Case Report - Diabetes Management (2018) Volume 8, Issue 3
A physician-patient’s perspective on lowering glycemic variability – Part I: The role of meal timing
- *Corresponding Author:
- Elsamma Chacko
Connecticut Valley Hospital
1000 Silver Street, Middletown, CT 06457, USA
E-mail: elsammac@msn.com
Abstract
A physician with a 19-year history of type 2 diabetes and impaired awareness of hypoglycemia sought to lower the hypoglycemia risk using continuous glucose monitoring to optimize the medications-meals-exercise triad. As part of the lifestyle modification the patient found distributing daily carbohydrate consumption skewed toward the morning, when physical activity is relatively high, lowered glycemic variability. She also found that the second meal could be the biggest meal of the day.
Keywords
Impaired awareness of hypoglycemia, continuous glucose monitor, resistance exercise, postprandial glucose
Introduction
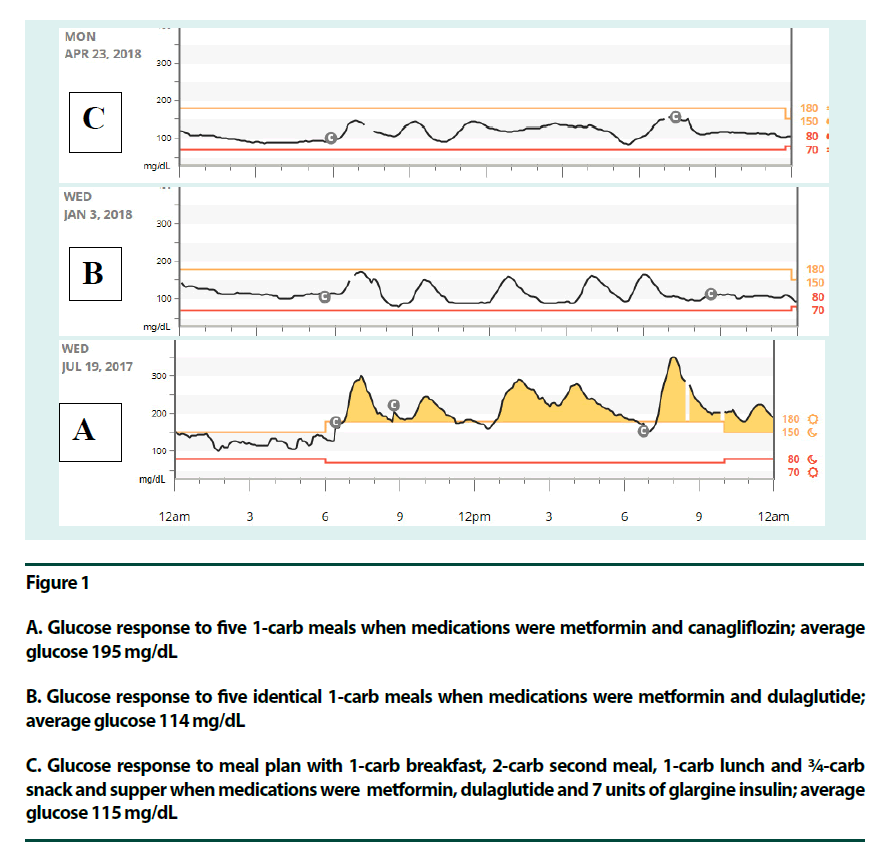
Lowering glycemic variability is thought to be more beneficial than lowering HbA1c or fasting blood glucose toward decreasing diabetes complications [1]. Lowering glycemic variability also means less hyperglycemia and hypoglycemia. The net, real-time response blood glucose levels have to medications, meals and exercise activities can be complex. The glucose response is dictated by the interplay of a large number of variables, including the individual’s state of diabetes, type and dosage of medications, meal timing and meal composition, and timing, intensity, duration and sequence of exercise. It is challenging to sort out the effects of one or a group of these variables on blood glucose levels under free-living conditions. Take meal plan, for example. How meal composition affects glycaemia, satiety and weight management is well documented in the literature [2-14] and in the official guidelines from diabetes organizations [15]. Balancing the meals by adding lean protein, extra fiber, healthy fat and vegetables has consistently shown glycaemia benefits. ADA guidelines, for example [15], recommend individualizing meal plans using the DASH diet [10], mediterranean diet [9], low fat diet, vegetarian/vegan diet [11,12], low carbohydrate diet [4-8] or diabetes plate method [15]. On the other hand, several studies have also shown the importance of meal timing in improving glycaemia [16-23], but they are not yet in the guidelines. Translational efforts in this area are exceedingly slow. This report details how a physician-patient adjusted meal timing to improve glycemic variability. (Part II reports on the role of exercise in this regard). The existence of a diurnal variation in glucose tolerance has been known since the 1970s [24]. Glucose levels are higher in the evening than in the morning. This effect is readily observed in older individuals. Decreased insulin levels are seen as contributing to the observed effect. Diminished physical activity during the evening hours is likely to add to the effect. One rather obvious tactic toward a more balanced glucose profile involves redistributing daily meals: eat the bigger meals in the early part of the day and go easy on carbohydrate intake towards the evening when physical activity tends to decline. Recent studies have lent signal support to this approach. Kahleova and colleagues show that two big meals a day, breakfast and lunch, are better than 6 small meals [16]. Jakubowicz and colleagues demonstrate that a big breakfast and small supper is better than small breakfast and big supper [17]. Mekary and colleagues show that skipping breakfast increases risk for type 2 diabetes [18]. Eating a breakfast itself decreases postprandial glucose of the second meal, the so called second-meal phenomenon [19,20]. Eating breakfast serves as the signal to our bodies to switch over to the incretin-insulin system from counter regulation. At this point free fatty acid levels go down. Much of the energy required for physical activity should now necessarily come from exogenous glucose, although muscle glycogen, endogenous glucose and free fatty acids would step in as fuel sources if needed. Two weight loss studies also showed early eaters doing better than late eaters [22,23]. The patient, who had been living with type 2 diabetes for 19 years, had also developed lately impaired awareness of hypoglycemia (IAH) [25]. After a second seizure episode, which came while driving to work in the morning, her endocrinologist prescribed continuous glucose monitoring (CGM) to closely monitor the medications-meals-exercise triad in near-real time and make defensive adjustments as needed. The patient was on metformin 1 gm twice a day and glargine insulin 36 units a day when she had the first seizure. The high insulin dose and not eating for eight hours on a busy day were identified as precipitating the first seizure. Insulin dose came down to 18 units when she started a breakfast-centered, low-carb – but otherwise balanced – meal plan which called for eating every 3-4 h [26]. The second seizure was related to exercise: it came ~2½ hours after a 10 min resistance exercise (RE) before her daily post-meal walk. Eating within two h after RE helped. The insulin dose came down further, to 7 units, when dulaglutide (0.75 mg/week) was added to the med regimen. Figure 1A shows the patient’s glucose profile on a meal plan with five 1-carb meals a day (75 gm carbohydrates total) while the medications were metformin and canagliflozine (SGLT-2 inhibitor): diurnal variation in glucose tolerance was significant. Postprandial glucose (PPG) of 6 pm supper (348 mg/dLor 19.3 mmol/L) was a lot bigger than the 6 am breakfast (294 mg/dL or 16.317 mmol/L). Fasting glucose was also high (163 mg/dL or 9.1 mmol/L), presumably due to high glucagon levels, side effect of SGLT-2 inhibitor [27]. These effects, however, disappeared when a DPP-4 inhibitor (linagliptin), GLP-1 R agonist (dulaglutide) or insulin was added to metformin and canagliflozin as a third agent or to metformin as a second agent. Figure 1B shows glucose response to five identical 1-carb meals when medications were metformin and dulaglutide: no significant diurnal variation in glucose tolerance was seen. In the absence of CGM, patients can personalize meal plan by adjusting the carb count using the guidelines: here, PPG <180 mg/dL (9.9 mmol/L) was used for breakfast as recommended by American Diabetes Association (ADA). For smaller meals PPG <140 mg/dL (7.77 mmol/L), which happens to be the recommendation by American Association of Clinical Endocrinologists (AACE). Figure 1C shows the glycaemia response to the patient’s personalized meal plan with two bigger meals in the morning and two smaller meals in the evening: breakfast was a 1-carb meal (1 egg scrambled, a slice of whole grain toast and a cup of coffee). The second meal was identical to the breakfast but had an extra cup of 1% milk making it a 2-carb meal. The rest of the meals were a 1-carb lunch followed by a ¾-carb evening snack and supper. Total carb intake was 5½ carbs (82.5 gm carbohydrates) per