Clinical Trial Teport - Interventional Cardiology (2011) Volume 3, Issue 4
A new standard of care for inoperable, severe aortic stenosis: implications of the PARTNER trial
- Corresponding Author:
- Susheel K Kodali
Columbia University Medical Center, 161 Fort Washington Avenue
5th Floor, New York, NY 10032, USA
Tel: +1 212 305 7060
Fax: +1 212 342 3660
E-mail: sk2427@columbia.edu
Abstract
Keywords
aortic stenosis, PARTNER, TAVR, transcatheter aortic valve replacement
With the aging of the population and the decline of rheumatic heart disease, calcific aortic stenosis (AS) has become the most common form of valvular heart disease requiring intervention in the developed world. The prevalence of calcific or ‘senile’ AS is estimated to be 2–8% among adults older than 65 years in the USA and Europe [1–3]. The prevalence continues to increase with age and may approach 30% in nonagenarians [4]. The pathophysiology of AS involves degeneration, inflammation and calcification of a normal tricuspid or congenitally bicuspid aortic valve [5]. Early in its course, the disease is often indolent, and patients are characteristically asymptomatic for a variable period of time. However, the disease progresses to cause obstruction to left ventricular outflow, concentric hypertrophy of the left ventricle with resultant hemodynamic effects including increases in wall stress and left ventricular and pulmonary artery pressures, and the eventual development of symptoms, including angina, syncope, and dyspnea. The onset of these cardinal symptoms marks a turning point in the course of severe AS, after which it becomes much more malignant. Disease progression accelerates and survival is extremely poor, averaging only 2–3 years with a high rate of sudden death [6,7].
Surgical aortic valve replacement (AVR) had previously been the only therapy demonstrated to improve both morbidity and mortality in patients with symptomatic, severe AS [7,8]. Multiple studies have demonstrated that the risk of surgery is relatively low in the absence of major comorbidities. Recent research has shown that the benefits of surgery are maintained even in the very elderly (age >80 years) and that the operative risk (in the absence of significant comorbidities) is not prohibitive in this group [9–13]. Thus, current American College of Cardiology (ACC), American Heart Association (AHA) and European Society of Cardiology (ESC) guidelines recommend AVR for all patients with symptomatic, severe AS [14,15]. In the absence of symptoms, additional class 1 indications for AVR include left ventricular systolic dysfunction and requirement for coronary artery bypass graft surgery or other surgery of the aorta or heart valves. Despite these guidelines, it is estimated that at least one-third of patients with symptomatic, severe AS do not undergo AVR for a variety of reasons, including severe left ventricular systolic dysfunction, advanced age and comorbid conditions [16]. Patients who are not candidates for surgery or who refuse surgery have a particularly poor prognosis with an average survival of less than 2 years [8,17–20].
No medical therapy has been shown to delay disease progression or to improve survival in patients with severe AS. Balloon aortic valvuloplasty (BAV) is a catheter-based therapy that involves inflating a balloon within a stenotic aortic valve to fracture calcifications, split fused commissures and increase orifice size [21]. BAV has been shown to modestly increase valve area, leading to a short-term improvement in symptoms [22–26]. However, these benefits are limited by rapid valvular restenosis and recurrence of symptoms, and therefore the absence of any long-term survival benefit [22,27–29]. Current guidelines support the use of BAV only as a bridge to surgery in hemodynamically unstable patients or as a palliative therapy in inoperable patients [14,15]. The failure of current medical therapy and BAV to significantly alter the prognosis of patients with severe AS has led to an intense research focus on novel percutaneous therapeutic strategies. In particular, transcatheter AVR (TAVR) has offered the tantalizing promise of achieving the benefits of surgical valve replacement, while obviating the need for extensive surgery, sternotomy, and cardiopulmonary bypass in high-risk operative candidates.
Introduction to the PARTNER trial
The first successful TAVR in a human being was performed in 2002 with a balloon-expandable, bovine pericardial valve that was delivered to the aortic position via the antegrade (transseptal) transfemoral vein approach [30]. Owing to technical difficulties and a high rate of procedural complications, this approach has now been supplanted by the retrograde transfemoral artery and antegrade transapical approaches [31,32]. There are currently two transcatheter heart valve systems that have undergone extensive clinical testing leading to CE mark (Conformité Européenne marking that is required for marketing in the European Economic Area) approval for use in Europe and abroad. The Edwards SAPIEN® valve system consists of a bovine pericardial tissue valve that is sewn into a balloon-expandable, stainless steel stent. The CoreValve (Medtronic) revalving system utilizes a porcine pericardial tissue valve that is mounted within a self-expanding nitinol stent. Pivotal, randomized trials are now comparing TAVR to standard therapy with both of these valve systems.
