Case Report - Interventional Cardiology (2020) Volume 12, Issue 1
Very late coronary thrombosis following bioresorobable vascular scaffold implantation for spontaneous coronary artery dissection
- Corresponding Author:
- Arif Al-Nooryani
Cardiology Department, Al Qassimi
Hospital, Sharjah, United Arab Emirates
E-mail: arif345@yahoo.com
Received date: February 11, 2020; Accepted date: February 21, 2020; Published date: February 28, 2020
Abstract
Abstarct: We present a case of acute coronary syndrome 6 years after successful bioresorbable vascular scaffold (BVS) implantation for right coronary artery spontaneous coronary artery dissection (SCAD). OCT demonstrated neoatherosclerotic intermediate lesion with organized thrombus, whereas fractional flow reserve documented hemodynamically significant lesion at the place of previous BVS.
Keywords
Spontaneous coronary artery dissection; Bioresorbable vascular scaffold; Late complications; Fractional flow reserve
Introduction
Spontaneous coronary artery dissection (SCAD) is rare but well recognized cause of acute coronary syndromes (ACS), mostly affecting young to middle-aged subjects preferable women [1]. By definition, SCAD refers to the acute development of a false lumen within the coronary artery wall which may compromise coronary flow by external compression of the true lumen. Pathophysiologically, SCAD is a distinct entity in ACS spectrum, and there are key differences in management and outcomes compared to ACS of atherosclerotic etiology [2,3]. Opposite to conventional ACS, conservative strategy is generally favored since revascularization in patients with SCAD is very challenging due to the presence of an underlying disrupted and friable coronary vessel wall, even leading to worse outcomes following percutanous coronary intervention (PCI) [2-4]. In a settings of usually absent atherosclerotic substrate, bioresorbable vascular scaffolds (BVS) due to its unique properties may be preferable option in comparison to caging metallic life-long stents, and may represent an attractive strategy when intervention for SCAD is necessary [5].
Case Report
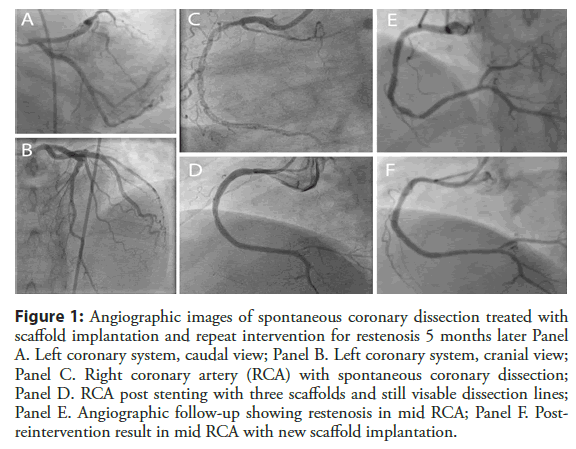
We present a case of 45 years old male patient admitted for chest pain syndrome following previous PCI procedures with BVS implantation. His current coronary artery disease risk profile included hypertension, smoking and diabetes mellitus type 2. Six years ago, he experienced acute coronary syndrome followed by invasive coronarography showing single vessel disease with long spiral dissection in right coronary artery (RCA) with slow flow to distal RCA - SCAD type 1 (Figure 1, panel C), with normal left coronary arteries (Figure 1, panels A and B). Due to limiting flow and ongoing chest pain ad hoc PCI was undertaken. Following numerous attempts with several coronary wires and using microcatheter support, distal true lumen wire position was finally achieved, and three overlapping Absorb BVS (Abbott Vascular, Abbott Park, Illinois) followed by post-dilatation, were implanted to cover the whole dissected segment from proximal to distal RCA (Absorb 3.5 × 28 mm, Absorb 3.0 × 28 mm and Absorb 2.5 × 28 mm). Post-stenting images showed good angiographic result with resultant TIMI 3 flow in RCA; still, some dissection lines were present in the mid portion of RCA (Figure 1, panel D) probably due to segmental wire passage in the false lumen and BVS implantation in the false lumen.
