L |
| Potassium | 3.7 | 3.6-5.1 mEq/L |
| Chloride | 101 | 101-111 mEQ/L |
| CO2 | 22 | 24-36 mEq/L |
| BUN | 9 | 8-26 mg/dL |
| Creatinine | 0.78 | 0.44-1.03 mg/dL |
| Glucose | 167 | 70-99 mg/dL |
| Calcium | 9.2 | 8.5-10.5 mg/dL |
Table 3. Basic Metabolic Panel.
| Lab | Result | Reference Value |
|---|---|---|
| UPEP | Negative | Negative |
| SPEP | Negative | Negative |
| Homocysteine | 7.4 | <11 mcmol/L |
| Cold agglutinins | <1:32 | <1: 32 |
Table 4. Additional Work Up.
| Year and Author Name | Patient demographics | Type of CNS involvement |
|---|---|---|
| 1982, Alexander | 37, F | SAH |
| 1994, Bragoni | 44, F | Ischemic stroke |
| 1994, Urban | 41, F | Venous sinus thrombosis |
| 1995, Giordano | 55, F | SAH |
| 1996, Nagahiro | 17, F | Ischemic stroke |
| 2002, Koh | 50, F | Ischemic stroke |
| 2009, Klingler | 46, F | SAH |
| 2010, Hayashi | 54, F | SAH |
| 2012, Asai | 52, F | Thalamic hemorrhage |
| 2012, Ishizaka | 66, F | SAH |
| 2013, Wang | 39, F | ICH |
| 2013, Mercurio | 43, F | Venous sinus thrombosis |
| 2013, Mercurio | 44, F | Venous sinus thrombosis |
| 2016, Ho TH | 50, F | Venous sinus thrombosis |
| 2018, present patient | 61, F | ICH |
Table 5. List of case reports.
One of the causes of ischemic stroke is internal carotid atherosclerosis. There have been several studies showing a strong connection between systemic rheumatic disease and early atherosclerosis [22]. A non-randomized study done by Ciccone et al. evaluated the differences in carotid intima-media thickness (C-IMT) in patients affected by autoimmune diseases [23]. A comparison between systemic Scleroderma (SSc) patients versus other connective tissue disease patients (such as systemic lupus erythematosus, polymyositis/dermatomyositis, antiphospholipid syndrome, mixed connective tissue disease, Sjogren’s syndrome) versus control patients showed no differences of incidence in terms of atheromatous plaques in both SSc and other connective tissue diseases with higher number of plaques than control group. These investigators demonstrated the abnormal function of the endothelial layer in SSc group predisposes to macrovascular changes such as increased C-IMT (which can lead to ischemic events) and microvascular changes (which can lead to Raynaud’s phenomenon). Thus, patients with systemic rheumatic disease are at risk for ischemic events by the increased number of atheromatous plaques compared to general population but not by increased thickness of the carotid intima-media.
Our patient was a middle age female with new onset of cognitive issues and headaches that developed ICH with secondary SAH. Although the cerebral angiogram and brain and leptomeningeal biopsies were negative for CNS vasculitis, this could not be excluded since this patient had no risk factors that could cause the ICH (such as diabetes, hypertension, cerebral aneurism or vessel spasm, exposure to NSAIDs), Bifrontal lobe involvement was also unusual. Previous reports regarding small CNS vessel vasculitis reported a sensitivity of only 75% for leptomeningeal biopsy [24].
Currently there is no standard of care for SS with CNS involvement; previous reports described the use of immunosuppressive therapy involving high dose pulse steroids, Rituximab or Cyclophosphamide [24]. Our patient did receive methylprednisolone 1 g for 3 consecutive days, followed by oral prednisone 1 mg/ kg that was tapered down over the course of 4 months. The patient’s mentation improved in one week and the confusion resolved. She remains without any neurological deficits.
Conclusion
Our case emphasizes the importance of a taking a detailed history in ICH cases, which may detect an autoimmune etiology. Although extremely rare, ICH can be seen in patients with primary SS and CNS involvement. Increasing awareness about the neurologic manifestations of SS may lead to prompt treatment and better prognosis of these patients.
Conflicts of interest'
None
Funding
None
Acknowledgement
No one
References
- Tobon GJ, Pers JO, Devauchelle-Pensec V, et al. Neurological Disorders in Primary Sjogren’s Syndrome. Autoimmune Dis. 2012, 11 (2011).
- Baer AN, Fox R, Romain PL. Clinical manifestations of Sjogren’s syndrome: Extraglandular disease.