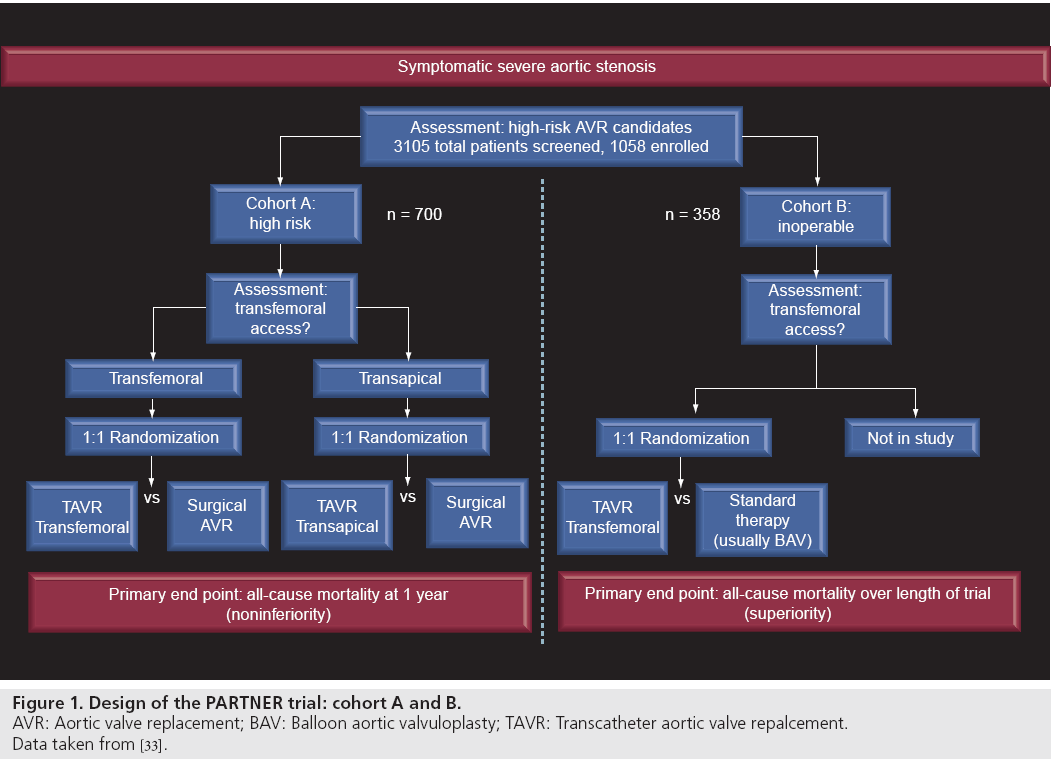
The Placement of Aortic Transcatheter Valves (PARTNER) trial was a multicenter, randomized clinical trial comparing TAVR with standard therapy in patients with severe symptomatic AS [33,34]. The trial was conducted in patients who were at high or prohibitive surgical risk for surgical AVR. Patients were enrolled into two cohorts based on their candidacy for AVR (Figure 1). Cohort A of the trial included patients who were considered to be surgical candidates despite elevated risk, as defined by a Society of Thoracic Surgeons (STS) risk score of greater than 10% or an estimated risk of mortality within 30 days of greater than 15%. Patients in this cohort were randomized to TAVR with the SAPIEN valve system (transapical or transfemoral approach based on vascular access) versus standard AVR. Cohort B enrolled patients who were determined not to be surgical candidates based on an estimated risk of death or serious, irreversible morbidity of greater than 50%. These patients were randomized to treatment with transfemoral TAVR with the SAPIEN valve system or standard medical therapy, which could include BAV. The results of cohort B of the PARTNER trial were published in the October 21, 2010 edition of the New England Journal of Medicine and represent the first randomized, controlled data on the safety and efficacy of TAVR.
Figure 1: Design of the PARTNER trial: cohort A and B. AVR: Aortic valve replacement; BAV: Balloon aortic valvuloplasty; TAVR: Transcatheter aortic valve repalcement.
Data taken from [33].