day. Medications for panel C was metformin, dulaglutide and 7 units of glargine insulin. The second-meal phenomenon [19,20] is clearly seen in all three panels of Figure 1. The carb-intake during breakfast was 1 carb (15 gm) in panels A, B & C. Carb intake of the second meal was increased from 1 carb in panels A & B to 2 carbs in panel C. It is clear that second meal could be the biggest meal of the day without compromising glucose levels. The meal plans as described here make sense physiologically. Meal plan in panel C can be ideal for non-exercise days: (breakfast could be bigger on exercise days). The carb count closely parallels the physical activity: as physical activity decreases, so does carbohydrate intake. The patient would not eat anything after 6 pm because a late dinner would result in elevated fasting glucose. Glycemic variability remains low. Hypoglycemia risk seems minimal likely on account of the lowered insulin dose and eating every 2-4 h. Moreover, the two bigger meals in the morning offered improved satiety during the active part of the day. A measure of self-experimentation goes hand in hand with self-management of chronic diseases. This is conspicuously so in the case of diabetes wherein levels of blood glucose depend on numerous variables. What is also true is that the average diabetes patient is not prepared to undertake the level of experimentation required for successful self-management. The perspective of this physician-patient with CGM has some merit: every variable except one can be kept constant while evaluation its effect on glucose and results can be reproduced. On the whole, adjusting carbohydrate intake as described here can be a valuable coping tool for people with diabetes. The downside here is the lack of statistical power. The applicability of such a calorie distribution in different populations should be confirmed by conventional studies for accelerated translation.
Figure 1
A. Glucose response to five 1-carb meals when medications were metformin and canagliflozin; average glucose 195 mg/dL
B. Glucose response to five identical 1-carb meals when medications were metformin and dulaglutide; average glucose 114 mg/dL
C. Glucose response to meal plan with 1-carb breakfast, 2-carb second meal, 1-carb lunch and ¾-carb snack and supper when medications were metformin, dulaglutide and 7 units of glargine insulin; average glucose 115 mg/dL
Summary
The patient confirmed that SGLT-2 inhibitors were better used with insulin or insulin secretagogues (DPP-4 inhibitors or GLP-1 R agonist) in this long-standing type 2 diabetes patient, likely due to low insulin levels. Minimizing insulin dose, from 36 units to 7 units, was a good approach in preventing hypoglycemia. Eating more carbs in the early part of the day and eating small meals or snacks every 2-4 h also helped toward lowering glycemic variability and hypoglycemia risk.
Acknowledgement
The authors thank Jorge Munoz, RN, APRN for his technical assistance with preparing the figures used in this report.
References
- Ceriello A, Kilpatrick E. Glycemic variability: both sides of the story. Diabetes. Care. 36(2), S272–275 (2013).
- Reynolds L, Douglas S, Kearney M et al. A pilot study examining the effects of consuming a high-protein vs normal-protein breakfast on free-living glycemic control in overweight/obese 'breakfast skipping' adolescents. Int. J. Obes. (Lond). 39(9), 1421–1424 (2015).
- Lim E, Hollingsworth K, Aribisala B et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 54(10), 2506–2514 (2011).