- Hayashi K, Morofuji Y, Suyama K, et al. Recurrence of Subarachnoid Hemorrhage Due to the Rupture of Cerebral Aneurysms in a Patient With Sjogren’s Syndrome. Neurol. Med. Chir. 50(8), 658–661 (2010).
- Alexander EL. Neurologic disease in Sjögren’s syndrome: mononuclear inflammatory vasculopathy affecting central/peripheral nervous system and muscle. A clinical review and update of immunopathogenesis. Rheum. Dis. Clin. North. Am. 19(4), 869–908 (1993).
- Delalande S, de Seze J, Fauchais AL, et al. Neurologic manifestations in primary Sjögren syndrome: a study of 82 patients. Med. 83(5), 280–291 (2004).
- Alexander EL, Malinow K, Lejewski JE, et al. Primary Sjögren’s Syndrome with Central Nervous System Disease Mimicking Multiple Sclerosis. Ann. Intern. Med. 104(3), 323–330 (1986).
- Vrethem M. Immunoglobulins within the central nervous system in primary Sjogren’s syndrome. J. Neurol. Sci. 100(1-2), 186–192 (1990).
- Pierot L. Asymptomatic cerebral involvement in Sjögren’s syndrome: MRI findings of 15 cases. Neuroradiology 35(5), 378–380 (1993).
- Morgen K. Central nervous system disease in primary Sjogrens syndrome: the role of magnetic resonance imaging. Semin. Arthritis. Rheum. 34(3), 623–630 (2004).
- Alexander EL, Craft C, Dorsch C, et al. Necrotizing arteritis and spinal subarachnoid hemorrhage in Sjögren syndrome. Ann. Neurol. 11(6), 632–635 (1982).
- Bragoni M, Di Piero V, Priori R, et al. Sjogren’s Syndrome Presenting as Ischemic Stroke. Stroke. 25(11), 2276–2279 (1994).
- Urban E, Jabbari B, Robles H. Concurrent cerebral venous sinus thrombosis and myeloradiculopathy in Sjögren’s syndrome. Neurology. 44(3), 554–556 (1994).
- Giordano MJ, Commins D, Silbergeld DL. Sjogren’s cerebritis complicated by subarachnoid hemorrhage and bilateral superior cerebellar artery occlusion: case report. Surg. Neurol. 43(1), 48–51 (1995).
- Nagahiro S, Mantani A, Yamada K, et al. Multiple cerebral arterial occlusions in a young patient with Sjögren’s syndrome: case report. Neurosurgery. 38(3), 592–595 (1996).
- Koh MS, Goh KY, Chen C, et al. Cerebral infarct mimicking glioma in Sjogren’s syndrome. Hong Kong. Med. J. 8(4), 292–294 (2002).
- Klingler JH, Glasker S, Shah MJ, et al. Rupture of a spinal artery aneurysm attributable to exacerbated Sjögren syndrome: case report. Neurosurgery. 64(5), E1010–E1011 (2009).
- Asai Y, Nakayasu H, Fusayasu E, et al. Moyamoya disease presenting as thalamic hemorrhage in a patient with neuromyelitis optica and Sjögren’s syndrome. J. Stroke. Cerebrovasc. Dis. 21(7), 619.e7–619.e9 (2012).
- Ishizaka S, Hayashi K, Otsuka M, et al. Syringomyelia and arachnoid cysts associated with spinal arachnoiditis following subarachnoid hemorrhage. Neurol. Med. Chir. 52(9), 686–690 (2012).
- Wang GQ, Zhang WW. Spontaneous intracranial hemorrhage as an initial manifestation of primary Sjögren’s syndrome: a case report. BMC. Neurol. 13, 100 (2013).
- Mercurio A, Altieri M, Saraceni VM, et al. Cerebral Venous Thrombosis Revealing Primary Sjögren Syndrome: Report of 2 Cases. Case. Rep. Med. 2013, 1–4 (2013).
- Ho TH, Hsu YW, Wang CW, et al. Cerebral Venous Sinus Thrombosis in A Patient with Sjögren’s Syndrome with Atypical Antibodies: A Case Report. Acta. Neurol. Taiwan. 25(2), 65–69 (2016).
- Kurmann RD, Mankad R. Atherosclerotic vascular disease in the autoimmune rheumatologic woman. Clin. Cardiol. 41, 258–263 (2018).
- Ciccone MM, Scicchitano P, Zito A, et al. Evaluation of differences in carotid intima-media thickness in patients affected by systemic rheumatic diseases. Intern. Emerg. Med. 10(7), 823–830 (2015).
- Haji-Ali RA, Calabrese LH. Primary angiitis of the central nervous system in adults.