Background and rationale
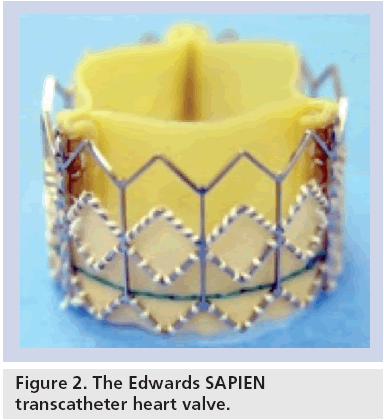
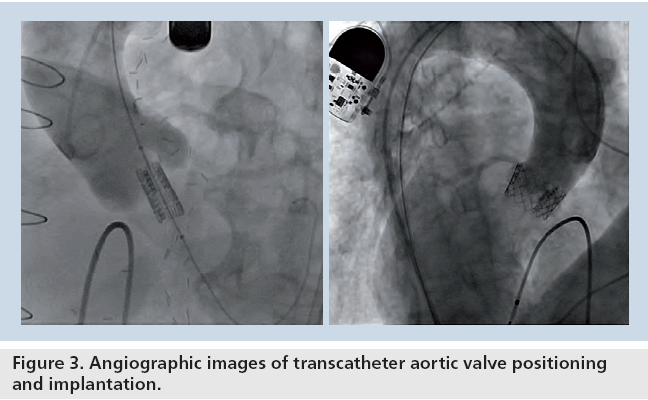
The Edwards SAPIEN transcatheter aortic valve studied in the PARTNER trial consists of a 23-mm or 26-mm diameter, trileaflet bovine pericardial valve that is hand sutured into a balloon- expandable, stainless-steel stent (Figure 2). For delivery, the sterile stent valve is crimped onto a standard balloon catheter. The device is introduced via a common femoral artery access site with either a 22 or 24 French sheath, depending on the valve size utilized. Following a standard balloon aortic valvuloplasty, the valve is advanced retrograde through the aorta under fluoroscopic guidance and is positioned within the native aortic valve (Figure 3). During deployment, rapid right ventricular pacing is performed to achieve mechanical asystole to minimize valve motion and migration. The procedure is typically performed with general anesthesia to facilitate transesophageal echocardiography, which is used to measure aortic annular dimensions, to assist with valve positioning, and to assess aortic regurgitation following deployment.
Several observational registries have shown retrograde transfemoral TAVR with the SAPIEN valve system to be safe and effective in patients considered inoperable or high-risk candidates for AVR. In initial feasibility trials, procedural success ranged from 86 to 93% and operative 30‑day mortality 7.3 to 12% [35–37]. Hemodynamic results were comparable to surgical AVR with minimal postprocedure valvular gradients and valve areas greater than 1.5 cm2. In these early feasibility studies, para-valvular aortic regurgitation has typically been mild with greater than 2+ regurgitation reported in only 5–10% of cases. However, procedural complications in these studies were frequent with major vascular complications ranging from 7–13% and cerebrovascular events ranging from 2.3 to 9.1% of cases. Other procedural complications, including annular rupture, coronary occlusion, and heart block occurred more rarely.
More recent observational studies of TAVR have reported improved procedural outcomes, likely reflecting advancements in patient selection, operator expertise, and valve platform technology. The SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) Registry, which was initiated to track outcomes after commercial approval of this device in Europe, reported improved procedural success of 95.1%, and 30‑day mortality of 6.3% [38]. Rates of procedural complications were similar to prior reports, including major vascular complications in 10.6% of cases and stroke in 2.4% of cases.
Limited data regarding long-term outcomes after TAVR with the SAPIEN system are available from the early observational series. These studies suggest that the initial hemodynamic results achieved with TAVR are durable for at least 1 year; valve areas have remained greater than 1.5 cm2 with no reports of structural valve deterioration, restenosis, or significant increases in paravalvular aortic regurgitation. 1-year mortality has ranged from 23.6 to 26%, and the majority of deaths have been non-valve related [35–37]. In fact, one of the series reported a valve-related 1-year mortality of less than 5% [37]. One recent study reported outcomes beyond 3 years for 70 patients who underwent successful TAVR and survived for at least 30 days [39]. Among these patients, survival was 57% and freedom from reoperation was 98.5%. There have been no reports of late valve deterioration, valve migration or stent fracture. Valve-related thromboembolic events appear to be rare, and long-term anticoagulation has not been routinely used. The favorable early and mid-term results of the initial observational studies of TAVR in high-risk patients paved the way for the current pivotal, randomized trials of this technology.