Figure 1: Angiographic images of spontaneous coronary dissection treated with scaffold implantation and repeat intervention for restenosis 5 months later Panel A. Left coronary system, caudal view; Panel B. Left coronary system, cranial view; Panel C. Right coronary artery (RCA) with spontaneous coronary dissection; Panel D. RCA post stenting with three scaffolds and still visable dissection lines in mid RCA; Panel E. Angiographic follow-up showing restenosis in mid RCA; Panel F. Post- reintervention result in mid RCA with new scaffold implantation.
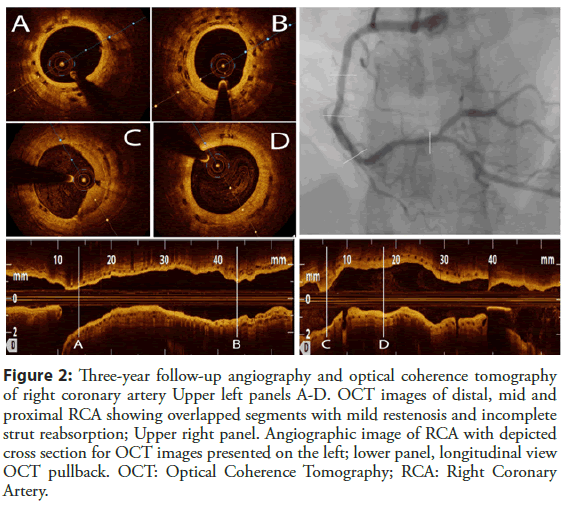
Patient was safely discharged on dual antiplatelet therapy of Aspirin and Clopidogrel, 100 mg and 75 mg daily, respectfully. Due to chest pain patient was readmitted 5 months later and repeat coronarography revealed in-stent restenosis in mid RCA (correspondent to the segment with still present dissection lines), which was treated with one more additional Absorb BVS implantation with optimization, covering only restenotic segment (Absorb 3.5 × 28 mm) (Figure 1, panels E and F). Due to atypical chest pain complaints, follow-up angiography was performed 2 and 3 years after index procedure (Figure 2) and showed diffuse mild in-stent restenosis in RCA which was confirmed with optical coherence tomography (OCT) imaging. OCT demonstrated neointimal formation with sings of early scaffold resorption and dismantling, with areas of 2 BVS layers in the place of BVS restenosis treated with another BVS (Figure 2).
Figure 2: Three-year follow-up angiography and optical coherence tomography of RCA Upper left panels A-D. OCT images of distal, mid and proximal RCA showing overlapped segments with mild restenosis and incomplete strut reabsorption; Upper right panel. Angiographic image of RCA with depicted cross section for OCT images presented on the left; lower panel, longitudinal view OCT pullback. OCT: Optical Coherence Tomography; RCA: Right Coronary Artery.
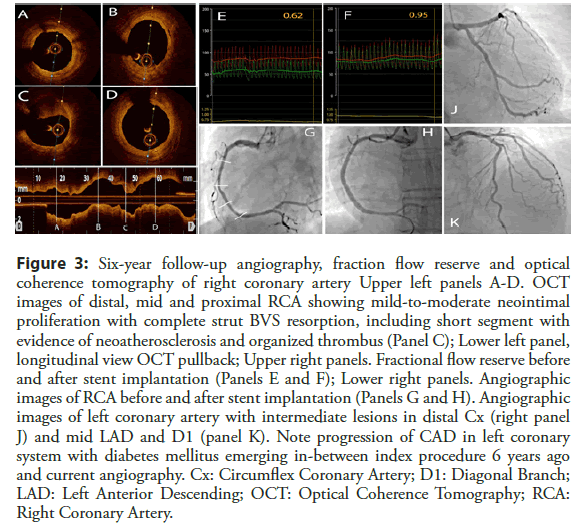
Conservative treatment was continued. Patient was free of angina symptoms for additional three years, but developed diabetes mellitus type 2. Two weeks ago he started to experience sudden chest pain of short duration in resting conditions, accompanied by dyspnea on exertion with variable frequency and severity. The patient underwent repeat angiography which showed new angiographically intermediate lesions of LAD, D1, OM branche, with mild-to-moderate and hazy lesion in mid RCA on the place of previously implanted BVS (Figure 3, Panel J and K). Invasive functional interrogation with fractional flow reserve (FFR) showed non-significant lesions in mid LAD (FFR 0.85), D1 (FFR 0.87), and OM2 (FFR 0.88), and surprisingly FFR of 0.62 in RCA (Figure 3, panel E).