- Tay J, Luscombie-Marsh N, Thomson C et al. Very low carbohydrate, low saturated fat diet for type 2 diabetes management: a randomized trial. Diabetes. Care. 37(11), 2909–2918 (2014).
- Fenton C, Fenton T. Dietary carbohydrate restriction: Compelling theory for further research. Nutrition. 32(1), 153 (2016).
- Snorgaard O, Poulson G, Anderson H et al. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ. 5(1), (2016).
- Neilsen J, Gando C, Joensson E et al. Low carbohydrate diet in type 1 diabetes, long-term improvement and adherence: A clinical audit. Diabetol. Metab. Syndr. 4, 23. (2012).
- Yancy W, Foy M, Chalecki A et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. 2, 34 (2005).
- Esposito K, Maiorino M, Bellastella G et al. Mediterranean diet for type 2 diabetes: cardio metabolic benefits. Endocrine 56(1), 27–32 (2017).
- Sacks F, Svetkey L, Vollmer W et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N. Eng. J. Med. 344(1), 3–10 (2001).
- Barnard N, Katcher H, Jenkins D et al. Vegetarian and Vegan diets in type 2 diabetes management. Nutr. Rev. 67(5), 255–263 (2009).
- Miller V, Mente A, Dehghan M et al. Fruit, vegetable and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 390 (10107), 2037–2049 (2017).
- Bozzetto L, Costabile G, Luongo D et al. Reduction in liver fat by dietary MUFA in type 2 diabetes is helped by enhanced hepatic fat oxidation. Diabetologia. 59(12), 2697–2701 (2006).
- Gannon M, Nuttall F. Control of blood glucose in type 2 diabetes without weight loss by modification of diet composition. Nutr. Metab. (Lond). 3, 16 (2006).
- Lifestyle Management: Standards of Medical Care in Diabetes – 2018. Diabetes. Care. 41(S1), 38–50 (2018).
- Kahleova H, Belinova L, Malinska H et al. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: a randomized crossover study. Diabetologia. 57(8), 1552–1560.
- Jakubowicz D, Wainstein J, Ahren B. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: a randomized clinical trial. Diabetologia 58(5), 912–919 (2015).
- Mekary R, Giovannucci E, Willett W et al. Eating patterns and type diabetes risk in men: breakfast omission, eating frequency, and snacking. Am. J. Clin. Nutr. 95(5), 1182–1189 (2012).
- Jovanovic A, Gerrard J, Taylor R. The second-meal phenomenon in type 2 diabetes. Diabetes. Care. 32(7), 1199-1201 (2009).
- Lee S, Tura A, Mari A et al. Potentiation of the early-phase insulin response by a prior meal contributes to the second-meal phenomenon in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 301(5), E984-90 (2011).
- Kuwata H, Iwasaki M, Shimizu S et al. Meal sequence and glucose excursion, gastric emptying and incretin secretion in type 2 diabetes: a randomized, controlled crossover, exploratory trial. Diabetologia. 59(3), 453–461 (2016).
- Madjd A, Yaylor M, Delavari A et al. Beneficail effect of high energy intake at lunch rather than dinner on weight loss in healthy obese women in a weight loss program: a randomized clinical trial. Am. J. Clin. Nutr. 104(4), 982–989 (2016).
- Garaulet M, Gomez-Abellan P, Alburquerque-Bejar J. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 37(4), 604–11 (2013).
- Zimmet P, Wall J, Rome R et al. Diurnal variation in glucose tolerance: Associated changes in plasma insulin, growth hormone, and non-esterified fatty acids. Br. Med. J. 1(5906), 485–488 (1974).
- Iqbal A, Heller S. The role of structured education in the management of hypoglycaemia. Diabetologia 61(4), 751–760 (2018).
- Chacko E, Awruch P, Swartz E. Breakfast-centered meal plan for people with diabetes: a modest cohort study under free-living conditions. Diabetes. Manag. 8(1), 32–37 (2018).
- Bonner C, Kerr-Conte J, Gmyr V et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat. Med. 21(5), 512– 517 (2015).