Design of the PARTNER Trial
Cohort B of the PARTNER trial was designed to compare transfemoral TAVR with standard therapy in patients with severe symptomatic AS, who were not considered to be candidates for AVR. Inclusion criteria included cardiac symptoms (New York Heart Association [NYHA] Class II or greater) and severe AS, which was defined as an aortic valve area of less than 0.8 cm2, a mean aortic valve gradient >40 mmHg, or a peak velocity >4.0 m/sec. Surgical risk assessment was carried out in each case by a cardiologist and two cardiovascular surgeons. Patients were considered to be inoperable if the estimated risk of either death within 30 days or a serious, irreversible condition exceeded 50%. Cardiovascular exclusion criteria included a bicuspid or noncalcified valve, significant aortic or mitral regurgitation (3+ or greater), severe left ventricular dysfunction (ejection fraction [EF] <20%), bulky calcifications near the coronary ostia, unrevascularized coronary artery disease, significant aortic vascular disease, and iliofemoral arteries that would not accommodate 22 or 24 French introducer sheaths. Subjects were also excluded on the basis of certain comorbid conditions, including severe chronic kidney disease (creatinine >3 milligrams per deciliter or requirement for dialysis), cerebrovascular accident or transient ischemic attack within 6 months, gastrointestinal hemorrhage within 3 months, bleeding diathesis, and life expectancy less than 12 months due to noncardiac disease.
Eligible patients were randomized to transfemoral TAVR with the SAPIEN aortic valve system or standard medical therapy. Patients in the standard therapy group were permitted to undergo BAV, but were prohibited from crossover to TAVR. Patients in the TAVR group were treated with heparin during the procedure and with dual antiplatelet therapy with aspirin and clopidogrel for at least 6 months if tolerated. All patients were followed for a minimum duration of 1 year. The primary end point was death from any cause during the duration of the trial. A coprimary end point was the hierarchical composite of death from any cause or repeat hospitalization due to valve- or procedure-related causes. Prespecified secondary end points included the rate of death from cardiovascular causes, the rate of repeat hospitalization, the NYHA functional class and 6‑min walk test performance. Safetyrelated end points included the rates of myocardial infarction, stroke, acute kidney injury, vascular complications, and bleeding. Of note, a prospectively-conducted quality-of-life substudy was conducted as part of the trial.
Results
The trial enrolled 358 patients at 21 sites between 2007 and 2009. The patients were randomized to TAVR (n = 179) or standard therapy (n = 179) and in the primary report of the trial were followed for a median duration of 1.6 years and a maximum duration of 2.8 years (follow-up is ongoing for 5 years). Overall, subjects were predominantly elderly (average age 83 years) and at high surgical risk (STS score 11.6 ± 6%). Comorbid conditions that contributed to patients being determined to be inoperable included oxygen-dependent respiratory failure (23.5%), frailty (23.1%), an extensively calcified (porcelain) aorta (15.1%) and chest wall deformity or irradiation (13.1%). At baseline, the two randomized groups were similar in terms of most identifiable characteristics, with the exception of a somewhat higher prevalence of atrial fibrillation (49 vs 33%) and COPD (52 vs 41%) in the standard therapy group.
Over 96% of the patients randomized to TAVR underwent the assigned procedure. The procedural outcomes and safety of TAVR in the study compare favorably with previously reported observational series [35–38]. Following TAVR, the mean aortic valve area increased from 0.6 to 1.5 cm2 and the mean gradient decreased from 45 to 11 mmHg at 30 days. Within 24 h of the procedure, two patients (1.1%) died, one patient (0.6%) had valve embolization, two patients (1.1%) required multiple valve implantations, and three patients (1.7%) had major strokes. The 30‑day mortality rate of 6.4% in the TAVR group was nearly identical to the rate of 6.3% reported from the SOURCE registry [38]. 84% of patients in the standard therapy group underwent BAV overall, including 64% within 30 days of randomization. In the standard therapy group, 4 patients (2.2%) underwent TAVR at nonparticipating sites abroad and 17 patients (9.5%) underwent AVR (AVR alone in 12 cases and AVR with placement of a conduit from the left ventricle to the aorta in 5 cases). There was no significant mortality difference between the TAVR and standard therapy groups at 30 days after randomization (5 and 2.8% respectively; p = 0.41).