Figure 3: Six-year follow-up angiography, fraction flow reserve and optical coherence tomography of RCA Upper left panels A-D. OCT images of distal, mid and proximal RCA showing mild-to-moderate neointimal proliferation with complete strut BVS resorption, including short segment with evidence of neoatherosclerosis and organized thrombus (Panel C); Lower left panel, longitudinal view OCT pullback; Upper right panels. Fractional flow reserve before and after stent implantation (Panels E and F); Lower right panels. Angiographic images of RCA before and after stent implantation (Panels G and H). Angiographic images of left coronary artery with intermediate lesions in distal Cx (right panel J) and mid LAD and D1 (panel K). Note progression of CAD in left coronary system with diabetes mellitus emerging in-between index procedure 6 years ago and current angiography. Cx: Circumflex Coronary Artery; D1: Diagonal Branch; LAD: Left Anterior Descending; OCT: Optical Coherence Tomography; RCA: Right Coronary Artery.
OCT imaging confirmed almost fully resorbed BVSs (Figure 3, lower panel), diffuse neointimal proliferation (Figure 3, panels A, B and D) with short in-stent eccentric restenosis in mid RCA showing signs of neo-atherosclerosis with superimposed organized thrombus (Figure 3, panel C). Since the patient had restenosis following BVS implantation, we decided to treat scaffold failure with 2 drug-eluting stent implantation with OCT guidance (Xience PRO 3.5 × 28 mm and 3.5 × 18 mm; Abbott Vascular, Abbott Park, Illinois) covering restenotic part (Figure 3, panels G and H). Repeat FFR showed optimal hemodynamic result (Figure 3, panel F). The patient was discharged on dual antiplatelet therapy, beta-blockers, combined statin and ezetimibe, and combination of metformine and DPP-4 inhibitors.
Discussion
Optimal treatment strategy in SCAD remains challenging due to different clinical and angiographic presentation with no randomized studies to guide practical decisions [2,3]. In cases of long lesions and limited flow with ongoing chest pain and myocardial ischemia, PCI, or even CABG, may be valuable option. However, success rate of PCI results is far from ideal being more than 50% in some large studies, later accompanied by stent thrombosis, PCI complications and even CABG emergency [2-4]. Biodegradable concept of BVS makes these devices attractive treatment option for patients with SCAD, being often very young and with a need to cover long segments of dissection. Few earlier case reports and series of patients confirmed excellent feasibility and short-to-midterm success of BVS in SCAD with no adverse effects in the follow-up [5]. Our case presents for the first time very late thrombosis in patient with overlapping BVS completely resorbed 6 years after index procedure.
In brief, BVS were designed to overcome limitations of metallic stents and allow long-term benefits by reducing late inflammation and thrombosis, with no caging of coronary arteries or jailing of the side branches [6]. Initial studies with BVS showed that the safety and efficacy of Absorb BVS is similar to best in class DES [7], however later mid-term follow-up [8,9] have questioned long-term safety of Absorb BVS due to higher rate of device thrombosis in comparison to DES, leading to interruption in clinical application and marketing of the device [6]. We have also demonstrated in the mid to long-term follow-up of the first 300 implanted BVS [10] that the outcome of BVS in high risk patients is respectable, but the patients with heart failure, multi-vessel and long BVS implantation were associated with worse outcome. In addition, although early intracoronary imaging studies [6] demonstrated favorable effects of vessel remodeling with late luminal enlargement and restoration of vasomotion, recent ABSORB Japan [11] study with 3 years serial multimodality imaging showed smaller luminal dimensions, greater neointimal formation, absent restoration of vasomotion, with intraluminal scaffold dismantling as one of the mechanisms of very late stent thrombosis. All these new imaging findings seems to be consistent with angiographic and OCT appearance of our patient, with continuous increase of neointimal formation over time, and very late scaffold thrombosis.