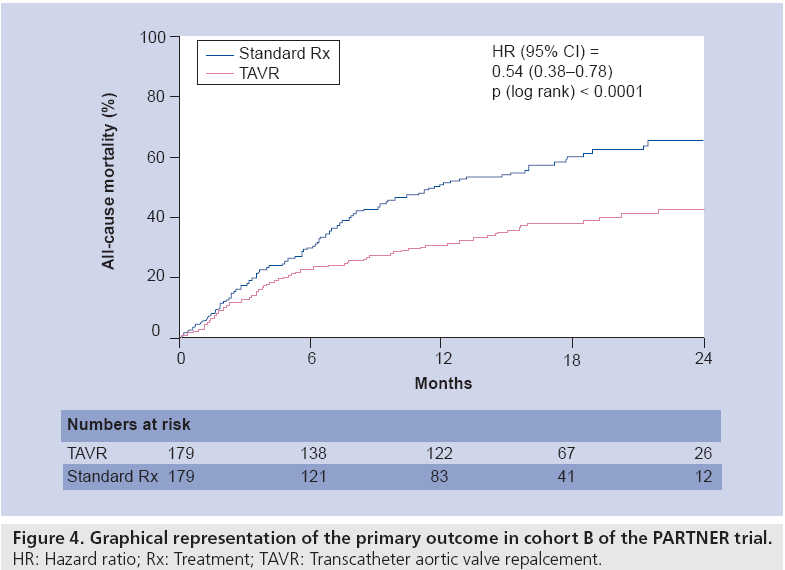
The primary end point of all-cause mortality at 1 year occurred in 30.7% of the TAVR group and 50.7% of the standard therapy group, representing an absolute mortality reduction of 20% (p < 0.001) (Figure 4). This corresponds with a number needed to treat with TAVR of only five patients to prevent one death compared to standard therapy. Cardiovascular death at 1 year was also reduced by TAVR, occurring in 20.5% of the TAVR group and 44.6% of the standard therapy group (p < 0.001). TAVR was also superior at 1 year with respect to the co-primary end point of the rate of the hierarchical composite of death from any cause or repeat hospitalization (p < 0.0001). Subgroup analyses demonstrated that the reduction in the primary end point was consistent across 10 major subgroups.
Certain important complications were more common in the TAVR group than the standard therapy group. Major strokes occurred in 5.0% of the TAVR group and 1.1% of the standard therapy group at 30 days (p = 0.06), but the composite of major stroke or death at 1 year still significantly favored TAVR (33 vs 51.3%, p < 0.001). Major vascular complications (16.2 vs 1.1%, p < 0.001) and major bleeding events (16.8 vs 3.9%, p < 0.001) at 30 days also occurred more frequently in the TAVR group. There were no statistically significant differences among the groups in the rates of other potentially important complications, including myocardial infarction, endocarditis, atrial fibrillation, permanent pacemaker placement, acute kidney injury and renal-replacement therapy.
Among patients who survived for 1 year, TAVR achieved durable hemodynamic results and was superior to standard therapy at improving symptoms. Echocardiographic follow-up of the TAVR group at 1 year showed no evidence of valve deterioration, no significant increase in valvular gradients, and no decrease in valve areas. Moderate or severe paravalvular regurgitation was present in 11.8% of patients at 30 days and 10.5% at 1 year. Overall, only 3 patients (1.7%) required repeat TAVR to treat significant aortic regurgitation. At 1 year, 74.8% of patients in the TAVR group were asymptomatic or had mild symptoms (NYHA class I or II) compared with 42% of patients in the standard therapy groups (p < 0.001). 6‑min walk test results were obtained at 1 year in a subgroup of patients and were also improved in the TAVR group, but were unchanged from baseline in the standard therapy group. In the prospectively conducted quality-of-life substudy of the trial, more favorable outcomes were observed with TAVR than with medical therapy; these data have been presented and are awaiting publication [40].
Summary
Severe AS is morbid condition with an average survival of only 2–3 years after the development of symptoms. Until recently, surgical AVR had been the only therapy demonstrated to improve mortality in this patient population. Standard medical therapy, including BAV, has been shown to result in a short-term improvement in symptoms, but has failed to offer any mortality benefit. Cohort B of the PARTNER trial compared TAVR with the SAPIEN valve system with standard therapy in patients with symptomatic, severe AS who were not candidates for AVR. In the trial, TAVR was superior to standard therapy in terms of the primary end point of overall mortality at 1 year. TAVR was also superior with respect to other important end points, including death from cardiovascular causes, death from any cause or repeat hospitalization, and death or major stroke. There were, however, higher 30‑day rates of major stroke, major vascular complications, and major bleeding in the TAVR group. At 1 year, TAVR was associated with improved symptoms and there was no evidence of valve deterioration or restenosis. This trial establishes TAVR as a new standard of care in inoperable patients with symptomatic severe AS.
Future perspective
The PARTNER trial is the first major randomized clinical trial of TAVR in patients with symptomatic, severe AS. Given the well-established benefits of AVR, it was necessary to study this unproven therapy in patients who were at elevated or prohibitive operative risk. The results of cohort B are promising in that they show a clear benefit of TAVR in patients who are not operative candidates. The results from cohort A will be published seperately and will compare transfemoral and transapical TAVR with standard AVR in high-risk surgical candidates. If the results continue to be favorable, it is likely that future studies will focus on the use of TAVR in increasingly low-risk patient populations. Further investigation will also be necessary to clarify the role of TAVR in patient groups who were excluded from the initial trials, such as patients with unrevascularized coronary artery disease or multiple valvular lesions, including significant mitral regurgitation. Studies in this group could address the intriguing possibility of hybrid procedures, such as TAVR with percutaneous coronary intervention or minimally invasive coronary artery bypass surgery or TAVR with percutaneous mitral valve intervention. Another patient group that will likely be the focus of ongoing investigation includes patients with failed tissue prosthetic valves in the aortic or mitral positions.
In parallel with expansion of the eligible patient population, transcatheter aortic valve technology can be expected to continue to evolve at a rapid pace. The current generation of the Edwards valve system (SAPIEN XT) has several important advantages over the versions employed in the PARTNER trial. The stent frame has a new geometry and is constructed from a thinner cobalt alloy, and the leaflet geometry has been redesigned to reduce stress and improve durability. These changes allow for a lower profile, 18–19 French delivery system which allows it to be used in smaller ilio-femoral vessels and may decrease the risk of vascular complications. The Sapien XT valve will be evaluated in the recently initiated PARTNER II trial. Additionally, the Medtronic CoreValve revalving system is also now being evaluated in a pivotal clinical trial. Other valve platforms are in earlier stages of development, but may also play an important role in the future of the field.
Given the advanced age and multiple comorbidities of many patients with AS, it will be important to address the impact of the procedure on quality of life. Early observational studies showed that TAVR can result in significant improvements in quality of life at 3 and 6 months after the procedure [41,42]. A study of the impact of TAVR on quality of life in the PARTNER trial was recently presented and showed that TAVR was superior to standard medical therapy at improving quality of life at 6 and 12 months in inoperable patients [40]. Similar quality of life analyses will also be carried out in cohort A of the PARTNER trial. Given the current intense focus on value in healthcare delivery, the cost–effectiveness of TAVR must be considered; a formal, prospectively- conducted cost–effectiveness analysis of the use of TAVR in the PARTNER trial is currently underway. As the use of TAVR expands to other patient populations, a continued focus on cost–effectiveness will be necessary to determine the best use of this breakthrough technology.
Information resources
▪ Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363(17), 1597–1607 (2010).
▪ Supplement to Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363(17), 1597–1607 (2010).
▪ Bonow RO, Carabello BA, Chatterjee K et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (Writing Committee to revise1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 52(13), e1–e142 (2008).
▪ Vahanian A, Baumgartner H, Bax J et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur. Heart J. 28(2), 230–268 (2007).
▪ Leon MB, Piazza N, Nikolsky E et al. Standardized end point definitions for transcatheter aortic valve implantation clinical trials: A consensus report from the Valve Academic Research Consortium. J. Am. Coll. Cardiol. 57(3), 253–269 (2011).
Financial and competing interests disclosure
Martin B Leon is a nonpaid member of scientific advisory board for Edwards Lifesciences and Medtronic. Susheel K Kodali is a Case Proctor /Consultant for Edwards Lifesciences. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Introduction, background & rationale of the Placement of Aortic Transcatheter Valves (PARTNER) trial
▪ Severe aortic stenosis (AS) is an extremely morbid disease with an average survival of only 2–3 years after the development of symptoms.
▪ Surgical aortic valve replacement (AVR) has been shown to improve both symptoms and survival in patients with symptomatic severe AS.
▪ Standard medical therapy and balloon aortic valvuloplasty may improve symptoms, but do not alter the prognosis of this disease.
▪ Early observational studies of transcatheter aortic valve replacement (TAVR) in patients with symptomatic severe aortic stenosis who are at high risk for AVR have shown favorable hemodynamic outcomes and have suggested improved survival relative to standard therapy.
Design of the PARTNER trial
▪ The PARTNER trial is a pivotal clinical trial that compared TAVR with the Edwards SAPIEN valve system to standard therapy in patients with severe symptomatic AS who were inoperable or high risk candidates for surgical AVR.
▪ Cohort B of the PARTNER trial randomized 358 patients with symptomatic, severe AS who were not candidates for surgical AVR to transfemoral TAVR versus standard medical therapy, including balloon aortic valvuloplasty.
Results
▪ The results of cohort B of the PARTNER Trial were published in the New England Journal of Medicine on 21 October 2010.
▪ The primary end point of all-cause mortality at 1 year occurred in 30.7% of the TAVR group and 50.7% of the standard therapy group (p < 0.001). This corresponds with a number needed to treat with TAVR of only five patients to prevent one death compared to standard therapy.