Data on the role of invasive functional testing in patients with in-stent restenosis are scarce, and absent in case of BVS restenotic lesions. The pathophysiologic and mechanistic nature of neo- atherosclerotic lesion following BVS implantation is different from natural atherosclerotic progression with possible different hemodynamic consequences. In addition, it has been previously shown that lesions of the same anatomic severity may have completely different hemodynamic significance in different segments of different coronary arteries - i.e. >80% of intermediate lesions in RCA, mid and distal Cx artery are in fact functionally non-significant [12]. Thus, the finding of highly hemodymanically significant mild to moderate lesion in RCA following 6 years following BVS implantation is remarkable, and could be easily underestimated.
Conclusion
Adequate treatment of SCAD in the presence if ongoing chest pain and myocardial ischemia remains challenging. Due to its disintegrated nature over time, BVS may be promising treatment strategy and alternative in patients with ongoing chest pain and angiographic presentation of SCAD [5]. Imaging following BVS implantation and especially in case of follow-up stent/scaffold failure is necessary to obtain good mechanistic understanding and achieve optimal result [11]. New generation of scaffolds with improved properties may show encouraging results in this lesion subset [13]. Despite different pathophysiologic mechanisms of coronary lesion progression, invasive functional evaluation remains recommended method of choice to confirm hemodynamic significance of the lesion.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Acknowledgment
The authors would like to thank Sister Rowaide Najeeb and Mrs. Kirti Sharma for generous help in preparing this case report.
References
- Saw J, Mancini GB, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. 2016;68:297-312.
- Adlam D, Alfonso F, Maas A, et al. European Society of Cardiology, acute cardiovascular care association, SCAD study group: A position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39:3353-3368.
- Hayes SN, Kim ESH, Saw J, et al. Spontaneous coronary artery dissection: Current state of the science: A scientific statement from the American heart Association. Circulation. 2018;137:e523-e557.
- Conrotto F, D’Ascenzo F, Cerrato E, et al. Safety and efficacy of drug eluting stents in patients with spontaneous coronary artery dissection. Int J Cardiol. 2017;238:105-109.
- Panoulas VF, Ielasi A. Bioresorbable scaffolds and drug-eluting balloons for the management of spontaneous coronary artery dissections. J Thorac Dis. 2016;8:e1328-e1330.
- Byrne RA, Stefanini GG, Capodanno D, et al. Report of an ESC-EAPCI Task Force on the evaluation and use of bioresorbable scaffolds for percutaneous coronary intervention: executive summary. Eur Heart J. 2018;39:1591-1601.
- Stone GW, Gao R, Kimura T, et al. 1-year outcomes with the Absorb bioresorbable scaffolds in patients with coronary artery disease: A patient-level, pooled meta-analysis. Lancet. 2016;387:1277-1289.
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): A 3-year, randomized, controlled, single-blind, multicenter clinical trial. Lancet. 2016;388:2479-2491.
- Wykrzikovska JJ, Kraak RP, Hofma SH, et al. Bioresorbable scaffolds versus metallic stents in routine PCI. N Engl J Med. 2017;376:2319-2328.
- Al Nooryani A, Elabbassi WN, AlBaba M, et al. Long term outcome of first 300 implanted Absorb vascular bioresorbable vascular scaffolds in an all-comers Middle East population. J Int Med Res. 2019;47:173-187.
- Onuma Y, Honda Y, Asano T, et al. Randomized comparison between everolimus-eliting bioresorbable scaffold and metallic stent. Modality imaging through 3 years. JACC Cardiovasc Interv. 2020;13:116-127.
- Koo BK, Yang HM, Doh JH, et al. Optimal intravascular ultrasound criteria and their accuracy for defining the functional significance of intermediate coronary stenoses of different locations. JACC Cardiovasc Interv. 2011;4:803-811.
- Hideo-Kajita A, Garcia-Garcia HM, Kolm P, et al. Comparison of clinical outcomes between Magmaris and Orsiro drug eluting stent at 12 months: Pooled patient level analysis from BIOSOLVE II-III and BIOFLOW II trials. Int J Cardiol. 2020;300:60-65.