▪ TAVR was also superior to standard therapy with respect to death from cardiovascular causes, death from any cause or repeat hospitalization, and death or major stroke, despite being associated with higher 30-day rates of major stroke, major vascular complications and major bleeding.
▪ TAVR improved symptoms more than standard therapy as reflected by superior 6‑min walk test results and fewer patients with New York Heart Association class III or IV symptoms at 1 year.
▪ The rates of major stroke, major vascular complications and major bleeding were increased at 30 days in the TAVR group.
Conclusions and future directions
▪ This trial establishes TAVR as the new standard of care for suitable patients with symptomatic severe AS who are not candidates for surgical AVR.
▪ With ongoing technological advancements and additional clinical trials, the population of patients with AS who are candidates for TAVR is likely to continue to expand.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Aronow WS, Kronzon I. Prevalence and severity of valvular aortic stenosis determined by Doppler echocardiography and its association with echocardiographic and electrocardiographic left ventricular hypertrophy and physical signs of aortic stenosis in elderly patients. Am. J. Cardiol. 67(8), 776–777 (1991).
- Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J. Am. Coll. Cardiol. 21(5), 1220–1225 (1993).
- Stewart BF, Siscovick D, Lind BK et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 29(3), 630–634 (1997).
- Tunick PA, Freedberg RS, Kronzon I. Cardiac findings in the very elderly: analysis of echocardiography in fifty-eight nonagenarians. Gerontology. 36(4), 206–211 (1990).
- Akat K, Borggrefe M, Kaden JJ. Aortic valve calcification: basic science to clinical practice. Heart 95(8), 616–623 (2009).
- Frank S, Johnson A, Ross J Jr. Natural history of valvular aortic stenosis. Br. Heart J. 35(1), 41–46 (1973).
- Ross J Jr, Braunwald E. Aortic stenosis. Circulation 38(1 Suppl.), 61–67 (1968).
- Schwarz F, Baumann P, Manthey J et al. The effect of aortic valve replacement on survival. Circulation 66(5), 1105–1110 (1982).
- Asimakopoulos G, Edwards MB, Taylor KM. Aortic valve replacement in patients 80 years of age and older: survival and cause of death based on 1100 cases: collective results from the UK Heart Valve Registry. Circulation 96(10), 3403–3408 (1997).
- Chiappini B, Bergonzini M, Gallieri S et al. Clinical outcome of aortic valve replacement in the elderly. Cardiovasc. Surg. 11(5), 359–365 (2003).
- Chiappini B, Camurri N, Loforte A, Di Marco L, Di Bartolomeo R, Marinelli G. Outcome after aortic valve replacement in octogenarians. Ann. Thorac. Surg. 78(1), 85–89 (2004).
- Gehlot A, Mullany CJ, Ilstrup D et al. Aortic valve replacement in patients aged eighty years and older: early and long-term results. J. Thorac. Cardiovasc. Surg. 111(5), 1026–1036 (1996).
- Sundt TM, Bailey MS, Moon MR et al. Quality of life after aortic valve replacement at the age of >80 years. Circulation 102(19 Suppl. 3), III70–III74 (2000).
- Bonow RO, Carabello BA, Chatterjee K et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 52(13), e1–e142 (2008).
- Vahanian A, Baumgartner H, Bax J et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur. Heart J. 28(2), 230–268 (2007).
- Iung B, Baron G, Butchart EG et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 24(13), 1231–1243 (2003).
- Kojodjojo P, Gohil N, Barker D et al. Outcomes of elderly patients aged 80 and over with symptomatic, severe aortic stenosis: impact of patient’s choice of refusing aortic valve replacement on survival. QJM 101(7), 567–573 (2008).
- Kvidal P, Bergstrom R, Horte LG, Stahle E. Observed and relative survival after aortic valve replacement. J. Am. Coll. Cardiol. 35(3), 747–756 (2000).
- O’Keefe JH Jr, Vlietstra RE, Bailey KR, Holmes DR Jr. Natural history of candidates for balloon aortic valvuloplasty. Mayo Clin. Proc. 62(11), 986–991 (1987).
- Schueler R, Hammerstingl C, Sinning JM, Nickenig G, Omran H. Prognosis of octogenarians with severe aortic valve stenosis at high risk for cardiovascular surgery. Heart 96(22), 1831–1836 (2010).
- McKay RG, Safian RD, Lock JE et al. Balloon dilatation of calcific aortic stenosis in elderly patients: postmortem, intraoperative, and percutaneous valvuloplasty studies. Circulation 74(1), 119–125 (1986).
- Kuntz RE, Tosteson AN, Berman AD et al. Predictors of event-free survival after balloon aortic valvuloplasty. N. Engl. J. Med. 325(1), 17–23 (1991).
- Letac B, Cribier A, Koning R, Lefebvre E. Aortic stenosis in elderly patients aged 80 or older. Treatment by percutaneous balloon valvuloplasty in a series of 92 cases. Circulation 80(6), 1514–1520 (1989).
- McKay RG. The Mansfield Scientific Aortic Valvuloplasty Registry: overview of acute hemodynamic results and procedural complications. J. Am. Coll. Cardiol. 17(2), 485–491 (1991).
- Safian RD, Berman AD, Diver DJ et al. Balloon aortic valvuloplasty in 170 consecutive patients. N. Engl. J. Med. 319(3), 125–130 (1988).
- Safian RD, Warren SE, Berman AD et al. Improvement in symptoms and left ventricular performance after balloon aortic valvuloplasty in patients with aortic stenosis and depressed left ventricular ejection fraction. Circulation 78(5 Pt 1), 1181–1191 (1988).
- Davidson CJ, Harrison JK, Leithe ME, Kisslo KB, Bashore TM. Failure of balloon aortic valvuloplasty to result in sustained clinical improvement in patients with depressed left ventricular function. Am. J. Cardiol. 65(1), 72–77 (1990).
- Lieberman EB, Bashore TM, Hermiller JB et al. Balloon aortic valvuloplasty in adults: failure of procedure to improve long-term survival. J. Am. Coll. Cardiol. 26(6), 1522–1528 (1995).
- Otto CM, Mickel MC, Kennedy JW et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 89(2), 642–650 (1994).
- Cribier A, Eltchaninoff H, Bash A et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 106(24), 3006–3008 (2002).
- Lichtenstein SV, Cheung A, Ye J et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 114(6), 591–596 (2006).
- Webb JG, Chandavimol M, Thompson CR et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 113(6), 842–850 (2006).
- Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363(17), 1597–1607 (2010).
- Supplement to: S, Leon MB, Smith CR et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363(17), 1597–1607 (2010).
- Kodali S. Long term results following transcatheter AVR with the Cribier-Edwards valve via the transfemoral approach: update from the REVIVE II and REVIVAL II trials. Presented at:Transcatheter Cardiovascular Therapeutics. Washington, DC, USA, 12–17 Otober 2008.
- Rodes-Cabau J, Webb JG, Cheung A et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J. Am. Coll. Cardiol. 55(11), 1080–1090 (2010).
- Webb JG, Altwegg L, Boone RH et al. Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes. Circulation 119(23), 3009–3016 (2009).
- Thomas M, Schymik G, Walther T et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 122(1), 62–69 (2010).
- Gurvitch R, Wood DA, Tay EL et al. Transcatheter aortic valve implantation: durability of clinical and hemodynamic outcomes beyond 3 years in a large patient cohort. Circulation 122(13), 1319–1327 (2010).
- Cohen DJ. Health related quality of life after transcatheter aortic valve implantation vs. non-surgical therapy among inoperable patients with severe aortic stenosis: results from the PARTNER trial. Presented at: American Heart Association Scientific Sessions 2010. American Heart Association (AHA). Chicago, Illinois, USA, 13–17 November 2010.
- Bekeredjian R, Krumsdorf U, Chorianopoulos E et al. Usefulness of percutaneous aortic valve implantation to improve quality of life in patients >80 years of age. Am. J. Cardiol. 106(12), 1777–1781 (2010).
- Krane M, Deutsch MA, Bleiziffer S et al. Quality of life among patients undergoing transcatheter aortic valve implantation. Am. Heart J. 160(3), 451–457 (2010).
▪ Discusses the pathophysiology and molecular mechanisms of calcific aortic stenosis (AS).
▪▪ The most recent American College of Cardiology and American Heart Association guidelines for the management of valvular heart disease, including AS.
▪▪ The most recent European Society of Cardiology guidelines for the management of valvular heart disease, including AS.
▪ This study shows that at least one-third of patients with symptomatic, severe AS do not undergo aortic valve replacement, clearly demonstrating that there is an unmet clinical need for new therapies.
▪▪ The results of cohort B of the Placement of Aortic Transcatheter Valves (PARTNER) trial establish transcatheter aortic valve replacement as the new standard of care for appropriate patients with severe, symptomatic AS who are not operative candidates.
▪ The supplement further describes the design of the PARTNER trial, including the complete inclusion and exclusion criteria.
▪ Analysis of the impact of transcatheter aortic valve replacement on quality of life among the patients in the PARTNER trial.